Abstract
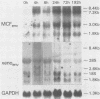
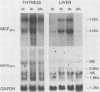
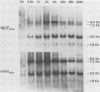
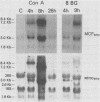
Northern (RNA) analyses were used to study the kinetics of induction of endogenous mink cell focus-forming (MCF) and xenotropic murine leukemia virus (MuLV)-related sequences in NFS and C57BL/6 mice injected with the polyclonal immune activators lipopolysaccharide (LPS), concanavalin A, and 8-bromoguanosine. All three mitogens induced 8.4-, 7.2-, 3.0-, and 1.8-kilobase (kb) MCF-related transcripts coordinately in the spleens of injected mice. Xenotropic MuLV-related expression was also rapidly induced in spleens by the three polyclonal immune activators, but in a noncoordinate manner: a distinct set of transcripts with different kinetics of expression was induced by each mitogen. MCF-related induction after LPS injection was both rapid and sustained; it began within 30 min and persisted for at least 8 days in the spleens of both NFS and C57BL/6 mice. LPS also caused prolonged induction of xenotropic transcripts in spleens of C57BL/6 but not NFS mice. The gld mutation, which causes polyclonal immune activation, induced 8.4-, 10.0-, and 13-kb MCF-related transcripts in C3H/HeJ mice without altering expression of 7.2-, 5.6-, 4.0-, 3.0-, or 1.8-kb MCF-related transcripts. The data demonstrate that individual endogenous MuLV-related transcripts can be induced coordinately or independently and suggest that expression of these transcripts is linked to early stages of lymphocyte activation.
Full text
PDF
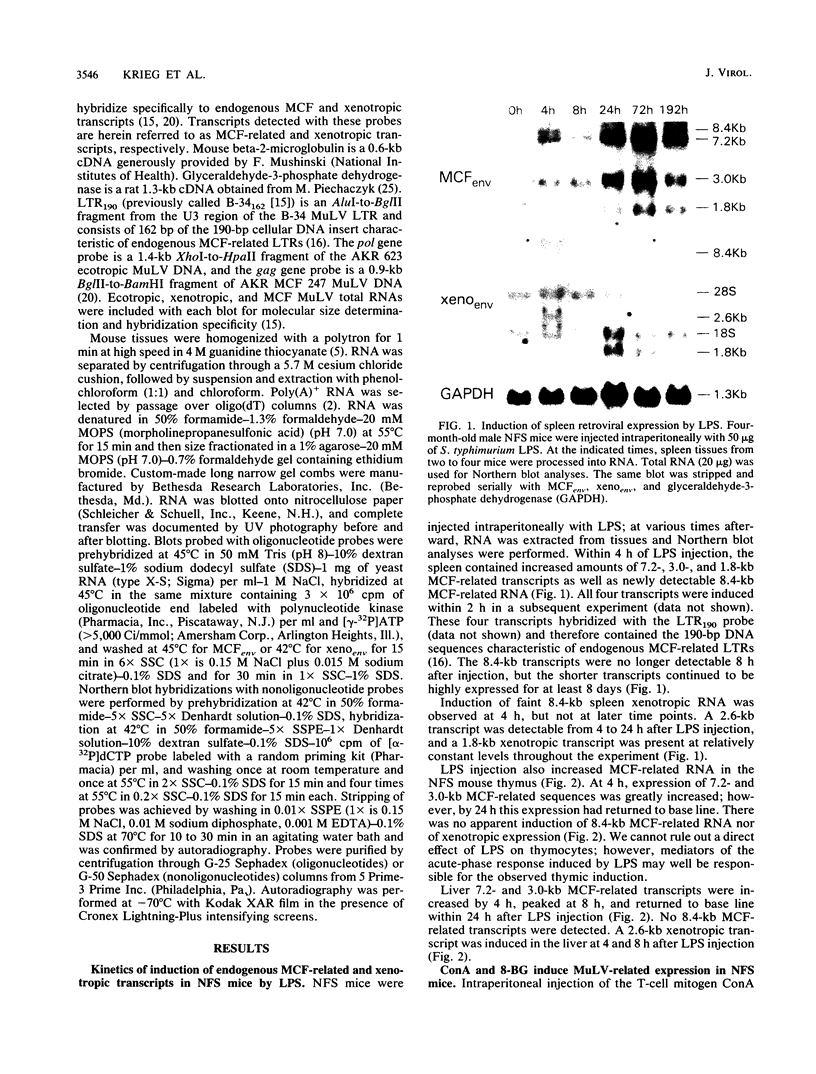
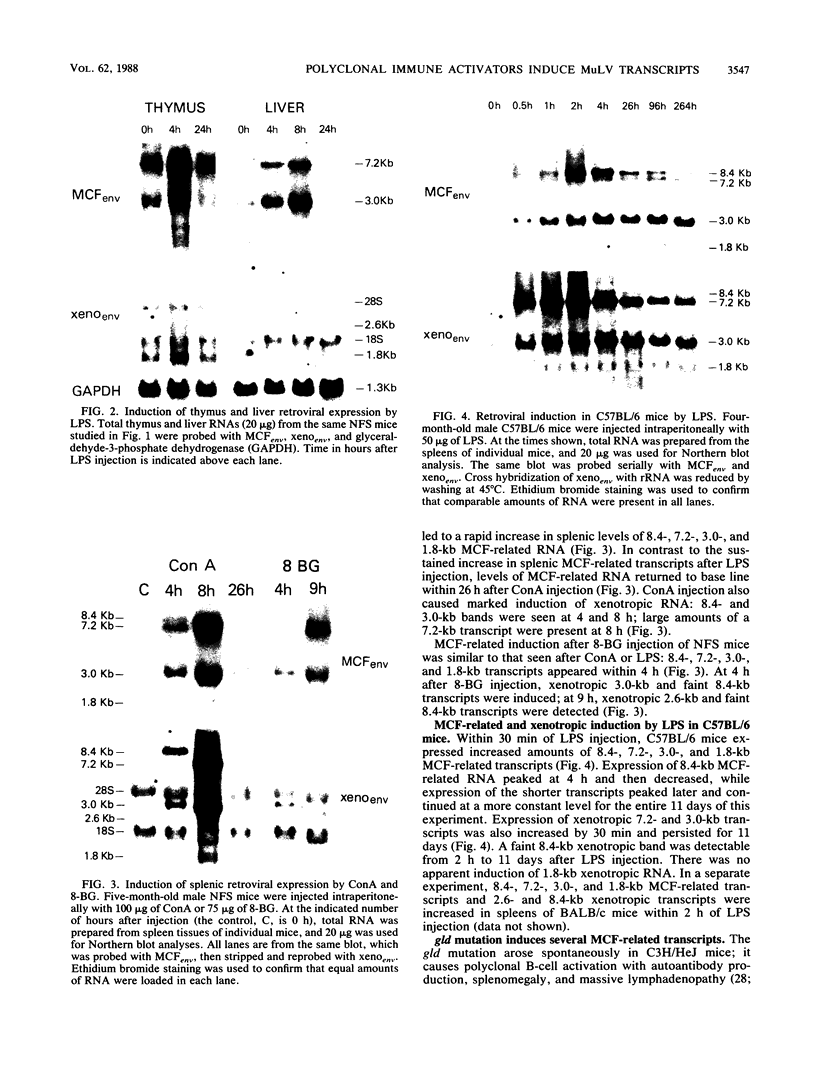
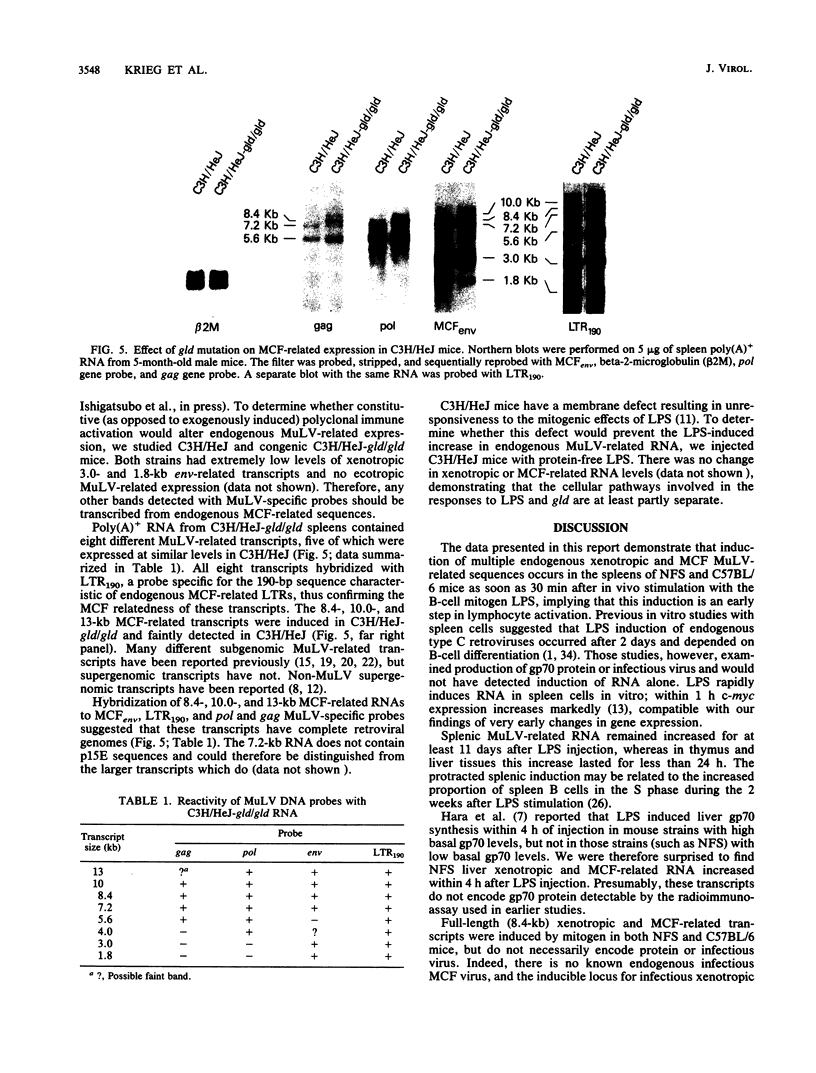


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberto B. P., Callahan L. F., Pincus T. Evidence that retrovirus expression in mouse spleen cells results from B cell differentiation. J Immunol. 1982 Dec;129(6):2768–2772. [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt C., Mileham K., Haas M., Nesbitt M. N., Harper M. E., Simon M. I. Chromosomal mapping of the mink cell focus-inducing and xenotropic env gene family in the mouse. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6298–6302. doi: 10.1073/pnas.80.20.6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Greenberger J. S., Phillips S. M., Stephenson J. R., Aaronson S. A. Induction of mouse type-C RNA virus by lipopolysaccharide. J Immunol. 1975 Jul;115(1):317–320. [PubMed] [Google Scholar]
