Abstract
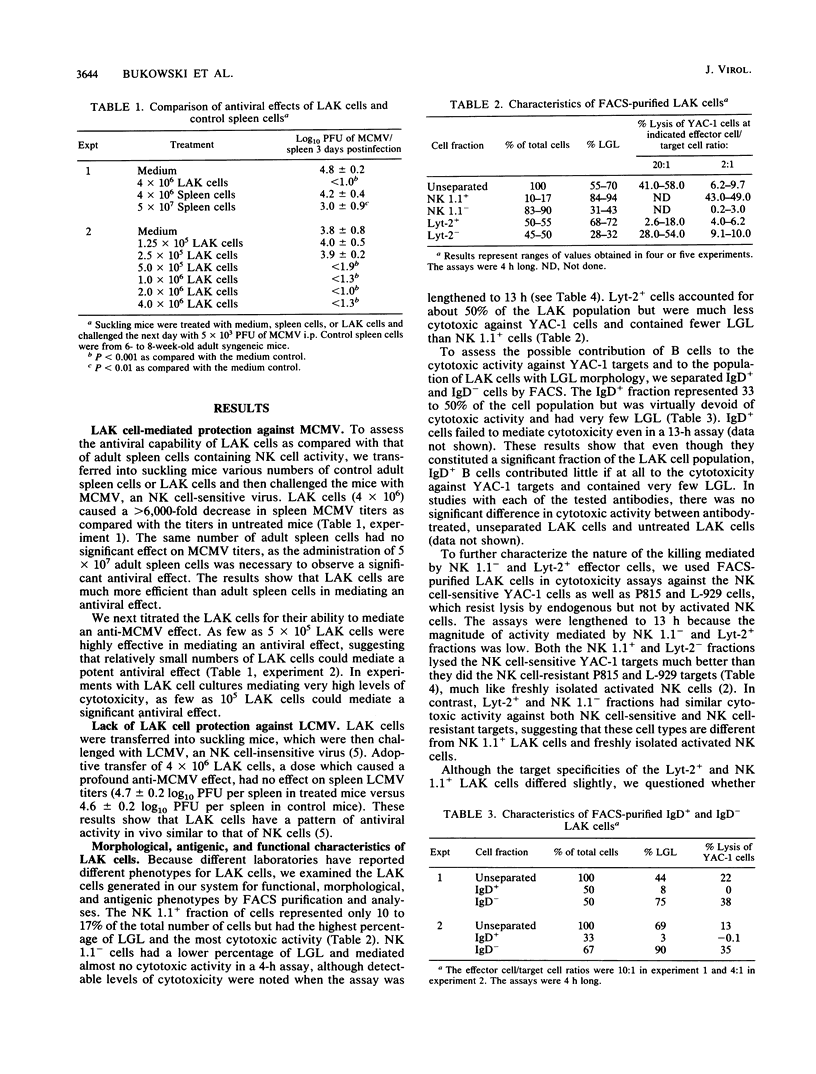
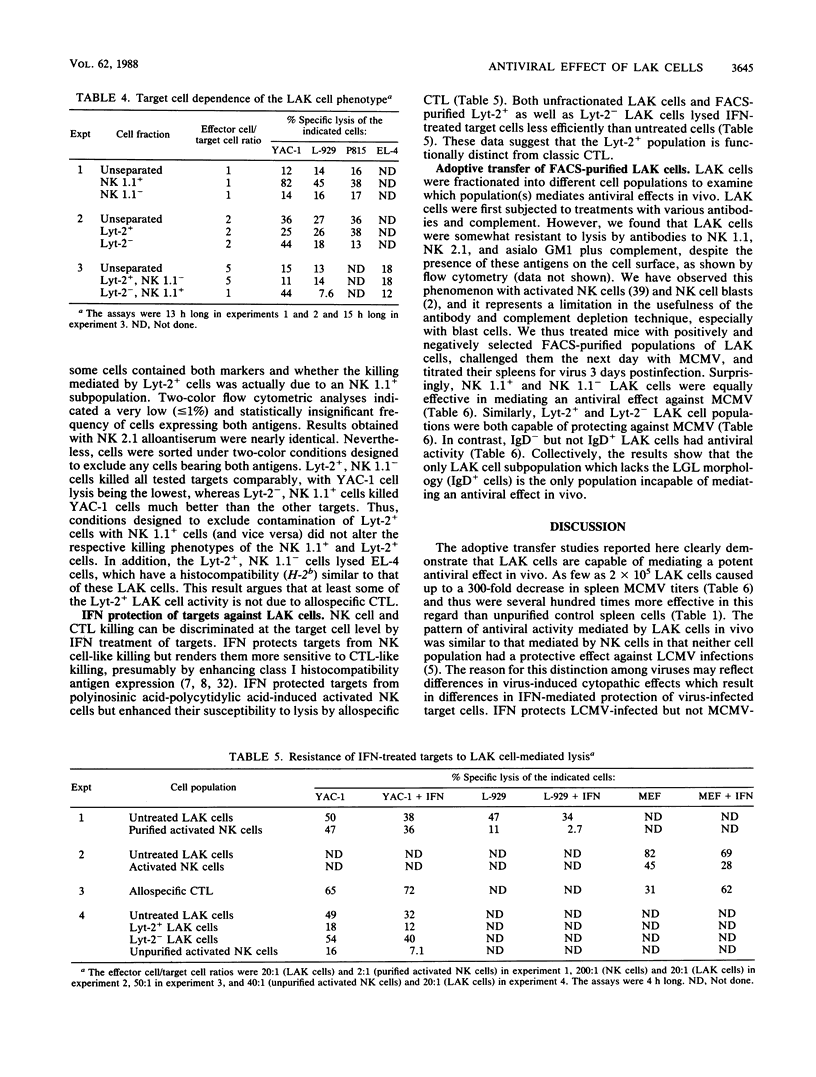
Lymphokine-activated killer (LAK) cells generated by cultivation of C57BL/6 mouse spleen cells in the presence of recombinant interleukin-2 were transferred into natural killer (NK) cell-deficient suckling mouse recipients. These mice were then challenged with either murine cytomegalovirus (MCMV) or lymphocytic choriomeningitis (LCMV) and sacrificed 3 days later. No interleukin 2 infusions were given. Mice receiving as few as 5 x 10(5) LAK cells had several 100-fold decreases in spleen MCMV titers as compared with untreated mice. This treatment had no effect on spleen LCMV titers. The LAK cell cultures contained 10 to 17% NK 1.1+, 50 to 55% Lyt-2+, and 33 to 50% immunoglobulin D+ cells. Double fluorescence labeling and in vitro cytotoxicity assays with fluorescence-activated cell sorting revealed at least two mutually exclusive killer cell populations. NK 1.1+ LAK cells resembled freshly isolated activated NK cells with regard to target cell range (YAC-1 cell killing greater than L-929, P815, and EL-4 cell killing), large granular lymphocyte (LGL) morphology, and decreased ability to lyse interferon (IFN)-treated target cells. Lyt-2+ LAK cells lysed the targets mentioned above but at lower levels and without the differences in susceptibility mentioned above. These Lyt-2+ LAK cells also had a decreased ability to lyse IFN-treated targets, in contrast to classic cytotoxic T lymphocytes, which lyse IFN-treated targets far more efficiently than untreated targets. Purified populations of LAK cells obtained by fluorescence-activated cell sorting were used in the antiviral protection model. The results showed that protection against MCMV could be mediated by NK 1.1+, NK 1.1-, Lyt-2+, Lyt-2-, and IgD- populations but not by IgD+ cells. The five protective populations all had in common the LGL phenotype and cytotoxic activity in vitro. The IgD+ population did not contain LGLs, lyse target cells in vitro, or mediate an antiviral effect in vivo. These results suggest that LAK cells may be therapeutically useful against certain virus infections (MCMV) but not others (LCMV) and that despite their heterogeneity in antigenic phenotype and cytotoxic activity, their pattern of antiviral activity in vivo resembles that of NK cells, which protect against MCMV but not LCMV.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andriole G. L., Mulé J. J., Hansen C. T., Linehan W. M., Rosenberg S. A. Evidence that lymphokine-activated killer cells and natural killer cells are distinct based on an analysis of congenitally immunodeficient mice. J Immunol. 1985 Nov;135(5):2911–2913. [PubMed] [Google Scholar]
