Abstract
In the present study we show that expression of the neural PKC-substrate B-50 (growth-associated protein [GAP-43]) in Rat-1 fibroblasts induced the formation of filopodial extensions during spreading. This morphological change was accompanied by an enhanced formation of peripheral actin filaments and by accumulation of vinculin immunoreactivity in filopodial focal adhesions, colocalizing with B-50. In time lapse experiments, the B-50–induced filopodial extensions were shown to stay in close contact with the substratum and appeared remarkably stable, resulting in a delayed lamellar spreading of the fibroblasts. The morphogenetic effects of the B-50 protein were entirely dependent on the integrity of the two N-terminal cysteines involved in membrane association (C3C4), but were not significantly affected by mutations of the PKC-phosphorylation site (S41) or deletion of the C terminus (177–226). Cotransfection of B-50 with dominant negative Cdc42 or Rac did not prevent B-50–induced formation of filopodial cells, whereas this process could be completely blocked by cotransfection with dominant negative Rho or Clostridium botulinum C3-transferase. Conversely, constitutively active Rho induced a similar filopodial phenotype as B-50. We therefore propose that the induction of surface extensions by B-50 in spreading Rat-1 fibroblasts depends on Rho-guanosine triphosphatase function.
INTRODUCTION
During neuronal outgrowth and regeneration, growth cones are responsible for guiding elongating axons and dendrites to their correct targets. Soluble or cell surface-bound guidance cues in the growth cone’s environment bind to receptors on filopodia and lamellipodia and trigger second messenger systems in the appropriate region of the growth cone leading to changes in cytoskeletal organization and function (reviewed by Goodman, 1996). Several studies have indicated that filopodia play a key role in signal detection by acting as autonomous sensors conveying information to the growth cone proper (Davenport et al., 1993). Growth cones that are depleted of their filopodia by including low concentrations of cytochalasins display disoriented pathfinding (Bentley and Toroian-Raymond, 1986; Chien et al., 1993). Furthermore, the direction of growth cone motility can be altered by single filopodial contacts (O’Connor et al., 1990; Gomez and Letourneau, 1994).
The initiation of neurite outgrowth is correlated with the increased expression of a relatively small subset of growth-associated proteins (GAPs). These include essential building blocks for neurite formation such as actin, α-tubulin, microtubule-associated proteins, and proteins thought to play a role in neurite outgrowth competence, such as the nervous tissue-specific protein B-50 (GAP-43, neuromodulin, F1). B-50 is primarily located at the inner leaflet of axonal and growth cone membranes of developing and regenerating neurons as well as in plastic regions of the adult brain (Oestreicher et al., 1997). The protein colocalizes to a great extent with the cortical actin cytoskeleton (Nielander et al., 1993; Widmer and Caroni, 1993; Aigner and Caroni, 1995) and has been shown to cosediment with f-actin in vitro (Hens et al., 1993; He et al., 1997).
Modulation of B-50 expression levels has been reported to have drastic effects on cell and growth cone morphology. Expression of B-50 in a variety of non-neuronal cells caused spontaneous formation of filopodia (Zuber et al., 1989; Widmer and Caroni, 1993). Overexpression of B-50 in neuronal cells also provoked filopodia formation (Nielander et al., 1993) as well as enhanced stimulus-induced neurite outgrowth (Yankner et al., 1990; Morton and Buss, 1992). Transgenic mice overexpressing B-50 displayed spontaneous sprouting of nerves at their terminal fields (Aigner et al., 1995) or axonal labyrinths composed of tightly packed sheaths of membranes at synaptic boutons (Holtmaat et al., 1995, 1997). Conversely, treatment of neuronal cells with antisense B-50 oligonucleotides or with anti-B-50 antibodies drastically reduced growth cone size and adherence as well as neurite outgrowth and steering capacity (Shea et al., 1991; Jap Tjoen San et al., 1992, 1995; Aigner and Caroni, 1993, 1995; Shea, 1994). Moreover, antisense-treated dorsal root ganglion growth cones displayed highly dynamic and unstable lamellar extensions and were strikingly devoid of local f-actin concentrations (Aigner and Caroni, 1995). Although B-50 might play an important role in neurite outgrowth, its presence is not a prerequisite. Dissociated hippocampal cells and PC12 cells were still able to grow out neurites in the presence of antisense B-50 oligoncleotides (Jap Tjoen San et al., 1992; Aigner and Caroni, 1993). In addition, neurite growth and connectivity in B-50 null mutant mice was grossly normal and cultured neurons from these mice extended axons to the same extent as neurons expressing B-50. However, depletion of B-50 caused severe guidance problems in outgrowing retinal axons (Strittmatter et al., 1995).
The mechanism through which B-50 influences cell or growth cone surface activity remains largely unknown. A potential Go activation domain has been proposed to reside within the first 10 amino acids of the protein (Sudo et al., 1992). B-50–induced filopodia formation in COS cells was reported to depend on the integrity of the membrane attachment site (C3C4) and of the presumed Go protein activation domain R6L9 (Strittmatter et al., 1994). The role of the phosphorylation state of the major PKC phosphorylation site (rat: S41) has been studied by expression of mutants mimicking permanently phosphorylated B-50 (S41 to D41) or unphosphorylated B-50 (S41 to A41). It was observed that these mutants exhibited modulatory effects on cell spreading when compared with the wild-type protein (Widmer and Caroni, 1993; Gamby et al., 1996; Meiri et al., 1996).
The Rho family of guanosine triphosphatases (GTPases), a subgroup of the Ras superfamily of small GTP-binding proteins, is centrally involved in the organization of the actin cytoskeleton (reviewed in Machesky and Hall, 1996; Symons, 1996). These GTPases function as molecular switches that cycle between an inactive, GDP-bound state and an active, GTP-bound form. The activity of Rho proteins is regulated by several accessory factors including nucleotide exchange factors, GTPase-activating proteins, and GDP-dissociation inhibitory proteins (Hall, 1994). Microinjection of activated forms of Cdc42, Rac, or Rho in quiescent Swiss 3T3 cells resulted in rapid formation of filopodia, lamellipodia, and stress fibers, respectively, accompanied by the formation of distinct focal adhesions (Nobes and Hall, 1995a). In the present study, we demonstrate that B-50 expression caused a striking rearrangement of the actin cytoskeleton in spreading Rat-1 fibroblasts, resulting in the formation of a filopodial morphology. These morphological changes could be blocked by interfering with endogenous Rho-GTPase function and were similar to changes induced by expression of constitutively active Rho. Our results thus indicate that B-50-induced formation of filopodia in spreading Rat-1 fibroblasts depends on Rho-GTPase function.
MATERIALS AND METHODS
Expression Constructs
cDNA coding for rat B-50 (−40 to +1085) was cloned into the eukaryotic expression vector pcDNA1 (Invitrogen, San Diego, CA). Mutations were introduced via oligonucleotide-directed, in vitro mutagenesis (Amersham, Arlington Heights, IL). A C-terminal deletion construct ([1–176]B-50) was obtained via religation of the wild-type B-50 expression construct upon PstI digestion. The pB-50/EGFP (enhanced green fluorescent protein) construct was obtained by cloning of a PCR product containing the open reading frame of the B-50 gene in pEGFP-N1 (Clontech, Palo Alto, CA), directly upstream of and in frame with the EGFP coding sequence. All constructs were completely sequenced to check for correct introduction of desired mutations and absence of errors. In transient cotransfections, myc-epitope tagged constructs expressing dominant negative and constitutively active forms of RhoA (gift from M. Symons), Cdc42Hs (gift from A. Hall), Clostridium botulinum C3-transferase (gift from R. Treisman) and Rac1 (gift from B.M.T. Burgering) were used.
