Abstract
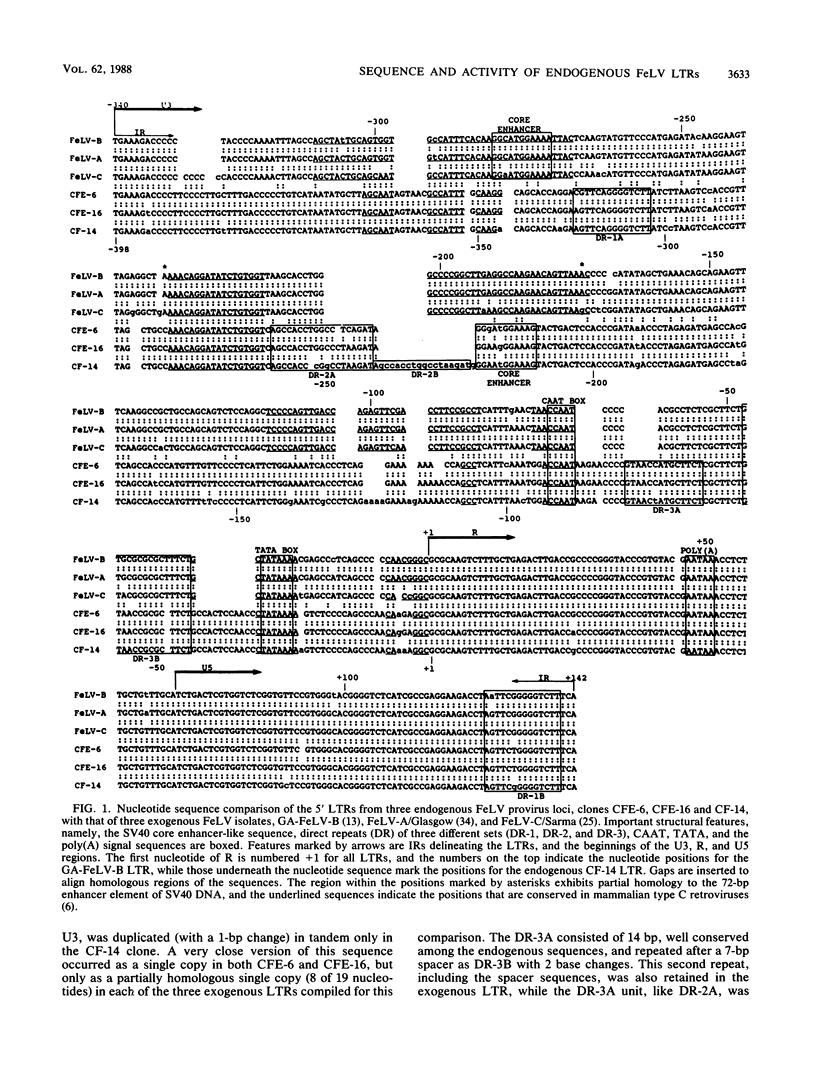
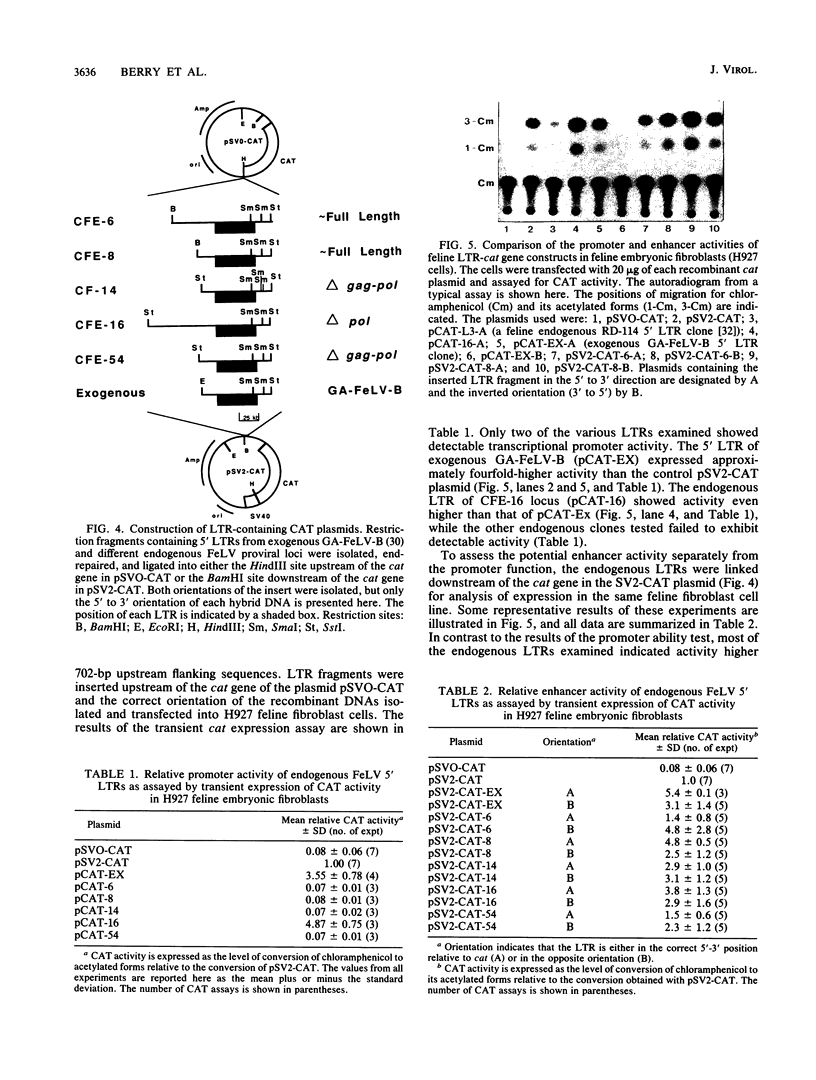
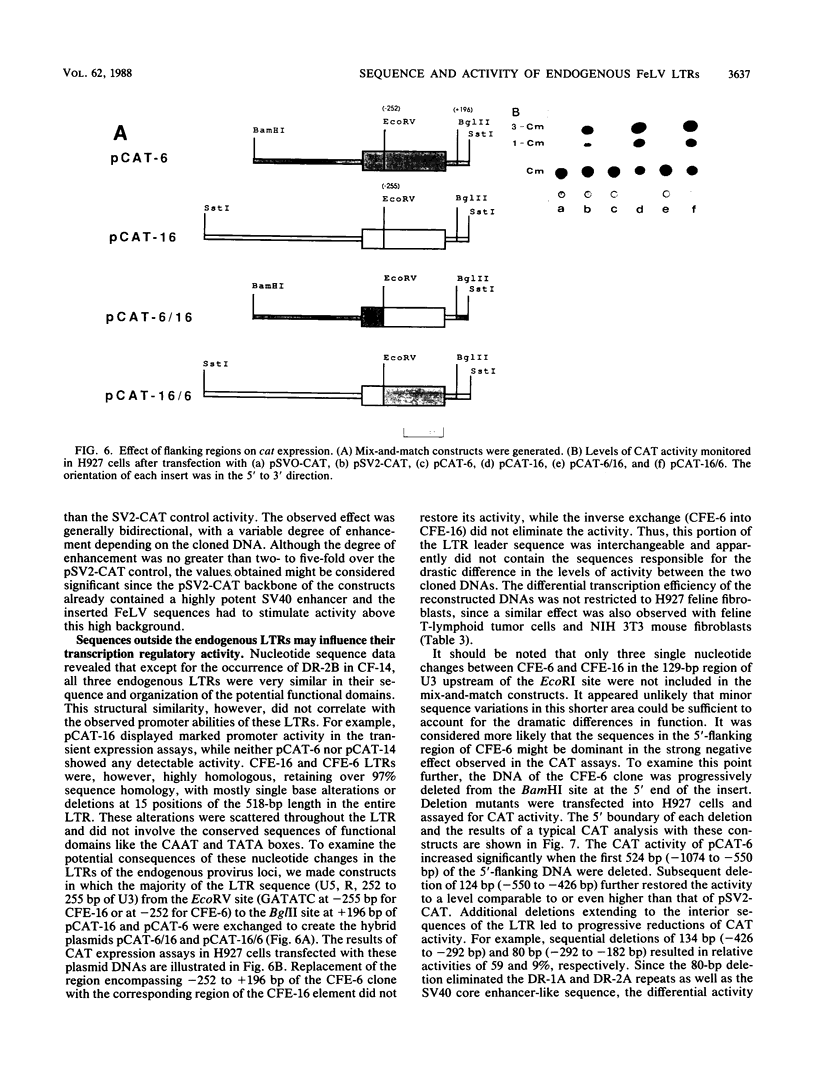
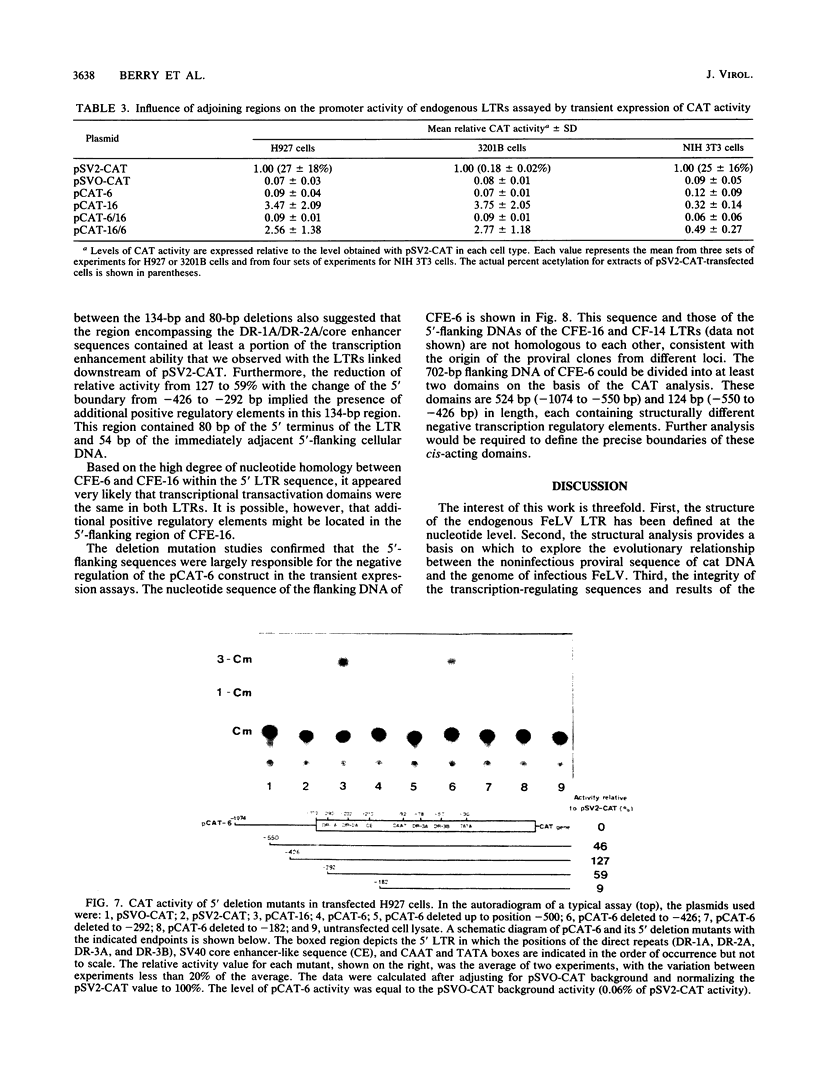
The nucleotide sequence of the 5' long terminal repeat (LTR) of three independent loci (CFE-6, CFE-16, and CF-14) of endogenous feline leukemia virus (FeLV) DNAs of the domestic cat genome was determined. The 3' LTR of the CFE-6 clone was also sequenced. The endogenous FeLV LTRs, which were very similar to each other in sequence and in organization of the functional domains, differed considerably from the exogenous FeLV LTR in the U3 region. The major differences in U3 included variations in sets of small (14 to 19 base pair) direct repeats, altered location of the simian virus 40 core enhancer-like sequence, and occurrence of three segments of largely nonhomologous sequences. There was extensive homology between endogenous and exogenous FeLV LTRs in sequences beginning from the TATA box through the R region down to the 3' end of the U5 region. The DNA sequence downstream of the 5' LTR encompassing the primer-binding site, leader, and almost to the end of the p15gag coding region, a point up to which the sequencing was carried out, also revealed a high degree of conservation. However, the detection of frameshift and nonsense mutations in this region of a nearly full-length endogenous provirus sequence (CFE-6) predicted its defectiveness and correlated with the lack of infectivity of this DNA. The functional studies of the endogenous LTRs, based on linkage to the bacterial cat gene and transient expression in feline cell lines, indicated that although the basic characteristics for promotion and enhancement of transcription were retained in each LTR, there was a