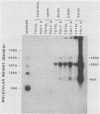
Abstract
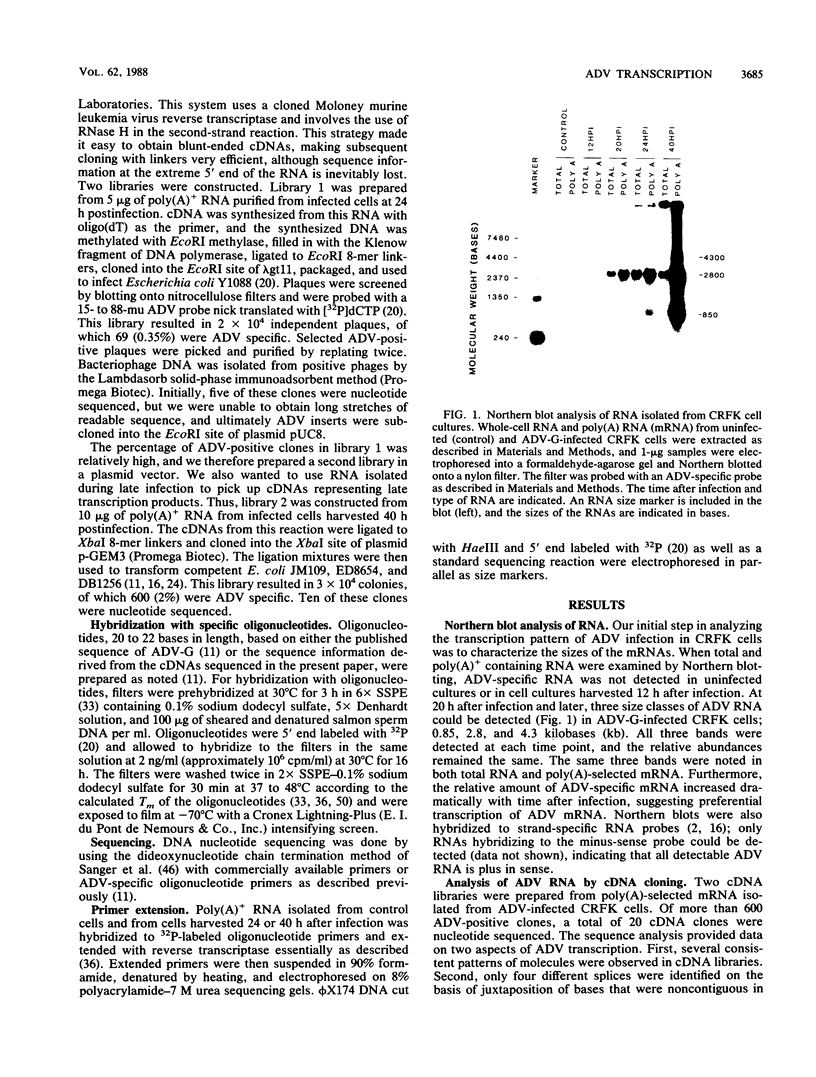
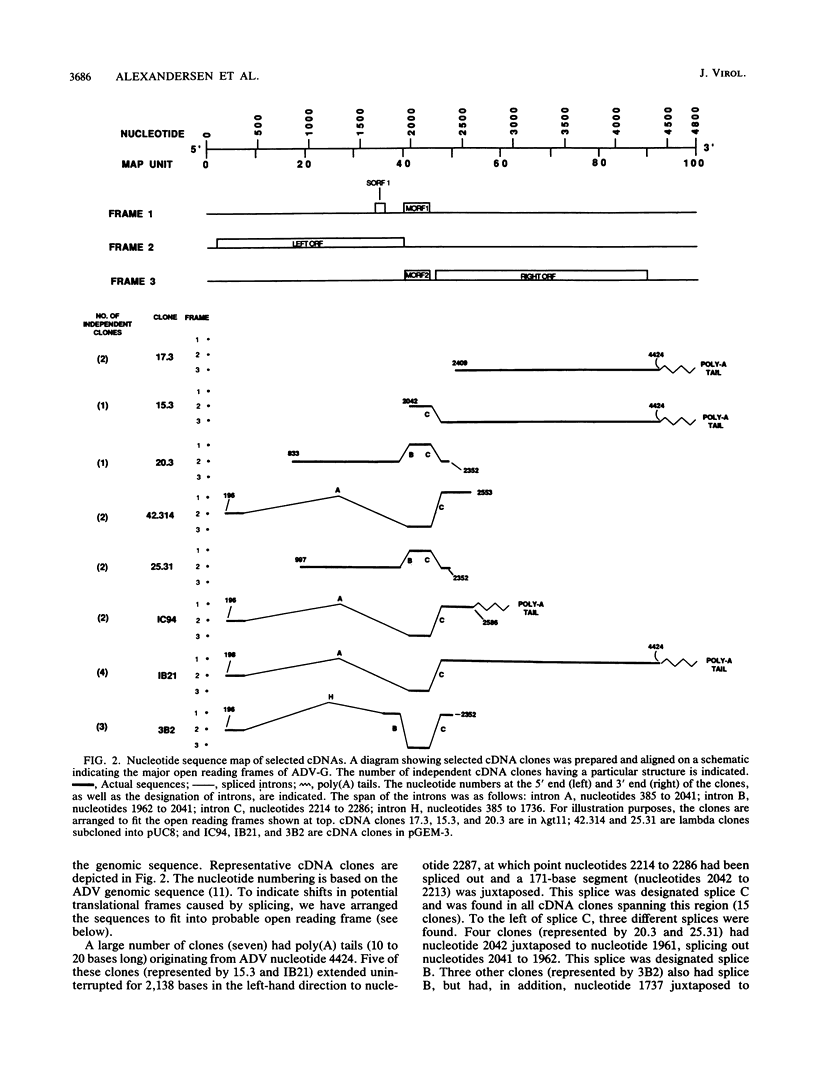
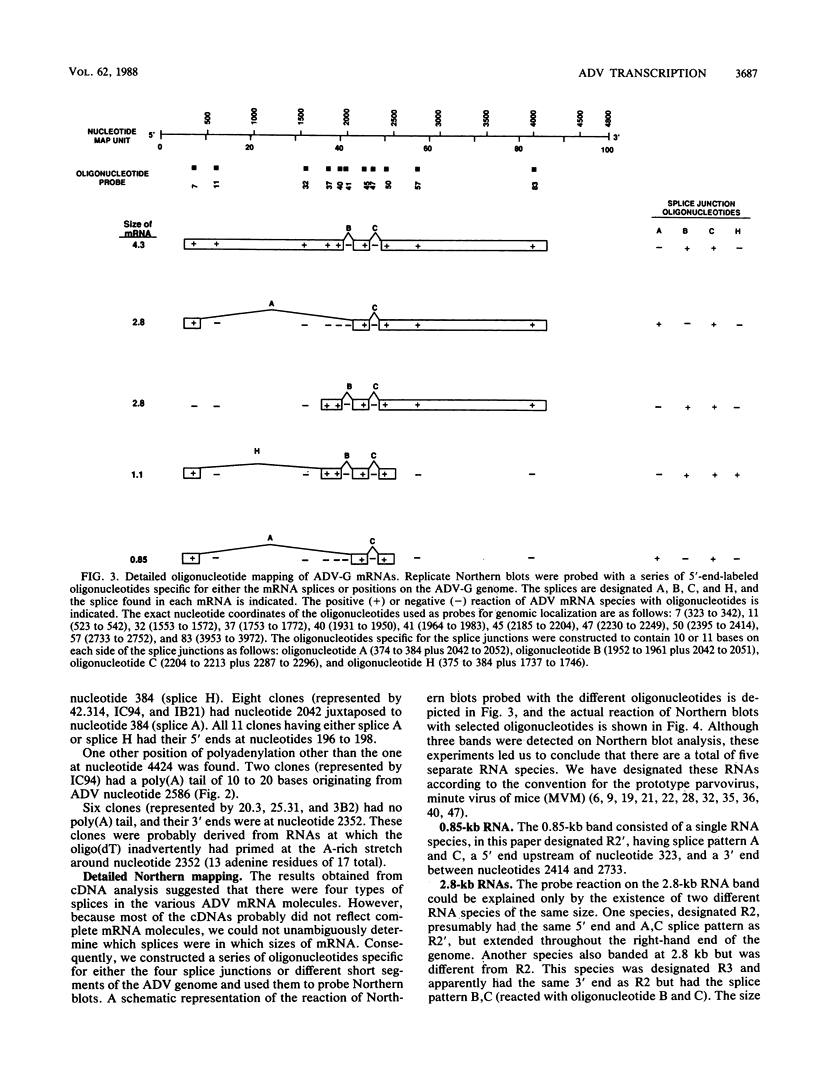
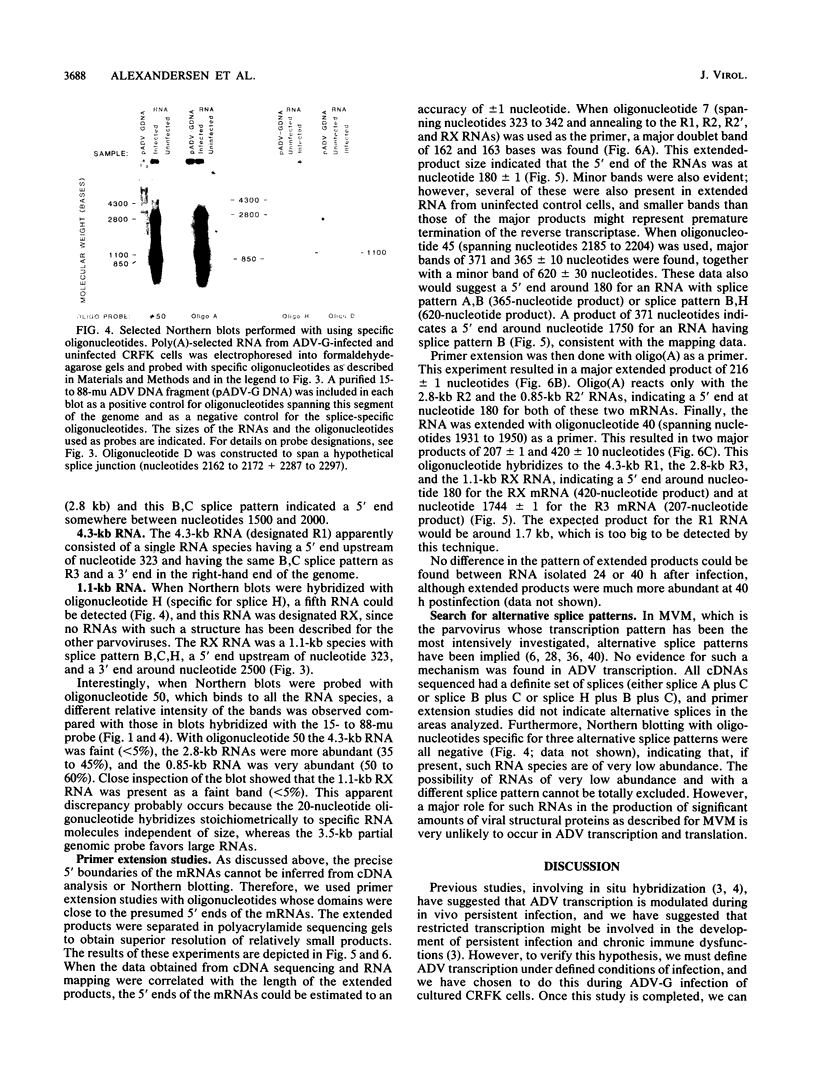
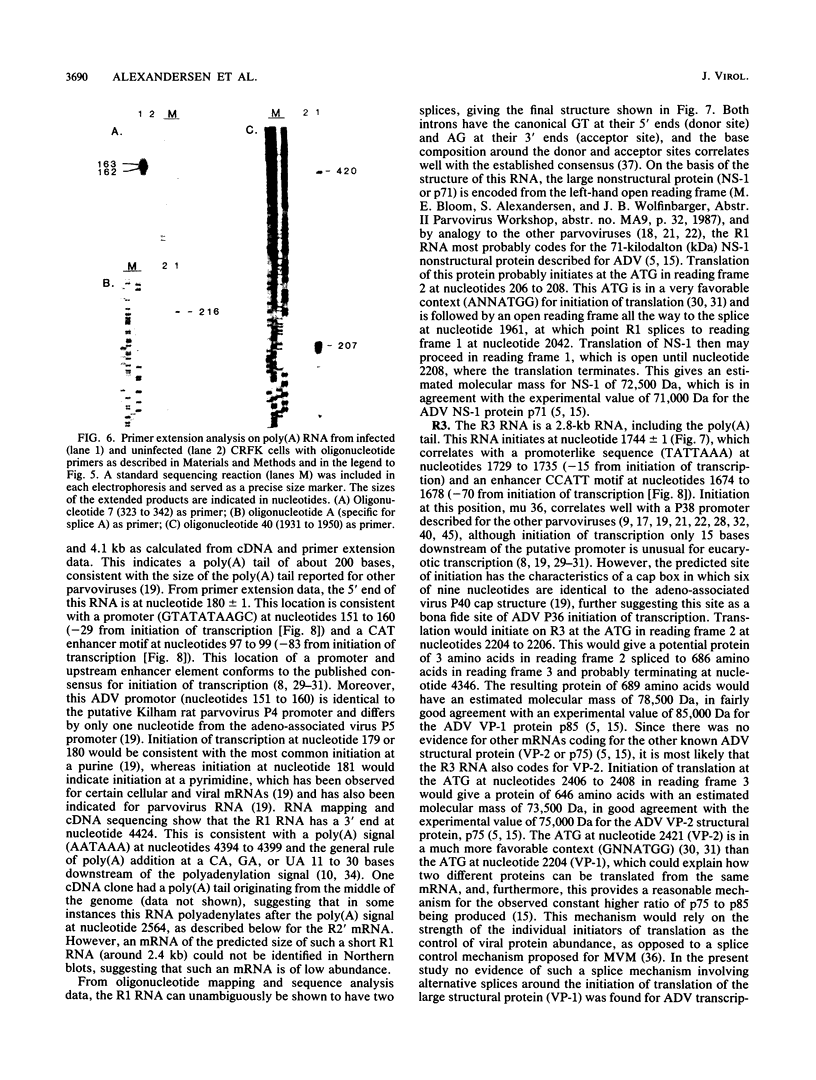
We studied the transcription program of Aleutian mink disease parvovirus (ADV) by using a combination of cDNA cloning and sequencing, primer extension, and Northern (RNA) blot hybridization with splice-specific oligonucleotides. The 4.8-kilobase ADV genome was transcribed in the rightward direction, yielding plus-sense polyadenylated transcripts of 4.3 (R1 RNA), 2.8 (R2), 2.8 (R3), 1.1 (RX), and 0.85 (R2') kilobases. Each RNA transcript had potential translation initiation sites within open reading frames, suggesting protein translation, and a scheme encompassing ADV structural and nonstructural proteins is proposed. Each of the five RNA transcripts had a characteristic set of splices and originated from a promoter at nucleotide 152 (map unit 3 [R1, R2, R2', and RX]) or at nucleotide 1729 (map unit 36 [R3]). The transcripts terminated with a poly(A) tail at one of two positions: either at map unit 53 (R2' and RX) or at map unit 92 (R1, R2, and R3). Similarities with and differences from the transcription maps of other parvoviruses are discussed, and possible roles of the unique features found in ADV transcription are related to the special pathogenic features of this virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S. Acute interstitial pneumonia in mink kits: experimental reproduction of the disease. Vet Pathol. 