- Hara I., Izui S., McConahey P. J., Elder J. H., Jensen F. C., Dixon F. J. Induction of high serum levels of retroviral env gene products (gp70) in mice by bacterial lipopolysaccharide. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4397–4401. doi: 10.1073/pnas.78.7.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman S. A., Coffin J. M. Efficient packaging of readthrough RNA in ALV: implications for oncogene transduction. Science. 1987 May 15;236(4803):845–848. doi: 10.1126/science.3033828. [DOI] [PubMed] [Google Scholar]
- Hoggan M. D., O'Neill R. R., Kozak C. A. Nonecotropic murine leukemia viruses in BALB/c and NFS/N mice: characterization of the BALB/c Bxv-1 provirus and the single NFS endogenous xenotrope. J Virol. 1986 Dec;60(3):980–986. doi: 10.1128/jvi.60.3.980-986.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izui S., McConahey P. J., Dixon F. J. Increased spontaneous polyclonal activation of B lymphocytes in mice with spontaneous autoimmune disease. J Immunol. 1978 Dec;121(6):2213–2219. [PubMed] [Google Scholar]
- Jakobovits A., Sharon N., Zan-Bar I. Acquisition of mitogenic responsiveness by nonresponding lymphocytes upon insertion of appropriate membrane components. J Exp Med. 1982 Oct 1;156(4):1274–1279. doi: 10.1084/jem.156.4.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N., Pfeifer-Ohlsson S., Kato M., Larsson E., Rydnert J., Ohlsson R., Cohen M. Tissue-specific expression of human provirus ERV3 mRNA in human placenta: two of the three ERV3 mRNAs contain human cellular sequences. J Virol. 1987 Jul;61(7):2182–2191. doi: 10.1128/jvi.61.7.2182-2191.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Khan A. S., Laigret F., Rodi C. P. Expression of mink cell focus-forming murine leukemia virus-related transcripts in AKR mice. J Virol. 1987 Mar;61(3):876–882. doi: 10.1128/jvi.61.3.876-882.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Martin M. A. Endogenous murine leukemia proviral long terminal repeats contain a unique 190-base-pair insert. Proc Natl Acad Sci U S A. 1983 May;80(9):2699–2703. doi: 10.1073/pnas.80.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S. Nucleotide sequence analysis establishes the role of endogenous murine leukemia virus DNA segments in formation of recombinant mink cell focus-forming murine leukemia viruses. J Virol. 