Cell Culture and Transfection Experiments
Wild-type Rat-1 cells were cultured in DMEM (high glucose: 4.5 g/l) supplemented with 10% FCS, 100 IU ml−1 penicillin (ICN Nutritional Biochemicals, Cleveland, OH), 100 μg ml−1 streptomycin (ICN), and 2 mM L-glutamine in a humidified atmosphere at 37°C and 7% CO2. Cells were plated in 3.5-cm culture dishes and transfected in the absence of serum with 5 μg wild- type or mutant B-50 expression constructs using lipofectin (Life Technologies, BRL, Gaithersburg, MD). Cotransfections were performed by transfecting 1:1 mixtures of B-50 and GTPase expression constructs. Medium was replenished 6 h after transfection, and cells were grown for an additional 24 h in the absence of serum and replated onto 12 mm uncoated, acid-washed glass coverslips using trypsin/EDTA. Forty minutes after replating, cells were fixed with 4% paraformaldehyde and 0.05% glutaraldehyde at 4°C for 20 min and processed for immunocytochemistry. Pharmacological agents, e.g., cytochalasin B (Sigma Chemical, St. Louis, MO, 0.05 μg/ml), wortmannin (Sigma, 10 nM), genistein (Sigma, 50 μM), or herbimycin A (Life Technologies, 500 ng/ml) were included immediately after replating.
Rat-1 cells stably expressing mutant Rac and Rho proteins are described elsewhere (Qiu et al., 1995a,b). In brief, a plasmid expressing a tetracycline-controlled transactivator (Gossen and Bujard, 1992) was transfected into Rat-1 cells in the presence of a G418 resistance marker. Subsequently, constructs expressing Rac or Rho mutants controlled by a hybrid element containing a tet operator and a cytomegalovirus minimal promoter were transfected in the presence of a puromycin resistance marker. These cells were grown in DMEM (high glucose) containing 10% FCS, 2 mM L-glutamine, 0.4 mg/ml G418, 1 μg/ml puromycin, and 2 μg/ml tetracycline. Tetracycline was withdrawn 48 h before replating to induce expression of the transfected genes. Transfection and replating of stably transfected cells were carried out as described for wild-type Rat-1 cells except for stable N19Rho transfected Rat-1 cells where a reduced serum concentration of 0.5% was used during transfection and subsequent experimentation.
Immunocytochemistry and Microscopy
Fixed cells were washed with 0.1 M phosphate buffer (PB) and incubated in 200 mM glycine in PB (PBG) for 20 min. Subsequently, coverslips were incubated 2 times in NaBH4 for 10 min to quench aldehyde groups. Aspecific binding sites were blocked by incubation in PBG containing 2% normal goat serum for 30 min at room temperature. Cells were incubated with the primary antibodies for 16 h at 4°C in PBG containing 0.05% Triton X-100 (PBG-T), thoroughly washed in PBG-T and incubated with secondary antibodies (60 min, 37°C). After washing with PBG-T, PB, and milli-Q, cells were embedded in Dabco/Mowiol and observed with an upright Leica TCS NT confocal laser scanning microscope (Heidelberg, Germany). B-50 immunoreactivity was visualized with the specific monoclonal antibody NM4 (Mercken et al., 1992; 1:4000) or with a polyclonal antibody, raised against full-length recombinant hexaHis-tagged B-50 (9527; 1:200). Vinculin immunoreactivity was detected using monoclonal anti-vinculin (1:100; Sigma) antibodies. Myc-epitope tags were detected with the monoclonal anti-myc antibody 9E10 (1:1000; Evan et al., 1985). F-actin was visualized by including FITC phalloidin or rhodamine phalloidin (Sigma) during incubation with the secondary antibody. In triple labeling experiments, fixed cells were incubated with 9527 and 9E10 and subsequently with goat anti-rabbit-TRITC, donkey anti-mouse-CY5, and FITC phalloidin. Appropriate control experiments were performed to verify the specificity of the signals.
Time lapse recordings were performed at 37°C and 7% CO2 in a microchamber mounted on an inverted Leica TCS NT confocal laser scanning microscope (Heidelberg, Germany). Twenty four hours after transfection, cells were trypsinized, replated on 24-mm glass coverslips, and transferred to the microscopic table. After identification of green fluorescing cells, time lapse recordings were started with time intervals of 30 s.
Quantification of Cellular Morphologies
In transient (co)transfection experiments, the percentage of filopodial cells was assessed by examining the f-actin staining of randomly selected transfected or untransfected cells, by an observer unaware of the identity of the transfected construct(s). Cells were regarded to be “filopodial” if they possessed at least 10 filopodia with lengths of at least 5 μm. One hundred cells were counted in each experiment, and experiments were performed in triplicate.
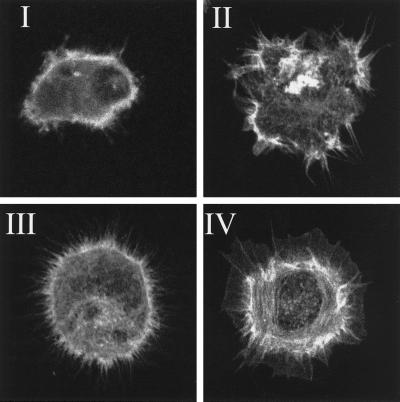
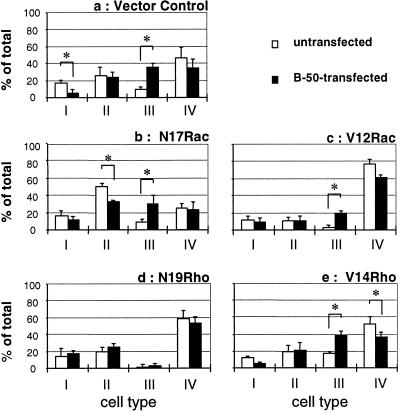
Quantitation of the morphology of the total fibroblast population in stably transfected N17Rac, V12Rac, N19Rho, V14Rho, and vector control cell lines was performed by categorizing morphologies into four classes (Figure 1): class I, cells with few protrusions; class II, well spread, irregularly shaped cells lacking a clear peripheral actin ring; class III, spheric cells with more than 10 filopodia of at least 5 μm; and class IV: cells with large lamellae or lamellipodia. At least 50 cells were counted in each experiment and experiments were performed in triplicate. Statistical analyses were performed using a two-tailed Student t test.
Figure 1.
Morphologies of Rat-1 fibroblasts during spreading, stained for f-actin. Class I, cells with few protrusions; Class II, irregularly shaped cells; Class III, filopodial cells; and Class IV, lamellar cells.
RESULTS
Fibroblasts Expressing B-50 Exhibit a Filopodial Phenotype during Spreading
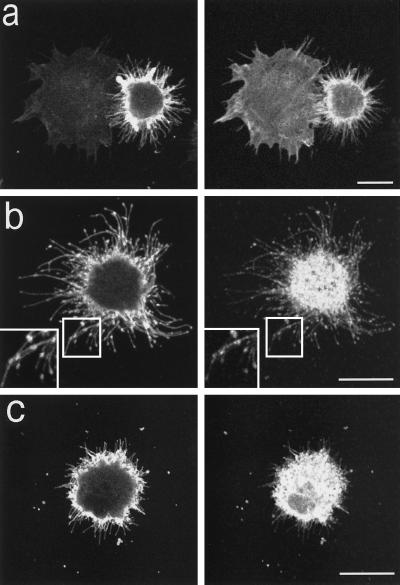
To study the morphogenetic effects of the B-50 protein, we performed transfection experiments in serum-starved Rat-1 fibroblasts, cells lacking endogenous B-50 expression. B-50–transfected cells were compared with untransfected or mock-transfected cells during spreading, 40 min after replating on glass coverslips and subsequent double labeling with anti-B-50 antibodies and f-actin–binding phalloidin. Transfected cells could readily be identified by their high levels of predominantly plasma membrane-associated B-50 immunoreactivity (Figure 2, left panels). The transfected fibroblasts exhibited a more intense phalloidin staining at the cellular periphery and a dramatic morphological change when compared with untransfected cells (Figure 2a, right panel). Cells expressing B-50 displayed reduced spreading areas and a remarkably spiny phenotype with high numbers of filopodia extruding from a spheric cell body (Figure 2a). These filopodia showed an intense staining for both B-50 immunoreactivity and phalloidin (Figure 2a, left and right panels). Upon quantification of the various cellular phenotypes in the f-actin channel (Figure 1), we determined that about 35% of the B-50 transfected cells adopted a strictly filopodial morphology (type III), compared with only a few percent of the mock-transfected cells (see Figure 4e). The mean minimal cell diameter of the whole cell population was significantly reduced from 17.3 ± 0.5 μm (untransfected cells) to 14.9 ± 0.4 μm (transfected cells; p < 0.01).
Figure 2.