- Biron C. A., Pedersen K. F., Welsh R. M. Purification and target cell range of in vivo elicited blast natural killer cells. J Immunol. 1986 Jul 15;137(2):463–471. [PubMed] [Google Scholar]
- Biron C. A., Turgiss L. R., Welsh R. M. Increase in NK cell number and turnover rate during acute viral infection. J Immunol. 1983 Sep;131(3):1539–1545. [PubMed] [Google Scholar]
- Bukowski J. F., Warner J. F., Dennert G., Welsh R. M. Adoptive transfer studies demonstrating the antiviral effect of natural killer cells in vivo. J Exp Med. 1985 Jan 1;161(1):40–52. doi: 10.1084/jem.161.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski J. F., Welsh R. M. Enhanced susceptibility to cytotoxic T lymphocytes of target cells isolated from virus-infected or interferon-treated mice. J Virol. 1986 Sep;59(3):735–739. doi: 10.1128/jvi.59.3.735-739.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski J. F., Welsh R. M. Inability of interferon to protect virus-infected cells against lysis by natural killer (NK) cells correlates with NK cell-mediated antiviral effects in vivo. J Immunol. 1985 Nov;135(5):3537–3541. [PubMed] [Google Scholar]
- Bukowski J. F., Welsh R. M. Interferon enhances the susceptibility of virus-infected fibroblasts to cytotoxic T cells. J Exp Med. 1985 Jan 1;161(1):257–262. doi: 10.1084/jem.161.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski J. F., Woda B. A., Habu S., Okumura K., Welsh R. M. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983 Sep;131(3):1531–1538. [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godeny E. K., Gauntt C. J. Involvement of natural killer cells in coxsackievirus B3-induced murine myocarditis. J Immunol. 1986 Sep 1;137(5):1695–1702. [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSHAW J. B., BETTS R. F., SIMON G., BOYNTON R. C. ACQUIRED CYTOMEGALOVIRUS INFECTION: ASSOCIATION WITH HEPATOMEGALY AND ABNORMAL LIVER-FUNCTION TESTS. N Engl J Med. 1965 Mar 25;272:602–609. doi: 10.1056/NEJM196503252721202. [DOI] [PubMed] [Google Scholar]
- Habu S., Fukui H., Shimamura K., Kasai M., Nagai Y., Okumura K., Tamaoki N. In vivo effects of anti-asialo GM1. I. Reduction of NK activity and enhancement of transplanted tumor growth in nude mice. J Immunol. 1981 Jul;127(1):34–38. [PubMed] [Google Scholar]