1984 Jun;50(3):864–871. doi: 10.1128/jvi.50.3.864-871.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A. M., Steinberg A. D., Khan A. S. Increased expression of novel full-length endogenous mink cell focus-forming-related transcripts in autoimmune mouse strains. Virology. 1988 Jan;162(1):274–276. doi: 10.1016/0042-6822(88)90422-9. [DOI] [PubMed] [Google Scholar]
- Laigret F., Repaske R., Boulukos K., Rabson A. B., Khan A. S. Potential progenitor sequences of mink cell focus-forming (MCF) murine leukemia viruses: ecotropic, xenotropic, and MCF-related viral RNAs are detected concurrently in thymus tissues of AKR mice. J Virol. 1988 Feb;62(2):376–386. doi: 10.1128/jvi.62.2.376-386.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Lerner R. A., Wilson M. C. Normal expression of polymorphic endogenous retroviral RNA containing segments identical to mink cell focus-forming virus. J Virol. 1985 Dec;56(3):691–700. doi: 10.1128/jvi.56.3.691-700.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Lerner R. A., Wilson M. C. The Gv-1 locus coordinately regulates the expression of multiple endogenous murine retroviruses. Cell. 1985 May;41(1):289–299. doi: 10.1016/0092-8674(85)90082-0. [DOI] [PubMed] [Google Scholar]
- Levy D. E., McKinnon R. D., Brolaski M. N., Gautsch J. W., Wilson M. C. The 3' long terminal repeat of a transcribed yet defective endogenous retroviral sequence is a competent promoter of transcription. J Virol. 1987 Apr;61(4):1261–1265. doi: 10.1128/jvi.61.4.1261-1265.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni C., Schumann G. Lipopolysaccharide induces C-type virus in short term cultures of BALB/c spleen cells. Nature. 1975 Mar 6;254(5495):60–61. doi: 10.1038/254060a0. [DOI] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveché E. S., Steinberg A. D. Flow cytometric analysis of in vivo activation of murine spleen cells. J Immunopharmacol. 1982;4(3):163–181. doi: 10.3109/08923978209026432. [DOI] [PubMed] [Google Scholar]
- Rossomando A., Meruelo D. Viral sequences are associated with many histocompatibility genes. Immunogenetics. 1986;23(4):233–245. doi: 10.1007/BF00373018. [DOI] [PubMed] [Google Scholar]
- Roths J. B., Murphy E. D., Eicher E. M. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984 Jan 1;159(1):1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P. Leukemia virus genomes in the chromosomal DNA of the mouse. Harvey Lect. 1978;71:173–192. [PubMed] [Google Scholar]