B-50–induced formation of filopodia and focal adhesions in spreading Rat-1 fibroblasts. Rat-1 fibroblasts were transfected with wild-type B-50-cDNA (in pcDNA1) using lipofectin and grown for 30 h in the absence of serum. Subsequently, cells were replated onto glass coverslips using trypsin/EDTA and fixed after 40 min. Cells were stained for B-50 (left panels) and for f-actin (a) or vinculin (b and c) (right panels) and observed by confocal laser scanning microscopy. Each figure shows a projection of several confocal planes. Inserts show enlargements of the indicated boxes. Scale bar, 10 μm.
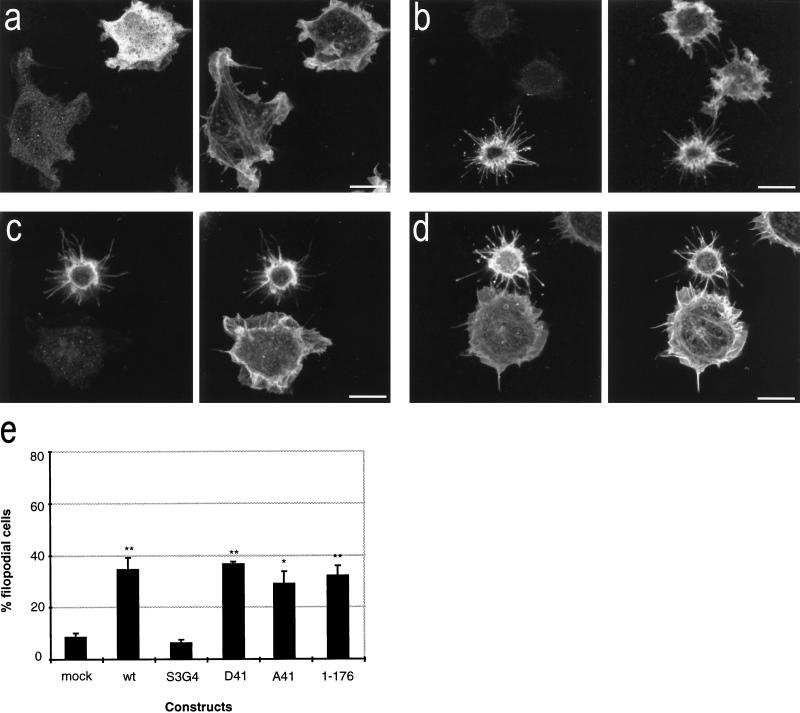
Figure 4.
Morphologies of cells expressing B-50 mutants. Rat-1 fibroblasts were transfected with [S3G4]B-50 (a), [D41]B-50 (b), [A41]B-50 (c), or [1–176]B-50 (d) (in pcDNA1) and processed as described in the legend to Figure 2. Cells were stained for B-50 (left panels) and for f-actin (right panels) and observed by confocal laser scanning microscopy. Typical examples for cells expressing the various constructs are shown. Scale bar, 10 μm. (e) Quantitation of filopodial cell formation (type III; Figure 1). Values represent means ± SEM from three independent experiments for each construct (**, p < 0.001; *, p < 0.01).
B-50–transfected filopodial cells exhibited striking punctate accumulations of B-50 immunoreactivity within the filopodial extensions (Figure 2b, left panel and inset). Similar B-50 accumulations were also seen in live pB-50/EGFP transfected cells (our unpublished observations), arguing against fixation of immunostaining artefacts. Double labeling experiments using polyclonal anti-B-50 antibodies and monoclonal anti-vinculin antibodies (Figure 2b, right panel) revealed an almost complete colocalization of B-50 with vinculin immunoreactivity within these spots (Figure 2b and inset), suggesting that they represent focal adhesion complexes. In contrast, no clear vinculin-immunoreactive complexes could be visualized at the cellular periphery of untransfected cells (our unpublished results). Occasionally, isolated B-50 and vinculin coimmunoreactive spots were observed in the vicinity of filopodial cells, probably left behind upon filopodial retraction (Figure 2c). No such vinculin-immunoreactive patches were observed surrounding untransfected cells, suggesting that the occurrence of these highly adhesive focal adhesions was due to the presence of B-50.
Dynamics of B-50-induced Filopodial Protrusions
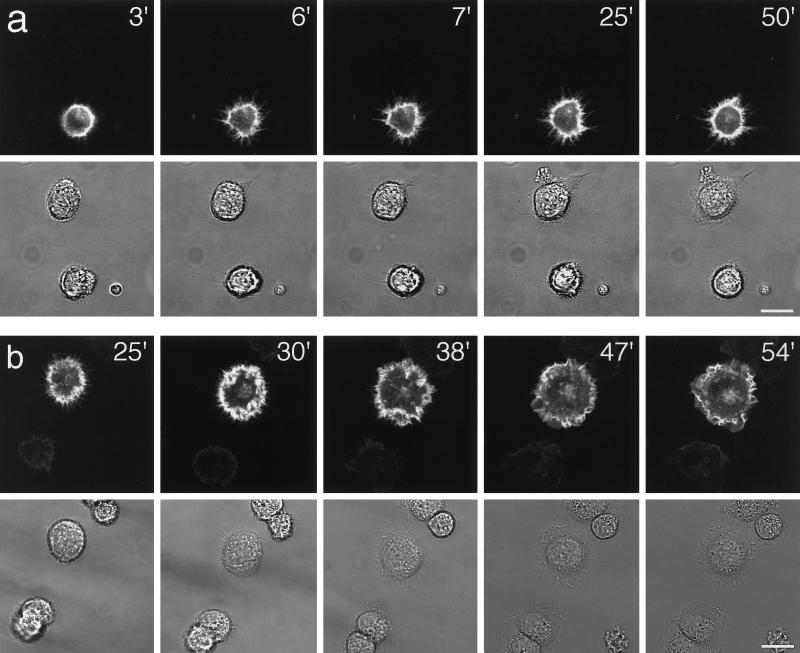
To examine the dynamics of B-50–induced filopodial extensions during spreading we performed time-lapse experiments on pB-50/EGFP–transfected Rat-1 cells. Transfected cells could be identified by the emission of bright green fluorescence, mainly localized at the cellular periphery and in filopodial extensions, upon exposure to blue light. The B-50/EGFP-fusion construct was as potent in the induction of filopodial cells as the wild-type B-50 construct (about 30% of transfected cells was strictly filopodial 40 min after replating), whereas EGFP-transfected control cells had a similar appearance as untransfected cells (our unpublished observations). Immediately after replating, transfected and untransfected cells appeared spherical or oval and often exhibited dynamic bleb formation and retraction. Upon attachment, transfected cells started to extend short, highly motile microspikes and filopodia that rapidly extended while staying in close contact with the glass surface (Figure 3a; compare time points after 3 and 6 min). Most of these filopodia were remarkably stable and remained present for more than 20 min (Figure 3a; compare time points after 6 and 25 min). At closer view, numerous protrusions appeared to cycle between periods of extension and retraction in which the tips frequently detached from and reattached to the surface of the coverslip as if exploring the substratum (our unpublished observations). The direction of the filopodial movements was predominantly perpendicular to the cell margin, and virtually no laterally swaying filopodia could be observed. Occasionally, small membrane veils, which were mostly short-lived, extended between filopodia. We did not observe the filopodial phenotype in untransfected or pEGFP-transfected cells. Rather, most untransfected cells started ruffling shortly after attachment (Figure 3a, upper cell).
Figure 3.
Dynamics of B-50/EGFP–induced filopodial extensions in living Rat-1 fibroblasts. Rat-1 fibroblasts were transfected as described in the legend to Figure 2. Upon trypsinization, cells were replated on 24-mm glass coverslips, mounted in a microchamber, and transferred to the microscopic stage of the confocal microscope where they were kept at 37°C and 7% CO2. Upper frames show GFP-fluorescence in pB-50/GFP-transfected cells; lower frames show the corresponding phase contrast images. (a) Frames at selected timepoints after replating showing the spontaneous extrusion of long, stable, substrate-attached filopodia in a pB-50/EGFP–transfected cell. (b) Time lapse sequence of a pB-50/EGFP–transfected cell showing the transition from a filopodial to a ruffled or lamellar phenotype. Note that untransfected cells do not show a filopodial phenotype preceding lamellar spreading. Scale bar, 10 μm.