- Hanshaw J. B. Congenital cytomegalovirus infection: a fifteen year perspective. J Infect Dis. 1971 May;123(5):555–561. doi: 10.1093/infdis/123.5.555. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T., Staal S., Djeu J. Y. Augmentation of natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic target cells. Int J Cancer. 1977 Apr 15;19(4):555–564. doi: 10.1002/ijc.2910190417. [DOI] [PubMed] [Google Scholar]
- Kalland T., Belfrage H., Bhiladvala P., Hedlund G. Analysis of the murine lymphokine-activated killer (LAK) cell phenomenon: dissection of effectors and progenitors into NK- and T-like cells. J Immunol. 1987 Jun 1;138(11):3640–3645. [PubMed] [Google Scholar]
- Koo G. C., Manyak C. L. Generation of cytotoxic cells from murine bone marrow by human recombinant IL 2. J Immunol. 1986 Sep 15;137(6):1751–1756. [PubMed] [Google Scholar]
- Koo G. C., Peppard J. R. Establishment of monoclonal anti-Nk-1.1 antibody. Hybridoma. 1984 Fall;3(3):301–303. doi: 10.1089/hyb.1984.3.301. [DOI] [PubMed] [Google Scholar]
- Lotzová E., Savary C. A., Herberman R. B. Induction of NK cell activity against fresh human leukemia in culture with interleukin 2. J Immunol. 1987 Apr 15;138(8):2718–2727. [PubMed] [Google Scholar]
- Macfarlan R. I., Burns W. H., White D. O. Two cytotoxic cells in peritoneal cavity of virus-infected mice: antibody-dependent macrophages and nonspecific killer cells. J Immunol. 1977 Nov;119(5):1569–1574. [PubMed] [Google Scholar]
- Macher A. M., Reichert C. M., Straus S. E., Longo D. L., Parrillo J., Lane H. C., Fauci A. S., Rook A. H., Manischewitz J. F., Quinnan G. V., Jr Death in the AIDS patient: role of cytomegalovirus. N Engl J Med. 1983 Dec 8;309(23):1454–1454. doi: 10.1056/NEJM198312083092312. [DOI] [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Rosenberg S. A. The anti-tumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo. J Immunol. 1985 Jul;135(1):646–652. [PubMed] [Google Scholar]
- Nedrud J. G., Collier A. M., Pagano J. S. Cellular basis for susceptibility to mouse cytomegalovirus: evidence from tracheal organ culture. J Gen Virol. 1979 Dec;45(3):737–744. doi: 10.1099/0022-1317-45-3-737. [DOI] [PubMed] [Google Scholar]
- Parker D. C., Wadsworth D. C., Schneider G. B. Activation of murine B lymphocytes by anti-immunoglobulin is an inductive signal leading to immunoglobulin secretion. J Exp Med. 1980 Jul 1;152(1):138–150. doi: 10.1084/jem.152.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986 Sep 1;164(3):814–825. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Salup R. R., Mathieson B. J., Wiltrout R. H. Precursor phenotype of lymphokine-activated killer cells in the mouse. J Immunol. 1987 Jun 1;138(11):3635–3639. [PubMed] [Google Scholar]