significant variation in the activity of the cat constructs. Reconstruction and deletion analyses with the CFE-6 5' LTR revealed the presence of strong transcription regulatory sequences in the 702-base-pair region immediately upstream of the 5' boundary of the endogenous LTR. These and related data suggest that in addition to the transcription-modulating elements occurring within the LTR, the cis-acting nucleotide sequences in the upstream cellular DNA may determine the overall efficiency of transcription of the defective endogenous FeLV provirus loci of the felid genome.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baluda M. A., Roy-Burman P. Partial characterization of RD114 virus by DNA-RNA hybridization studies. Nat New Biol. 1973 Jul 11;244(132):59–62. doi: 10.1038/newbio244059a0. [DOI] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Sherr C. J., Todaro G. J. Evolution of type C viral genes: origin of feline leukemia virus. Science. 1975 Nov 28;190(4217):886–888. doi: 10.1126/science.52892. [DOI] [PubMed] [Google Scholar]
- Busch M. P., Devi B. G., Soe L. H., Perbal B., Baluda M. A., Roy-Burman P. Characterization of the expression of cellular retrovirus genes and oncogenes in feline cells. Hematol Oncol. 1983 Jan-Mar;1(1):61–75. doi: 10.1002/hon.2900010108. [DOI] [PubMed] [Google Scholar]
- Casey J. W., Roach A., Mullins J. I., Burck K. B., Nicolson M. O., Gardner M. B., Davidson N. The U3 portion of feline leukemia virus DNA identifies horizontally acquired proviruses in leukemic cats. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7778–7782. doi: 10.1073/pnas.78.12.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H. R., Barker W. C. Nucleotide sequences of the retroviral long terminal repeats and their adjacent regions. Nucleic Acids Res. 1984 Feb 24;12(4):1767–1778. doi: 10.1093/nar/12.4.