The “stability” of the filopodial phenotype in pB-50/EGFP-transfected cells appeared to be different from cell to cell. Whereas some pB-50/EGFP- transfected cells exhibited lamellar ruffling shortly after attachment, others remained filopodial for at least 2 h. At 24 h after replating, the morphology of most pB-50/EGFP-transfected cells was indistinquishable from that of untransfected cells. We have depicted a typical sequence of the transition from a filopodial phenotype to a lamellar, spread phenotype in Figure 3b. The filopodial extensions were invaded by highly motile membranous veils that gruadually increased in size (Figure 3b; compare time points after 25 and 30 min). Filopodia were then engorged by these ruffles that gradually matured into lamellae surrounding the spread cell (Figure 3b; compare time points after 38 and 54 min). The time of initiation of this transition differed from cell to cell but, when started, was generally completed within 10–20 min. In contrast, untransfected cells progressed directly into a ruffling, lamellar phenotype without a preceding extension of high numbers of filopodia (Figure 3, a and b, lower panels). This suggests that B-50 delays membrane ruffling and veil formation during spreading by the introduction of a filopodial phenotype.
Functional Domains for Morphogenetic Activity of B-50
The involvement of the membrane-targeting domain of B-50 in the cell surface activity of the protein was studied in cells transfected with a construct in which the two N-terminal cysteines had been mutated to prevent membrane attachment (C3C4 → S3G4). [S3G4]B-50 transfected cells displayed a mainly cytosolic distribution of B-50 immunoreactivity and were morphologically indistinguishable from untransfected controls, i.e., did not show a filopodial phenotype or reduced spreading (compare Figure 4a to Figure 2a). The number of filopodial cells was as low as in mock-transfected cells (Figure 4e), demonstrating that the N-terminal cysteine residues are critical for the morphogenetic activity of B-50.
To examine the potential modulatory effects of the PKC phosporylation site (S41), controlling calmodulin binding to B-50, we have compared the morphologies of cells expressing the PKC site mutants [D41]B-50 (mimicking permanently phosphorylated B-50 and unable to bind calmodulin [Chapman et al., 1991]; Figure 4a), [A41]B-50 (mimicking permanently unphosphorylated B-50, binding calmodulin at higher affinity [Meiri et al., 1996; Figure 4b]), and wild-type B-50 (Figure 2). We did not observe significant differences as a consequence of these mutations in the number of B-50–induced filopodial cells (Figure 4e), in the number of extended filopodia, in the intensity of phalloidin staining, or in the spreading areas (our unpublished results), arguing against a major role of the PKC phosphorylation site in the morphogenetic activity of B-50 during spreading.
Since the C terminus of B-50 has been inferred to contain structural elements essential for the morphogenetic activity of the B-50 protein (Wiederkehr et al., 1997), we have examined the morphological changes in cells transfected with a mutant construct lacking amino acids 177–226. Expression of [1–176]B-50 induced a similar filopodial cell type (Figure 4c) as the wild-type protein (Figure 2a) and was quantitively equally potent (Figure 4e), ruling out a critical role for this domain in the morphogenetic function of B-50 during spreading.
Involvement of Rho-like GTPases in B-50–induced Morphological Changes
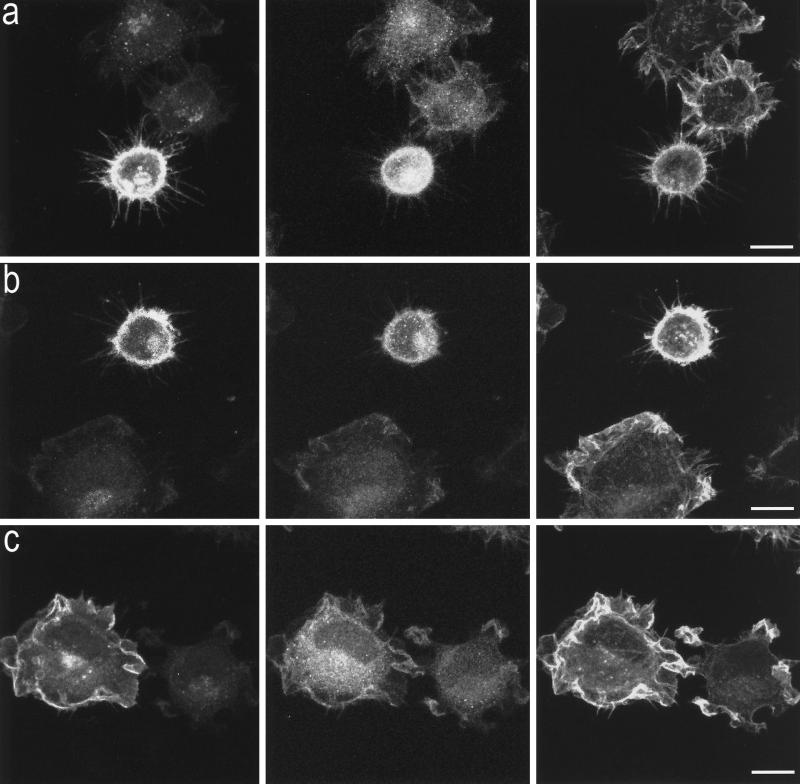
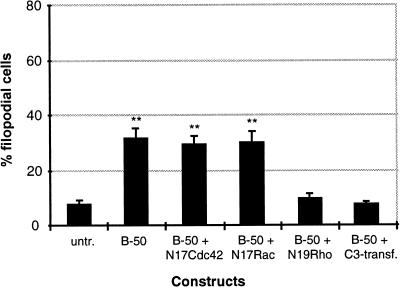
The actin cytoskeleton of Swiss 3T3 fibroblast has been shown to be tightly controlled by a group of Rho-like GTPases (Nobes and Hall, 1995). To determine whether the B-50–induced formation of filopodia and focal adhesions in spreading Rat-1 fibroblasts was controlled by members of the family of Rho-like GTPases, we have made use of expression constructs encoding dominant negative forms of these GTPases to specifically block endogenous GTPase function. Cells were transiently cotransfected with B-50 and myc-epitope–tagged constructs of dominant negative Cdc42 (N17Cdc42: Figure 5a), Rac (N17Rac; Figure 5b), or Rho (N19Rho; Figure 5c). Forty minutes after replating, cells were fixed and triple stained with antibodies against B-50 (left panels), myc epitopes (middle panels), and f-actin–binding phalloidin (right panels). This allowed us to examine changes in the actin cytoskeleton of succesfully cotransfected cells versus untransfected cells (Figure 5). The percentages of filopodial cells were quantified (Figure 6) as described in MATERIALS AND METHODS.
Figure 5.
Effects of interference with Cdc42, Rac, or Rho function on the B-50–induced morphological changes. Rat-1 fibroblasts were cotransfected with wild-type B-50/GAP-43 and myc-epitope–tagged N17Cdc42 (a), N17Rac (b), or N19Rho (c) and processed as described in the legend to Figure 2. Cells were triple-stained for B-50 (left panels), myc-epitopes (middle panels), and f-actin (right panels) and visualized by confocal laser scanning microscopy. Scale bar, 10 μm.
Figure 6.
Quantitation of filopodial cells after cotransfection with B-50 and GTPase inhibitors. Rat-1 fibroblasts were treated as described in the legend to Figure 5. Percentages of filopodial cells (type III, Figure 1) were scored by examining the f-actin staining. Values represent means ± SEM from three independent experiments for each construct (**, p < 0.001).
Cotransfected cells expressing high levels of both B-50 and N17Cdc42 displayed an enhanced phalloidin staining accompanied by a large number of long filopodia as well as reduced spreading area (Figure 5a, lower cell) comparable to cells transfected with B-50 alone (Figure 2a). Similarly, cells cotransfected with B-50 and N17Rac exhibited increased levels of cortical f-actin, a filopodial phenotype, and reduced spreading (Figure 5B, upper cell). However, when Rat-1 fibroblasts were cotransfected with B-50 and dominant negative Rho (N19Rho), we no longer observed induction of a filopodial phenotype, and the cells spread as well as untransfected control cells (Figure 5c, left cell). Apparently, the expression of dominant negative Rho prevented the cells to adopt a B-50–induced phenotype. A similar interference with the induction of a filopodial phenotype by B-50 was obtained upon cotransfection of B-50 with a construct expressing C3 transferase (our unpublished results), a C. botulinum ADP-ribosyl transferase that specifically blocks the Rho-GTPase through ADP ribosylation (Sekine et al., 1989). Quantification of the percentage of filopodial cells (Figure 6) confirmed that blocking endogenous Cdc42 with N17Cdc42 or endogenous Rac with N17Rac did not change the percentages of filopodial cells induced by B-50, either alone or cotransfected with an empty control vector (pcDNA3; our unpublished results). In contrast, interference with Rho function, either with N19Rho or with C3 transferase, interfered completely with the morphogenetic effect of B-50 and lowered the percentage of filopodial cells to that of untransfected control cells.