1986 Sep;23(5):579–588. doi: 10.1177/030098588602300506. [DOI] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J. Evidence of restricted viral replication in adult mink infected with Aleutian disease of mink parvovirus. J Virol. 1988 May;62(5):1495–1507. doi: 10.1128/jvi.62.5.1495-1507.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Wolfinbarger J., Race R. E. In situ molecular hybridization for detection of Aleutian mink disease parvovirus DNA by using strand-specific probes: identification of target cells for viral replication in cell cultures and in mink kits with virus-induced interstitial pneumonia. J Virol. 1987 Aug;61(8):2407–2419. doi: 10.1128/jvi.61.8.2407-2419.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Uttenthal-Jensen A., Aasted B. Demonstration of non-degraded Aleutian disease virus (ADV) proteins in lung tissue from experimentally infected mink kits. Brief report. Arch Virol. 1986;87(1-2):127–133. doi: 10.1007/BF01310549. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Gardiner E. M., Tattersall P. DNA sequence of the lymphotropic variant of minute virus of mice, MVM(i), and comparison with the DNA sequence of the fibrotropic prototype strain. J Virol. 1986 Feb;57(2):656–669. doi: 10.1128/jvi.57.2.656-669.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra S. P., Rose J. A., Hardy M., Baroudy B. M., Anderson C. W. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimhon M., Gabarro-Arpa J., Ehrlich R., Reiss C. Physical characteristics in eucaryotic promoters. Nucleic Acids Res. 1983 Jul 11;11(13):4521–4540. doi: 10.1093/nar/11.13.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Labow M. A. Parvovirus gene regulation. J Gen Virol. 