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Donahue P. R., Hoover E. A., Beltz G. A., Riedel N., Hirsch V. M., Overbaugh J., Mullins J. I. Strong sequence conservation among horizontally transmissible, minimally pathogenic feline leukemia viruses. J Virol. 1988 Mar;62(3):722–731. doi: 10.1128/jvi.62.3.722-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Mullins J. I. Nucleotide sequence of the envelope gene of Gardner-Arnstein feline leukemia virus B reveals unique sequence homologies with a murine mink cell focus-forming virus. J Virol. 1983 Jun;46(3):871–880. doi: 10.1128/jvi.46.3.871-880.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guilhot S., Hampe A., D'Auriol L., Galibert F. Nucleotide sequence analysis of the LTRs and env genes of SM-FeSV and GA-FeSV. Virology. 1987 Nov;161(1):252–258. doi: 10.1016/0042-6822(87)90194-2. [DOI] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Even J., Sherr C. J., Galibert F. Nucleotide sequences of feline sarcoma virus long terminal repeats and 5' leaders show extensive homology to those of other mammalian retroviruses. J Virol. 1983 Jan;45(1):466–472. doi: 10.1128/jvi.45.1.466-472.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover E. A., Mullins J. I., Quackenbush S. L., Gasper P. W. Experimental transmission and pathogenesis of immunodeficiency syndrome in cats. Blood. 1987 Dec;70(6):1880–1892. [PubMed] [Google Scholar]
- Laprevotte I., Hampe A., Sherr C. J., Galibert F. Nucleotide sequence of the gag gene and gag-pol junction of feline leukemia virus. J Virol. 1984 Jun;50(3):884–894. doi: 10.1128/jvi.50.3.884-894.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niman H. L., Akhavi M., Gardner M. B., Stephenson J. R., Roy-Burman P. Differential expression of two distinct endogenous retrovisus genomes in developing tissues of the domestic cat. J Natl Cancer Inst. 1980 Mar;64(3):587–594. [PubMed] [Google Scholar]