Activated Rho Induces Filopodia Formation in Spreading Rat-1 Fibroblasts
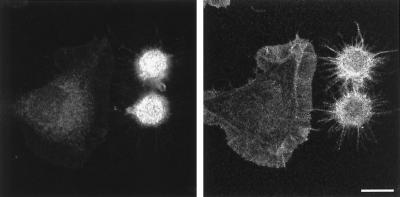
Since Rho activation has been linked to formation of stress fibers and focal adhesions in quiescent fibroblasts (Ridley and Hall, 1992; Nobes and Hall, 1995), we tested whether introduction of constitutively active Rho (V14Rho) would cause filopodia formation in Rat-1 fibroblasts during spreading. Upon transient transfection with myc-epitope–tagged V14Rho, 62 ± 5.3% (mean ± SEM) of the transfected spreading fibroblasts (Figure 7, left panel) acquired a strictly filopodial phenotype with enhanced f-actin staining (Figure 7, right panel), very similar to the phenotype of B-50–transfected cells (Figure 2a). Moreover, the V14Rho transfected cells displayed a comparable reduction in spreading area when compared with untransfected cells. Cotransfection of V14Rho with B-50 did not lead to a further increase in the percentage of filopodial cells (54 ± 4.1%; mean ± SEM).
Figure 7.
Morphological changes induced by expression of activated Rho in Rat-1 fibroblasts during spreading. Rat-1 fibroblasts were transfected with myc-epitope–tagged V14Rho and processed as described in the legend to Figure 2. Cells were stained for myc-epitopes (left panels) and FITC-coupled phalloidin (right panels) and observed by confocal laser scanning microscopy. Scale bar, 10 μm.
Shifts in Morphological Categories in Fibroblasts Stably Transfected with Rac and Rho Mutants as a Consequence of B-50 Expression
The dominant cellular phenotype of the vector control cell line during spreading was that of ruffled or lamellar cells (type IV; Figure 1), with the remainder of the cells mainly adopting a more irregular (type II) or round-up morphology (type I; Figure 8a, open bars). Transfection of this vector control cell line with B-50 caused a large increase in the number of filopodial cells (type III) from 9% to 36% (Figure 8a). This was accompanied mainly by a decrease in the number of round-up (type I) and lamellar (type IV) cells. Cells stably expressing dominant negative Rac (N17Rac) displayed a mainly irregular morphology (type II) and a 47% reduction in the number of lamellar cells when compared with vector control cells (type IV; Figure 8, a and b; open bars). Upon transfection with B-50, a morphological shift to a filopodial phenotype (type III) was observed with a concomitant decrease in irregular cells (type II; Figure 8b, filled bars). This confirms data of the transient cotransfection assay (Figures 5 and 6) showing that interference with Rac function did not block the B-50–induced formation of filopodial cells during spreading. Cells expressing constitutively active Rac (V12Rac) displayed a predominantly lamellar phenotype (type IV; Figure 8c, open bars). Transfection of B-50 in these cells caused a large increase in the percentage of filopodial cells (type III) with a concomitant reduction in the percentage of lamellar cells (type IV; Figure 8c, filled bars). Cells stably transfected with N19Rho exhibited an overall phenotype that was very similar to that of vector control cells, indicating that inhibition of Rho function by N19Rho did not cause a drastic alteration in the cellular morphology during spreading (compare Figure 8a to Figure 8d, open bars). These cells did not respond with any significant morphological change upon transfection with B-50, giving further evidence for a critical role for the Rho-GTPase in the morphogenetic function of B-50 (Figure 8d, filled bars). A cell line stably expressing constitutively active Rho (V14Rho) displayed an about twofold increase in the percentage of filopodial cells (type III) when compared with vector control cells and a larger increase when compared with N19Rho cells, whereas both V14Rho and N19Rho were without effect on formation of lamellar cells (type IV; Figure 8, a, d, and e, open bars). Transfection of B-50 in V14Rho cells caused an increase in the amount of filopodial cells (type III) with a concomitant reduction in the percentage of lamellar (type IV) and round-up (type I) cells. Overall, these data confirm that B-50–induced formation of filopodial cells depends on the activation state of Rho-GTPase but not of Rac-GTPase.
Figure 8.
B-50–induced shifts in morphological categories of Rat-1 fibroblasts stably expressing mutant Rac- or Rho-GTPase constructs. Rat-1 fibroblasts stably expressing control plasmids (a), N17Rac (b), V12Rac (c), N19Rho (d), or V14Rho (e) were transfected with B-50 and processed as described in the legend to Figure 2. Untransfected (open bars) or wild-type B-50 transfected cells (closed bars) were classified into four morphological categories based on their f-actin staining (see Figure 1). Values represent means ± SEM from three independent experiments for each cell line (*, p < 0.05).
DISCUSSION
We have used Rat-1 fibroblasts to study the morphogenetic effects of the neural phosphoprotein B-50 during spreading. The results presented in this paper show that expression of B-50 induces a rearrangement of the fibroblast actin cytoskeleton accompanied by the extrusion of substrate-attached filopodia. The morphological changes induced by B-50 were completely prevented by coexpression of dominant negative Rho (N19Rho) or C3 transferase, whereas cells expressing constitutively active Rho (V14Rho) resembled the B-50–induced filopodial morphology. Our results thus indicate that B-50 induces a Rho-GTPase–dependent rearrangement of the fibroblast cytoskeletal architecture during cell spreading.
B-50 Expression Causes a Rearrangement of the Fibroblast Actin Cytoskeleton
Serum starvation of fibroblasts causes a loss of stress fibers, ruffles, and actin-rich lamellipodia, thereby providing a pool of actin monomers, available for polymerization upon an appropriate stimulus (Machesky and Hall, 1997). Ectopic expression of B-50 in serum-starved Rat-1 fibroblasts resulted in increased peripheral f-actin levels during spreading when compared with untransfected control cells, a process that was accompanied by extensive protrusion of f-actin–positive filopodia (Figure 2a). Inclusion of the actin- depolymerizing drug cytochalasin B (0.05 μg/ml) during spreading completely prevented the B-50–induced cortical cytoskeletal changes (our unpublished results), arguing for the dependence of these changes on new actin filament formation. In vitro, B-50 has been shown to cosediment with f-actin (Hens et al., 1993; He et al., 1997). Moreover, phosphorylated B-50 was shown to stabilize long actin filaments, whereas unphosphorylated B-50 reduced filament length and increased the critical concentration for actin polymerization, an effect that could be potentiated by calmodulin binding (He et al., 1997). Therefore, it was postulated that B-50 may directly regulate actin dynamics depending on its phosphorylation state and on calmodulin binding. In the present study, we did not observe clear morphological differences between cells expressing [D41]B-50 (mimicking phosphorylated B-50) or unphosphorylatable [A41]B-50 compared with the wild-type protein (Figure 4, b, c, and e). Thus, the PKC phosphorylation site (S41) is not critically involved in the B-50–induced rearrangement of the Rat-1 fibroblast cytoskeletal architecture during initial spreading, which is in agreement with previous reports (Strittmatter et al., 1994).