1987 Mar;68(Pt 3):601–614. doi: 10.1099/0022-1317-68-3-601. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Perryman S., Lechner D., Wolfinbarger J. B. Nucleotide sequence and genomic organization of Aleutian mink disease parvovirus (ADV): sequence comparisons between a nonpathogenic and a pathogenic strain of ADV. J Virol. 1988 Aug;62(8):2903–2915. doi: 10.1128/jvi.62.8.2903-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Kaaden O. R., Huggans E., Cohn A., Wolfinbarger J. B. Molecular comparisons of in vivo- and in vitro-derived strains of Aleutian disease of mink parvovirus. J Virol. 1988 Jan;62(1):132–138. doi: 10.1128/jvi.62.1.132-138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Aasted B., Wolfinbarger J. B. Analysis of Aleutian disease virus infection in vitro and in vivo: demonstration of Aleutian disease virus DNA in tissues of infected mink. J Virol. 1985 Sep;55(3):696–703. doi: 10.1128/jvi.55.3.696-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Analysis of Aleutian disease of mink parvovirus infection using strand-specific hybridization probes. Intervirology. 1987;27(2):102–111. doi: 10.1159/000149727. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Characterization of Aleutian disease virus as a parvovirus. J Virol. 1980 Sep;35(3):836–843. doi: 10.1128/jvi.35.3.836-843.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Identification of a nonvirion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982 Aug;43(2):608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell M. C., Beard C., Astell C. R. In vitro identification of a B19 parvovirus promoter. Virology. 1987 Apr;157(2):534–538. doi: 10.1016/0042-6822(87)90296-0. [DOI] [PubMed] [Google Scholar]