- Niman H. L., Gardner M. B., Stephenson J. R., Roy-Burman P. Endogenous RD-114 virus genome expression in malignant tissues of domestic cats. J Virol. 1977 Sep;23(3):578–586. doi: 10.1128/jvi.23.3.578-586.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niman H. L., Stephenson J. R., Gardner M. B., Roy-Burman P. RD-114 and feline leukaemia virus genome expression in natural lymphomas of domestic cats. Nature. 1977 Mar 24;266(5600):357–360. doi: 10.1038/266357a0. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Donahue P. R., Quackenbush S. L., Hoover E. A., Mullins J. I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988 Feb 19;239(4842):906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintrell N., Varmus H. E., Bishop J. M., Nicholson M. O., McAllister R. M. Homologies among the nucleotide sequences of the genomes of C-type viruses. Virology. 1974 Apr;58(2):568–575. doi: 10.1016/0042-6822(74)90090-7. [DOI] [PubMed] [Google Scholar]
- Rhode B. W., Emerman M., Temin H. M. Instability of large direct repeats in retrovirus vectors. J Virol. 1987 Mar;61(3):925–927. doi: 10.1128/jvi.61.3.925-927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Gasper P. W., Nicolson M. O., Mullins J. I. Molecular analysis and pathogenesis of the feline aplastic anemia retrovirus, feline leukemia virus C-Sarma. J Virol. 1986 Oct;60(1):242–250. doi: 10.1128/jvi.60.1.242-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sarma P. S., Log T., Skuntz S., Krishnan S., Burkley K. Experimental horizontal transmission of feline leukemia viruses of subgroups A, B, and C. J Natl Cancer Inst. 1978 Apr;60(4):871–874. doi: 10.1093/jnci/60.4.871. [DOI] [PubMed] [Google Scholar]