Mutation of the N-terminal cysteines, essential for correct membrane targeting (Liu et al., 1994; Aarts et al., 1995), resulted in a complete loss of the morphogenetic activity of B-50 during cell spreading (Figure 4, a and e). No change in f-actin level or distribution was observed in cells expressing [S3G4]B-50 when compared with mock-transfected cells (Figure 4, a and e), indicating that B-50 affects actin filament assembly and/or organization only if localized at the cellular cortex. Recently, Wiederkehr et al. (1997) reported that targeting of chick B-50 to punctate structures at the cell surface of 3T3-fibroblasts required both an intact N-terminal acylation domain as well as (an) element(s) in the C terminus (60–236). The same elements would be involved in the B-50–induced extrusion of filopodia and blebs (Wiederkehr et al., 1997). The C-terminal segment of B-50 harbors a highly conserved casein kinase II domain (Pisano et al., 1988; Apel et al., 1991) that might be involved in binding f-actin (Hens et al., 1993; He et al., 1997). In the present study, we did not observe apparent morphological differences or changes in f-actin levels between cells expressing [1–176]B-50, missing the casein kinase II domain, or the wild-type construct (Figure 4, d and e), arguing against a critical role for the distal C-terminal segment (177–226) in B-50–induced cytoskeletal changes in spreading fibroblasts.
An unexpected finding was the concentration of B-50 immunoreactivity at discrete spots within the filopodial extensions where it colocalized with vinculin immunoreactivity, indicating that these spots represent focal adhesions (Figure 3, b–d). Focal adhesion proteins play an important role in the structural integrity of actin-based growth cone extensions, such as filopodia. Vinculin-deficient PC12 cells extended highly unstable filopodia and lamellipodia accompanied by a strongly diminished neurite outgrowth (Varnum-Finney and Reichardt, 1994). Moreover, focal inactivation of vinculin in growth cones by microscale chromophore-assisted laser inactivation resulted in a decreased integrity of growth cone filopodia (Sydor et al., 1996). The presence of filopodial focal adhesions in spreading Rat-1-fibroblasts could similarly enhance the stability of B-50–induced filopodia. Indeed, our time lapse experiments showed that the B-50–induced filopodial protrusions were remarkably stable and remained in close contact with the substratum (Figure 3a). Occasionally, we observed B-50 immunoreactivity colocalizing with vinculin immunoreactivity in isolated, substrate-attached patches surrounding transfected cells (Figure 2c), indicating that B-50 itself is a constituent of focal adhesions. Similar, highly adhesive B-50 immunoreactive patches have been described to be left behind upon dislodgement of isolated growth cones with gentle streams of culture medium and upon neurite retraction in transfected PC12 cells (Meiri and Gordon-Weeks, 1990; Nielander et al., 1993). Several other studies also point to a role for B-50 in membrane–substrate adhesiveness. Depletion of B-50 in various neuronal cell cultures caused a dramatic decrease in growth cone spreading and adhesion with highly dynamic and unstable lamellar extensions (Aigner and Caroni, 1993, 1995; Shea, 1994; Jap Tjoen San et al., 1995). Moreover, the reduction of cell adherence in a B-50–deficient PC12 cell line was restored upon reintroduction of B-50 (Meiri et al., 1996). B-50–induced formation of focal adhesions, as was observed in this study, may thus provide a molecular basis for the stimulatory effects of the protein on cell or growth cone adhesion.
B-50–induced Filopodia Formation Requires Rho-GTPase Activity
The dramatic changes in the cortical actin cytoskeleton that occurred as a consequence of B-50 expression prompted us to examine the contribution of Rho-like GTPases in B-50–induced morphological changes. These Ras-related GTP-binding proteins have been implicated to function in numerous cellular processes including the control of actin filament formation and focal adhesion assembly, endocytosis, and cell division (Symons, 1996; Machesky and Hall, 1996). In quiescent 3T3 fibroblasts, introduction of activated forms of Cdc42 or Rac has been shown to cause formation of filopodia and lamellipodia, respectively (Nobes and Hall, 1995). The activity of these GTPases can be blocked by the introduction of dominant negative GTPases which, in analogy to N17Ras, are locked in an inactive conformation and inhibit endogenous GTPases by competing with guanine nucleotide exchange factors (Hall, 1994). The introduction of dominant negative Cdc42 (N17Cdc42) did not prevent B-50–induced filopodia formation in spreading Rat-1 fibroblasts (Figures 5 and 6), arguing against a Cdc42-dependent mechanism. Moreover, the lack of inhibitory effect of dominant negative Rac (N17Rac) also rules out a role for this GTPase in the B-50–induced formation of filopodial cells during spreading (Figures 5, 6, and 8). In contrast, transient coexpression of dominant negative Rho (N19Rho) or C3 transferase, as well as stable transfection of N19Rho, completely blocked B-50–induced formation of filopodial cells (Figures 5, 6, and 8), implicating a crucial role for the Rho-GTPase in the morphogenetic effect of the B-50 protein. The inhibitory effects of these antagonists of Rho function are not likely caused by either cytoskeletal collapse or by an overall impairment of actin filament formation. First, under the experimental conditions used, no increased cell rounding was observed in C3 transferase- transfected, serum-starved cells when compared with mock-transfected cells during spreading (our unpublished results), a phenomenon reported to occur in quiescent cells grown in the presence of serum (Chardin et al., 1989; Lang and Bertoglio, 1995). Second, f-actin levels were not overtly affected in C3 transferase or N19Rho-transfected fibroblasts (our unpublished results) and moreover, a small percentage of transiently cotransfected cells still acquired a filopodial phenotype (Figure 6). Finally, overall morphology and f-actin staining of the stable N19Rho cells during spreading were very similar to that of vector control cells (Figure 8; see also Qiu et al., 1995b).
In quiescent Swiss 3T3 fibroblasts, microinjection of activated Rho was shown to cause enhanced formation of actin filaments mainly organized into stress fibers (Ridley and Hall, 1992; Machesky and Hall, 1997) whereas we observed that introduction of activated Rho (V14Rho) into spreading Rat-1 fibroblasts resulted in enhanced formation of actin filaments accompanied by the formation of numerous filopodia and a decreased spreading area, without apparent induction of stress fibers (Figure 7). This implies that the regulation of actin-based structures by Rho-like GTPases diverges depending on cell type and experimental conditions used (see also Symons, 1996; Jin and Strittmatter, 1997).
At present, it is not yet clear how the Rho-GTPase signaling pathway is involved in the B-50–induced formation of focal adhesions and filopodia. Our cotransfection experiments with inhibitors of Rho function indicate, however, that Rho-GTPase function is indispensable for B-50–induced morphological changes in spreading Rat-1 fibroblasts. The observation that V14Rho caused a similar morphological change suggests that the Rho-GTPase functions downstream of B-50 to induce a filopodial morphology during spreading. The relative amount of activated Rho protein may subsequently determine the extent of the morphological change. Absence of activatable Rho prohibited the formation of filopodial cells (Figure 8d); low levels of activated Rho (V14Rho) caused a modest increase (Figure 8e), whereas the presence of high levels of V14Rho (transient transfection assay) caused a dramatic increase in filopodial cell formation, concomitant with enhanced f-actin levels. Expression of B-50 resulted in an intermediate response both in filopodial cell formation (35%) as well as in peripheral f-actin content (Figures 2, 4e, and 8). Transfection of either B-50 or V14Rho induced an increase in peripheral f-actin levels accompanied with a filopodial phenotype in spreading Rat-1 cells (Figures 2, 7, and 8), implying that they make use of a similar mechanism. This is further substantiated by the observation that B-50 expression could not further increase the percentage of filopodial cells after transient transfection with V14Rho. Altogether, this indicates that B-50 is directly dependent on Rho-GTPase function to induce formation of filopodial cells.
Microinjection studies in Swiss 3T3 fibroblasts (Ridley and Hall, 1992) and stable transfections of Rat1 fibroblasts (Qiu et al., 1995a) have demonstrated that expression of constitutively active Rac (V12Rac) induces while dominant negative Rac (N17Rac) strongly inhibits ruffling and lamellipodia formation. In agreement with these findings, we observed a large increase in the percentage of lamellar cells in the V12Rac cell line (+65%) and a strong reduction in the N17Rac cell line (−47%) during cell spreading when compared with vector control cells (Figure 8, a, b, and c, open bars). These percentages were not significantly affected upon expression of B-50 (75% and 34%, respectively; Figure 8, a,b, and c, black bars), indicating that B-50 does not interfere with the Rac-mediated lamellipodia formation. Since the activation state of the Rac-GTPase did not significantly affect the B-50–induced formation of filopodial cells either, it must be assumed that B-50 and Rac act in separate pathways to induce formation of filopodia and lamellipodia, respectively.