- Carlson J. O., Lynde-Maas M. K., Shen Z. D. A nonstructural protein of feline panleukopenia virus: expression in Escherichia coli and detection of multiple forms in infected cells. J Virol. 1987 Feb;61(2):621–624. doi: 10.1128/jvi.61.2.621-624.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Race R., Wehrly K., Nishio J., Bloom M., Lechner D., Bergstrom S., Robbins K., Mayer L., Keith J. M. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature. 1985 May 23;315(6017):331–333. doi: 10.1038/315331a0. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Organization of nonstructural genes of the autonomous parvovirus minute virus of mice. J Virol. 1986 Jun;58(3):724–732. doi: 10.1128/jvi.58.3.724-732.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Crandell R. A., Fabricant C. G., Nelson-Rees W. A. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK). In Vitro. 1973 Nov-Dec;9(3):176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- DeLange A. M., Reddy M., Scraba D., Upton C., McFadden G. Replication and resolution of cloned poxvirus telomeres in vivo generates linear minichromosomes with intact viral hairpin termini. J Virol. 1986 Aug;59(2):249–259. doi: 10.1128/jvi.59.2.249-259.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham J. R., Henson J. B., Crawford T. B., Padgett G. A. The epizootiology of aleutian disease. Front Biol. 1976;44:135–158. [PubMed] [Google Scholar]