- Saga Y., Tung J. S., Shen F. W., Boyse E. A. Alternative use of 5' exons in the specification of Ly-5 isoforms distinguishing hematopoietic cell lineages. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5364–5368. doi: 10.1073/pnas.84.15.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann G., Moroni C. Mitogen induction of murine C-type viruses. I. Analysis of lymphoid cell subpopulations. J Immunol. 1976 Apr;116(4):1145–1150. [PubMed] [Google Scholar]
- Speck N. A., Baltimore D. Six distinct nuclear factors interact with the 75-base-pair repeat of the Moloney murine leukemia virus enhancer. Mol Cell Biol. 1987 Mar;7(3):1101–1110. doi: 10.1128/mcb.7.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Moroni C. Phenotypic mixing of retroviruses in mitogen-stimulated lymphocytes: analysis of xenotropic and defective endogenous mouse viruses. J Gen Virol. 1984 Feb;65(Pt 2):317–326. doi: 10.1099/0022-1317-65-2-317. [DOI] [PubMed] [Google Scholar]
- Stoye J. P., Moroni C. Proliferation and differentiation requirements for the induction of two retroviral loci during B-cell activation. J Gen Virol. 1985 Jan;66(Pt 1):109–120. doi: 10.1099/0022-1317-66-1-109. [DOI] [PubMed] [Google Scholar]
- Thomas C. Y., Coffin J. M. Genetic alterations of RNA leukemia viruses associated with the development of spontaneous thymic leukemia in AKR/J mice. J Virol. 1982 Aug;43(2):416–426. doi: 10.1128/jvi.43.2.416-426.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung J. S., Saga Y., Boyse E. A. The incongruous Ly-5 phenotype of lpr/lpr and gld/gld T cells. Immunogenetics. 1987;25(2):126–129. doi: 10.1007/BF00364279. [DOI] [PubMed] [Google Scholar]
- Wecker E. Expression of endogenous viral antigens during immune response. Med Microbiol Immunol. 1977;164(1-3):231–238. doi: 10.1007/BF02121317. [DOI] [PubMed] [Google Scholar]
- Wejman J. C., Taylor B. A., Jenkins N. A., Copeland N. G. Endogenous xenotropic murine leukemia virus-related sequences map to chromosomal regions encoding mouse lymphocyte antigens. J Virol. 1984 Apr;50(1):237–247. doi: 10.1128/jvi.50.1.237-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfert R. L., Goodman M. G., Weigle W. O. Demonstration of activation-specific interactions among B lymphocyte membrane proteins by a photoreactive cross-linking agent. J Immunol. 1984 Jun;132(6):2703–2708. [PubMed] [Google Scholar]