To date, the molecular events that underly Rho-mediated cytoskeletal changes remain largely unknown. An increasing number of proteins that bind Rho in a GTP-dependent manner (Rho-effector proteins) have been identified including protein kinases, phospholipid kinases, phospholipases, and a number of proteins of still unknown function (Machesky and Hall, 1996; Symons, 1996). Inclusion of inhibitors of tyrosine kinases (herbimycin A [500 ng/ml]; genistein [50 μM]) or of phosphatidylinositol 3-kinase (wortmannin [10 nM]) did not block B-50–induced filopodial cell formation (our unpublished results) arguing against a role for these potential Rho-effector kinases (Machesky and Hall, 1996) in the B-50–induced morphological changes. An interesting Rho effector molecule represents phosphatidylinositol-4-phosphate-5-kinase (Chong et al., 1994), which generates phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid that has been reported to control actin polymerization and focal adhesion assembly by regulating the activity of many cortical actin-associated proteins such as profilin, gelsolin, cofilin, and vinculin, proteins of the ezrin/radixin/moesin family, and MARCKS (Janmey, 1994; Gilmore and Burridge, 1996; Tsukita et al., 1997). PIP2 may also be directly involved in activation of the Rho-GTPase by enhancing GDP release (Zheng et al., 1996). This is of particular interest since B-50 has been implicated to influence the turnover of PIP2 (Jolles et al., 1980; Van Hooff et al., 1988). However, these modulatory effects on PIP2 levels were dependent on the phosphorylation state of B-50, whereas no apparent morphological differences were observed in cells expressing different phosphorylation site mutants (Figure 4). It thus remains unclear if or how the phosphatidylinositol metabolism is involved in the morphogenetic function of the B-50 protein.
Recent evidence suggests that GTP-binding proteins of the Rho subfamily also play important roles in various aspects of axonal and dendritic outgrowth (reviewed in Luo et al., 1997). Because of the clear similarities in membrane and cytoskeletal dynamics between fibroblasts and growth cones (Bray and Hollenbeck, 1988; Bray and White, 1988), it is tempting to speculate that the neural protein B-50 influences growth cone morphology and behavior through a Rho-dependent organization of actin filaments and focal adhesions. Interestingly, both depletion of B-50 via antisense oligonucleotides (Aigner and Caroni, 1995) as well as introduction of C3 transferase (Jin and Strittmatter, 1997) in dorsal root ganglion neurons compromised formation of well spread, adhesive growth cones (with filopodia) and caused increased outgrowth rates, supporting the idea of a concerted action of B-50 and Rho in the control of growth cone spreading and neurite growth.
ACKNOWLEDGMENTS
We thank Drs. R.-G. Qiu and M. Symons for kindly providing the stable Rat-1 cell lines expressing V14Rho, N19Rho, V12Rac, N17Rac, and vector. We are grateful to Mrs. A.J. van Rozen for help in the morphological quantifications and to Mrs. J.E. Biewenga for the B-50/EGFP construct. We thank Dr. A.B. Oestreicher for the B-50 antibodies and for helpful discussions. Finally, we thank Drs. M. Symons, A. Hall, and R. Treisman for the various constructs and Dr. B.M.T. Burgering for the Rac1 constructs and the 9E10 antibodies. This work was supported by NWO grant 903–42–006 and by the Prinses Beatrix fonds, grant 95–1008.
REFERENCES
- Aarts LHJ, Van der Linden JAM, Hage WJ, Van Rozen AJ, Oestreicher AB, Gispen WH, Schotman P. N-terminal cysteines essential for Golgi sorting of B-50 (GAP-43) in PC12 cells. Neuroreport. 1995;6:969–972. doi: 10.1097/00001756-199505090-00005. [DOI] [PubMed] [Google Scholar]
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Aigner L, Caroni P. Depletion of 43-kD growth-associated protein in primary sensory neurons leads to diminished formation and spreading of growth cones. J Cell Biol. 1993;123:417–429. doi: 10.1083/jcb.123.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner L, Caroni P. Absence of persistent spreading, branching, and adhesion in GAP-43-depleted growth cones. J Cell Biol. 1995;128:647–660. doi: 10.1083/jcb.128.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel ED, Litchfield DW, Clark RH, Krebs EG, Storm DR. Phosphorylation of neuromodulin (GAP-43) by casein kinase II; identification of phosphorylation sites and regulation by calmodulin. J Biol Chem. 1991;16:10544–10551. [PubMed] [Google Scholar]
- Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- Bray D, Hollenbeck PJ. Growth cone motility and guidance. Annu Rev Cell Biol. 1988;4:43–61. doi: 10.1146/annurev.cb.04.110188.000355. [DOI] [PubMed] [Google Scholar]
- Bray D, White JG. Cortical flow in animal cells. Science. 1988;239:883–888. doi: 10.1126/science.3277283. [DOI] [PubMed] [Google Scholar]
- Chapman ER, Au D, Alexander KA, Nicolson TA, Storm DR. Characterization of the calmodulin binding domain of neuromodulin.J. Biol Chem. 1991;266:207–213. [PubMed] [Google Scholar]
- Chardin P, Boquet P, Madaule P, Popoff MR, Rubin EJ, Gill DM. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien CB, Rosenthal DE, Harris WA, Holt CE. Navigational errors made by growth cones without filopodia in the embryonic Xenopus brain. Neuron. 1993;11:237–251. doi: 10.1016/0896-6273(93)90181-p. [DOI] [PubMed] [Google Scholar]
- Chong LD, Traynor-Kaplan A, Bokoch GM, Schwartz MA. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Davenport RW, Dou P, Rehder V, Kater SB. A sensory role for neuronal growth cone filopodia. Nature. 1993;361:721–724. doi: 10.1038/361721a0. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsey G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamby C, Waage MC, Allen RG, Baizer L. Analysis of the role of calmodulin binding and sequestration in neuromodulin (GAP-43) function. J Biol Chem. 1996;271:26698–26705. doi: 10.1074/jbc.271.43.26698. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4–5-bisphosphate. Nature. 1996;381:531–535. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Letourneau PC. Filopodia initiate choices made by sensory neuron growth cones at laminin/fibronectin borders in vitro. J Neurosci. 1994;14:5959–5972. doi: 10.1523/JNEUROSCI.14-10-05959.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CS. Mechanisms and molecules that control growth cone guidance. Annu Rev Neurosci. 1996;19:341–377. doi: 10.1146/annurev.ne.19.030196.002013. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- He Q, Dent EW, Meiri KF. Modulation of actin filament behavior by GAP-43 (neuromodulin) is dependent on the phosphorylation status of serine 41, the protein kinase C site. J Neurosci. 1997;17:3515–3524. doi: 10.1523/JNEUROSCI.17-10-03515.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hens JJH, Benfenati F, Nielander HB, Valtorta F, Gispen WH, De Graan PNE. B-50/GAP-43 binds to actin filaments without affecting actin polymerization and filament organization. J Neurochem. 1993;61:1530–1533. doi: 10.1111/j.1471-4159.1993.tb13649.x. [DOI] [PubMed] [Google Scholar]
- Holtmaat AJGD, Dijkhuizen PA, Oestreicher AB, Romijn HJ, Van der Lugt NMT, Berns A, Margolis FL, Gispen WH, Verhaagen J. Directed expression of the growth-associated protein B-50/GAP-43 to olfactory neurons in transgenic mice results in changes in axon morphology and extraglomerular fiber growth. J Neurosci. 1995;15:7953–7965. doi: 10.1523/JNEUROSCI.15-12-07953.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat AJGD, Hermens WTJMC, Sonnemans MAF, Giger RJ, Van Leeuwen FW, Kaplitt MG, Oestreicher AB, Gispen WH, Verhaagen J. Adenoviral vector-mediated expression of B-50/GAP-43 induces alterations in the membrane organization of olfactory axon terminals in vivo. J Neurosci. 1997;17:6575–6586. doi: 10.1523/JNEUROSCI.17-17-06575.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA. Phosphoinositides and calcium as regulators of cellular actin assembly and disassembly. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- Jap Tjoen San ERA, Schmidt-Michels MH, Oestreicher AB, Gispen WH, Schotman P. Inhibition of nerve growth factor-induced B-50/GAP-43 expression by antisense oligomers interferes with neurite outgrowth of PC12 cells. Biochem Biophys Res Commun. 1992;187:839–846. doi: 10.1016/0006-291x(92)91273-s. [DOI] [PubMed] [Google Scholar]