- Selgrade M. J., Osborn J. E. Divergence of mouse brain interferon responses following virulent or avirulent Newcastle disease virus inoculation. Proc Soc Exp Biol Med. 1973 May;143(1):12–18. doi: 10.3181/00379727-143-37243. [DOI] [PubMed] [Google Scholar]
- Stein-Streilein J., Guffee J. In vivo treatment of mice and hamsters with antibodies to asialo GM1 increases morbidity and mortality to pulmonary influenza infection. J Immunol. 1986 Feb 15;136(4):1435–1441. [PubMed] [Google Scholar]
- Trinchieri G., Santoli D. Anti-viral activity induced by culturing lymphocytes with tumor-derived or virus-transformed cells. Enhancement of human natural killer cell activity by interferon and antagonistic inhibition of susceptibility of target cells to lysis. J Exp Med. 1978 May 1;147(5):1314–1333. doi: 10.1084/jem.147.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Lu S. D., Mark D. F. Site-specific mutagenesis of the human interleukin-2 gene: structure-function analysis of the cysteine residues. Science. 1984 Jun 29;224(4656):1431–1433. doi: 10.1126/science.6427925. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978 Jul 1;148(1):163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Zinkernagel R. M. Heterospecific cytotoxic cell activity induced during the first three days of acute lymphocytic choriomeningitis virus infection in mice. Nature. 1977 Aug 18;268(5621):646–648. doi: 10.1038/268646a0. [DOI] [PubMed] [Google Scholar]
- Welsh R. M. Regulation of virus infections by natural killer cells. A review. Nat Immun Cell Growth Regul. 1986;5(4):169–199. [PubMed] [Google Scholar]
- Wong C. Y., Woodruff J. J., Woodruff J. F. Generation of cytotoxic T lymphocytes during coxsackievirus B-3 infection. III. Role of sex. J Immunol. 1977 Aug;119(2):591–597. [PubMed] [Google Scholar]
- Yang H., Welsh R. M. Induction of alloreactive cytotoxic T cells by acute virus infection of mice. J Immunol. 1986 Feb 15;136(4):1186–1193. [PubMed] [Google Scholar]
- Yang H., Yogeeswaran G., Bukowski J. F., Welsh R. M. Expression of asialo GM1 and other antigens and glycolipids on natural killer cells and spleen leukocytes in virus-infected mice. Nat Immun Cell Growth Regul. 1985;4(1):21–39. [PubMed] [Google Scholar]
- Yang J. C., Mulé J. J., Rosenberg S. A. Murine lymphokine-activated killer (LAK) cells: phenotypic characterization of the precursor and effector cells. J Immunol. 1986 Jul 15;137(2):715–722. [PubMed] [Google Scholar]