- Hadlow W. J., Race R. E., Kennedy R. C. Comparative pathogenicity of four strains of Aleutian disease virus for pastel and sapphire mink. Infect Immun. 1983 Sep;41(3):1016–1023. doi: 10.1128/iai.41.3.1016-1023.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Sahli R., McMaster G. K., Hirt B. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus. J Virol. 1986 Sep;59(3):564–573. doi: 10.1128/jvi.59.3.564-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P. Enhancer elements. Cell. 1983 Jun;33(2):313–314. doi: 10.1016/0092-8674(83)90410-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Regulation of protein synthesis in virus-infected animal cells. Adv Virus Res. 1986;31:229–292. doi: 10.1016/S0065-3527(08)60265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labieniec-Pintel L., Pintel D. The minute virus of mice P39 transcription unit can encode both capsid proteins. J Virol. 1986 Mar;57(3):1163–1167. doi: 10.1128/jvi.57.3.1163-1167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt M. A., Imperiale M. J., Ali H., Nevins J. R. Requirement of a downstream sequence for generation of a poly(A) addition site. Cell. 1984 Jul;37(3):993–999. doi: 10.1016/0092-8674(84)90433-1. [DOI] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. R., Ward D. C. Three splicing patterns are used to excise the small intron common to all minute virus of mice RNAs. J Virol. 1986 Dec;60(3):1170–1174. doi: 10.1128/jvi.60.3.1170-1174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Ayub J., Hao Y. S., Kurtzman G., Shimada T., Young N. Novel transcription map for the B19 (human) pathogenic parvovirus. J Virol. 1987 Aug;61(8):2395–2406. doi: 10.1128/jvi.61.8.2395-2406.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett G. A., Gorham J. R., Henson J. B. Epizootiologic studies of Aleutian disease. I. Transplacental transmission of the virus. J Infect Dis. 1967 Feb;117(1):35–38. doi: 10.1093/infdis/117.1.35. [DOI] [PubMed] [Google Scholar]
- Pintel D., Dadachanji D., Astell C. R., Ward D. C. The genome of minute virus of mice, an autonomous parvovirus, encodes two overlapping transcription units. Nucleic Acids Res. 1983 Feb 25;11(4):1019–1038. doi: 10.1093/nar/11.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D. Aleutian disease: a persistent parvovirus infection of mink with a maximal but ineffective host humoral immune response. Prog Med Virol. 1986;33:42–60. [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. 3. Immune complex arteritis. Am J Pathol. 1973 May;71(2):331–344. [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd trans-Activation of parvovirus P38 promoter by the 76K noncapsid protein. J Virol. 1985 Sep;55(3):886–889. doi: 10.1128/jvi.55.3.886-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D. C., Kurstak E., Tattersall P. Characteristics and taxonomy of Parvoviridae. Intervirology. 1985;23(2):61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- Srivastava A., Lusby E. W., Berns K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983 Feb;45(2):555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Tal J., Carter B. J. Negative and positive regulation in trans of gene expression from adeno-associated virus vectors in mammalian cells by a viral rep gene product. Mol Cell Biol. 1986 Aug;6(8):2884–2894. doi: 10.1128/mcb.6.8.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]