- Jap Tjoen San ERA, Van Rozen AJ, Nielander HB, Oestreicher AB, Gispen WH, Schotman P. Expression levels of B-50/GAP-43 in PC12 cells are decisive for the complexity of their neurites and growth cones. J Mol Neurosci. 1995;6:185–200. doi: 10.1007/BF02736764. [DOI] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci. 1997;17:6256–6236. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles J, Zwiers H, Van Dongen CJ, Schotman P, Wirtz KWA, Gispen WH. Modulation of brain polyphosphoinositide metabolism by ACTH-sensitive protein phosphorylation. Nature. 1980;286:623–625. doi: 10.1038/286623a0. [DOI] [PubMed] [Google Scholar]
- Lang P, Bertoglio J. Inhibition of lymphocyte-mediated cytotoxicity by Clostridium botulinum C3 transferase. Methods Enzymol. 1995;256:320–327. doi: 10.1016/0076-6879(95)56037-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fisher DA, Storm DR. Intracellular sorting of neuromodulin (GAP-43) mutants modified in the membrane targeting domain. J Neurosci. 1994;14:5807–5817. doi: 10.1523/JNEUROSCI.14-10-05807.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo LQ, Jan LY, Jan YN. Rho family small GTP-binding proteins in growth cone signalling. Curr Opin Neurobiol. 1997;7:81–86. doi: 10.1016/s0959-4388(97)80124-9. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Rho: A connection between membrane receptor signalling and the cytoskeleton. Trends Cell Biol. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J Cell Biol. 1997;138:913–926. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri KF, Gordon-Weeks PR. GAP-43 in growth cones is associated with areas of membrane that are tightly bound to substrate and is a component of a membrane skeleton subcellular fraction. J Neurosci. 1990;10:256–266. doi: 10.1523/JNEUROSCI.10-01-00256.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri KF, Hammang JP, Dent EW, Baetge EE. Mutagenesis of ser41 to ala inhibits the association of GAP-43 with the membrane skeleton of GAP-43-deficient PC12B cells: effects on cell adhesion and the composition of neurite cytoskeleton and membrane. J Neurobiol. 1996;29:213–232. doi: 10.1002/(SICI)1097-4695(199602)29:2<213::AID-NEU7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Mercken M, Lubke U, Vandermeeren M, Gheuens J, Oestreicher AB. Immunocytochemical detection of the growth associated protein B-50 by newly characterized monoclonal antibodies in human brain and muscle. J Neurobiol. 1992;23:309–321. doi: 10.1002/neu.480230310. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Buss TN. Accelerated differentiation in response to retinoic acid after retrovirally mediated gene transfer of GAP-43 into mouse neuroblastoma cells. Eur J Neurosci. 1992;4:910–916. doi: 10.1111/j.1460-9568.1992.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Nielander HB, French P, Oestreicher AB, Gispen WH, Schotman P. Spontaneous morphological changes by overexpression of the growth-associated protein B-50/GAP-43 in a PC12 cell line. Neurosci Lett. 1993;162:46–50. doi: 10.1016/0304-3940(93)90556-z. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac and Cdc-42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- O’Connor TP, Duerr JS, Bentley D. Pioneer growth cone steering decisions mediated by single filopodial contacts in situ. J Neurosci. 1990;10:3935–3946. doi: 10.1523/JNEUROSCI.10-12-03935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreicher AB, De Graan PNE, Gispen WH, Verhaagen J, Schrama LH. B-50, the growth associated protein-43: modulation of cell morphology and communication in the nervous system. Prog Neurobiol. 1997;53:627–686. doi: 10.1016/s0301-0082(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Pisano MR, Hegazy MG, Reimann EW, Dokas LA. Phosphorylation of protein B-50 (GAP-43) from adult rat brain cortex by casein kinase II. Biochem Biophys Res Commun. 1988;155:1207–1212. doi: 10.1016/s0006-291x(88)81268-3. [DOI] [PubMed] [Google Scholar]
- Qiu R-G, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- Qiu R-G, Chen J, McCormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Sekine A, Fujiwara M, Narumiya S. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J Biol Chem. 1989;264:8602–8605. [PubMed] [Google Scholar]
- Shea TB. Delivery of anti-GAP-43 antibodies into neuroblastoma cells reduces growth cone size. Biochem Biophys Res Commun. 1994;203:459–464. doi: 10.1006/bbrc.1994.2204. [DOI] [PubMed] [Google Scholar]
- Shea TB, Perrone-Bizzozero NI, Beermann ML, Benowitz LI. Phospholipid-mediated delivery of anti-GAP-43 antibodies into neuroblastoma cells prevents neuritogenesis. J Neurosci. 1991;11:1685–1690. doi: 10.1523/JNEUROSCI.11-06-01685.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter SM, Fankhauser C, Huang PL, Mashimo H, Fishman MC. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell. 1995;80:445–452. doi: 10.1016/0092-8674(95)90495-6. [DOI] [PubMed] [Google Scholar]
- Strittmatter SM, Valenzuela D, Fishman MC. An amino-terminal domain of the growth-associated protein GAP-43 mediates its effects on filopodial formation and cell spreading. J Cell Sci. 1994;107:195–204. doi: 10.1242/jcs.107.1.195. [DOI] [PubMed] [Google Scholar]
- Sudo Y, Valenzuela D, Becksickinger AG, Fishman MC, Strittmatter SM. Palmitoylation alters protein activity - blockade of Go stimulation by GAP-43. EMBO J. 1992;11:2095–2102. doi: 10.1002/j.1460-2075.1992.tb05268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydor AM, Su AL, Wang FS, Xu A, Jay DG. Talin and vinculin play distinct roles in filopodial motility in the neuronal growth cone. J Cell Biol. 1996;134:1197–1207. doi: 10.1083/jcb.134.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons M. Rho family GTPases: the cytoskeleton and beyond. Trends Biochem Sci. 1996;21:178–181. [PubMed] [Google Scholar]
- Tsukita S, Yonemura S, Tsukita Sh. ERM proteins: head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem Sci. 1997;22:53–58. doi: 10.1016/s0968-0004(96)10071-2. [DOI] [PubMed] [Google Scholar]
- Van Hooff COM, De Graan PNE, Oestreicher AB, Gispen WH. B-50 phosphorylation and polyphosphoinositide metabolism in nerve growth cone membranes. J Neurosci. 1988;8:1789–1795. doi: 10.1523/JNEUROSCI.08-05-01789.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum-Finney B, Reichardt LF. Vinculin-deficient PC12 cell lines extend unstable lamellipodia and filopodia and have a reduced rate of neurite outgrowth. J Cell Biol. 1994;127:1071–1084. doi: 10.1083/jcb.127.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer F, Caroni P. Phosphorylation-site mutagenesis of the growth-associated protein GAP-43 modulates its effects on cell spreading and morphology. J Cell Biol. 1993;120:503–512. doi: 10.1083/jcb.120.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A, Staple J, Caroni P. The motility-associated proteins GAP-43, MARCKS, and CAP-23 share unique targeting and surface activity-inducing properties. Exp Cell Res. 1997;236:103–116. doi: 10.1006/excr.1997.3709. [DOI] [PubMed] [Google Scholar]
- Yankner BA, Benowitz LI, Villa-Komaroff L, Neve RL. Transfection of PC12 cells with the human GAP-43 gene: effects on neurite outgrowth and regeneration. Mol Brain Res. 1990;7:39–44. doi: 10.1016/0169-328x(90)90071-k. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Glaven JA, Wu WJ, Cerione RA. Phosphatidylinositol 4,5-bisphosphate provides an alternative to guanine nucleotide exchange factors by stimulating the dissociation of GDP from Cdc42Hs. J Biol Chem. 1996;271:23815–23819. doi: 10.1074/jbc.271.39.23815. [DOI] [PubMed] [Google Scholar]
- Zuber MX, Goodman DW, Karns LR, Fishman MC. The neuronal growth-associated protein GAP-43 induces filopodia in non-neuronal cells. Science. 1989;244:1193–1195. doi: 10.1126/science.2658062. [DOI] [PubMed] [Google Scholar]