- Snyder H. W., Jr, Hardy W. D., Jr, Zuckerman E. E., Fleissner E. Characterisation of a tumour-specific antigen on the surface of feline lymphosarcoma cells. Nature. 1978 Oct 19;275(5681):656–658. doi: 10.1038/275656a0. [DOI] [PubMed] [Google Scholar]
- Soe L. H., Devi B. G., Mullins J. I., Roy-Burman P. Molecular cloning and characterization of endogenous feline leukemia virus sequences from a cat genomic library. J Virol. 1983 Jun;46(3):829–840. doi: 10.1128/jvi.46.3.829-840.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe L. H., Shimizu R. W., Landolph J. R., Roy-Burman P. Molecular analysis of several classes of endogenous feline leukemia virus elements. J Virol. 1985 Dec;56(3):701–710. doi: 10.1128/jvi.56.3.701-710.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spodick D. A., Ghosh A. K., Parimoo S., Roy-Burman P. The long terminal repeat of feline endogenous RD-114 retroviral DNAs: analysis of transcription regulatory activity and nucleotide sequence. Virus Res. 1988 Feb;9(2-3):263–283. doi: 10.1016/0168-1702(88)90035-4. [DOI] [PubMed] [Google Scholar]
- Spodick D. A., Soe L. H., Roy-Burman P. Genetic analysis of the feline RD-114 retrovirus-related endogenous elements. Virus Res. 1984 Oct;1(7):543–555. doi: 10.1016/0168-1702(84)90012-1. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Warnock M., Wheeler A., Wilkie N., Mullins J. I., Onions D. E., Neil J. C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. J Virol. 1986 Jun;58(3):825–834. doi: 10.1128/jvi.58.3.825-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]





