Abstract
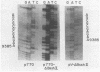
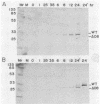
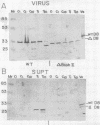
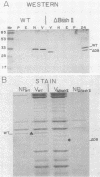
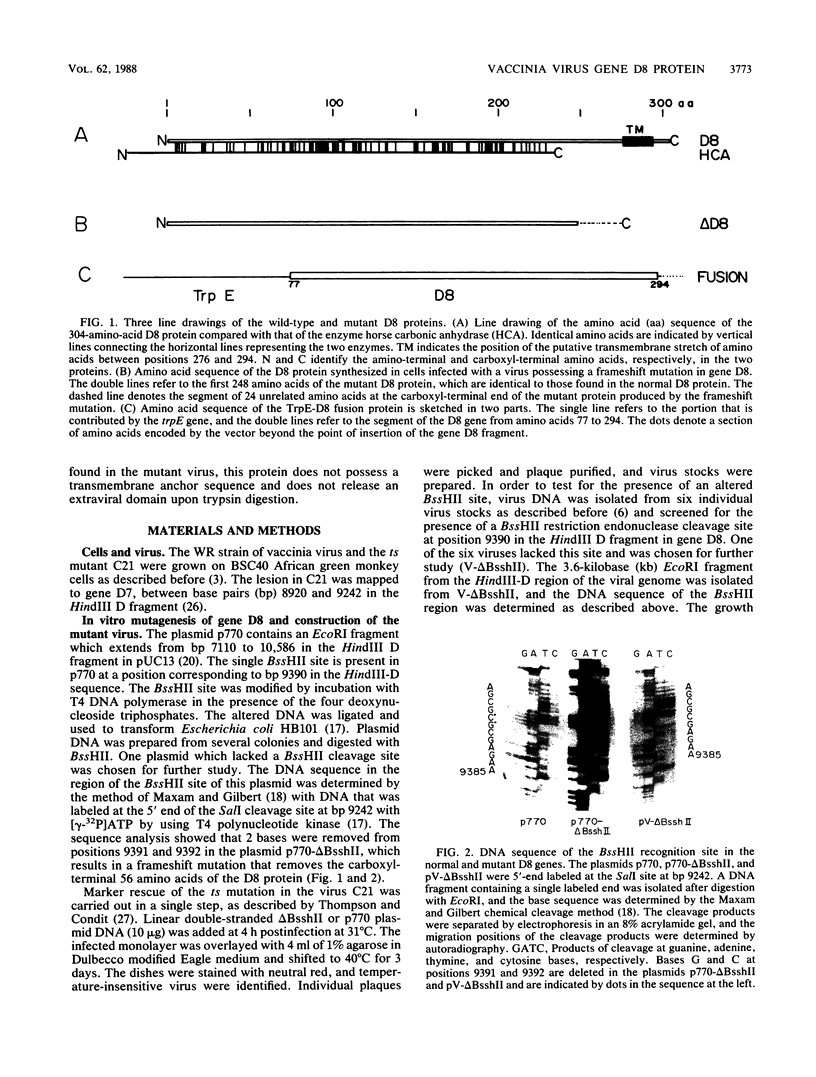
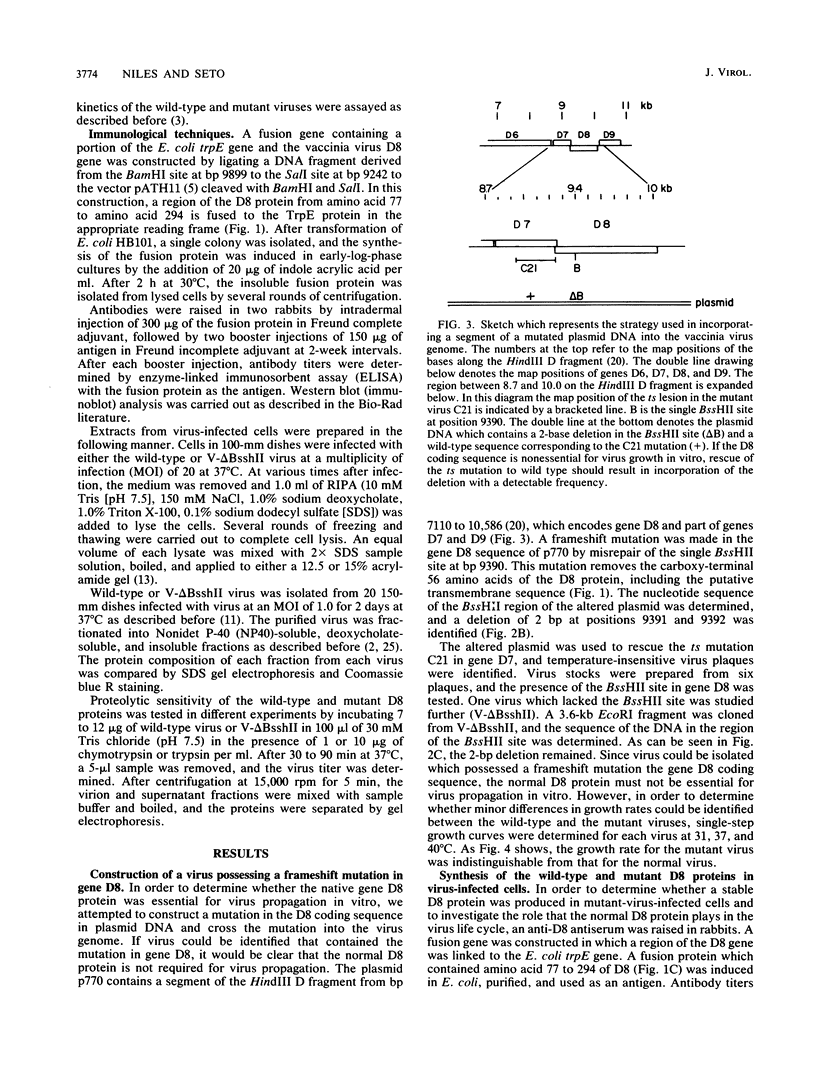
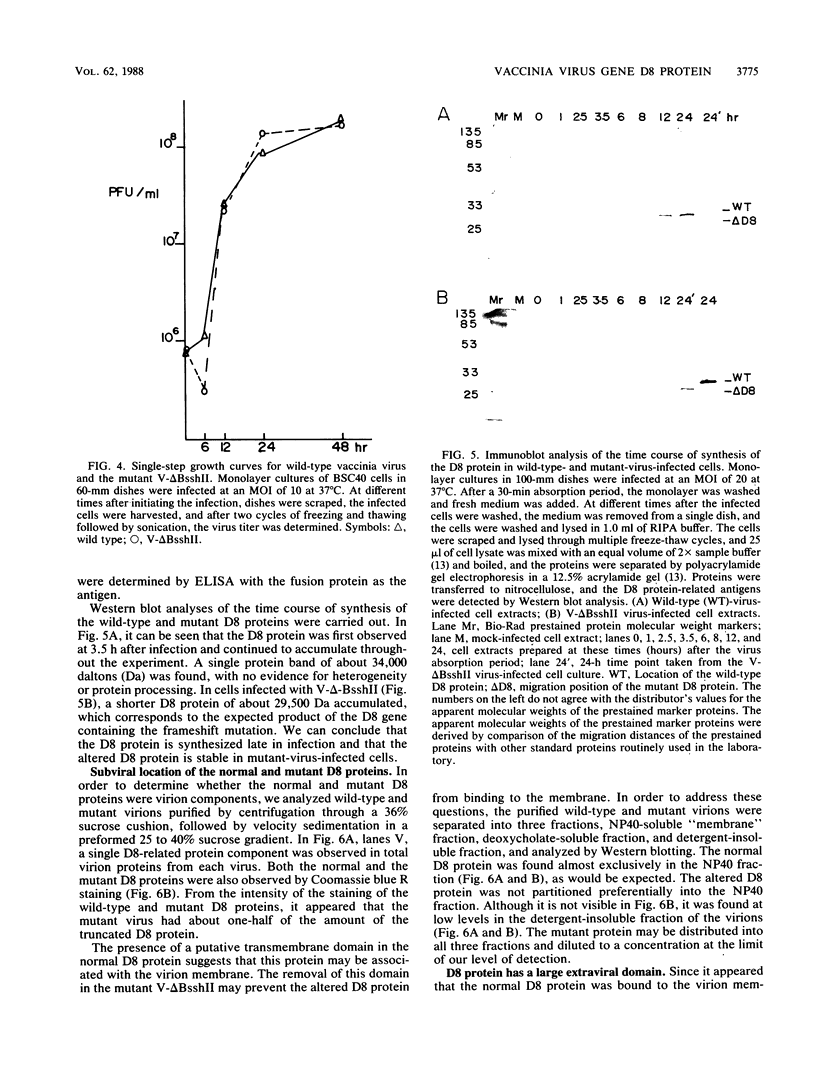
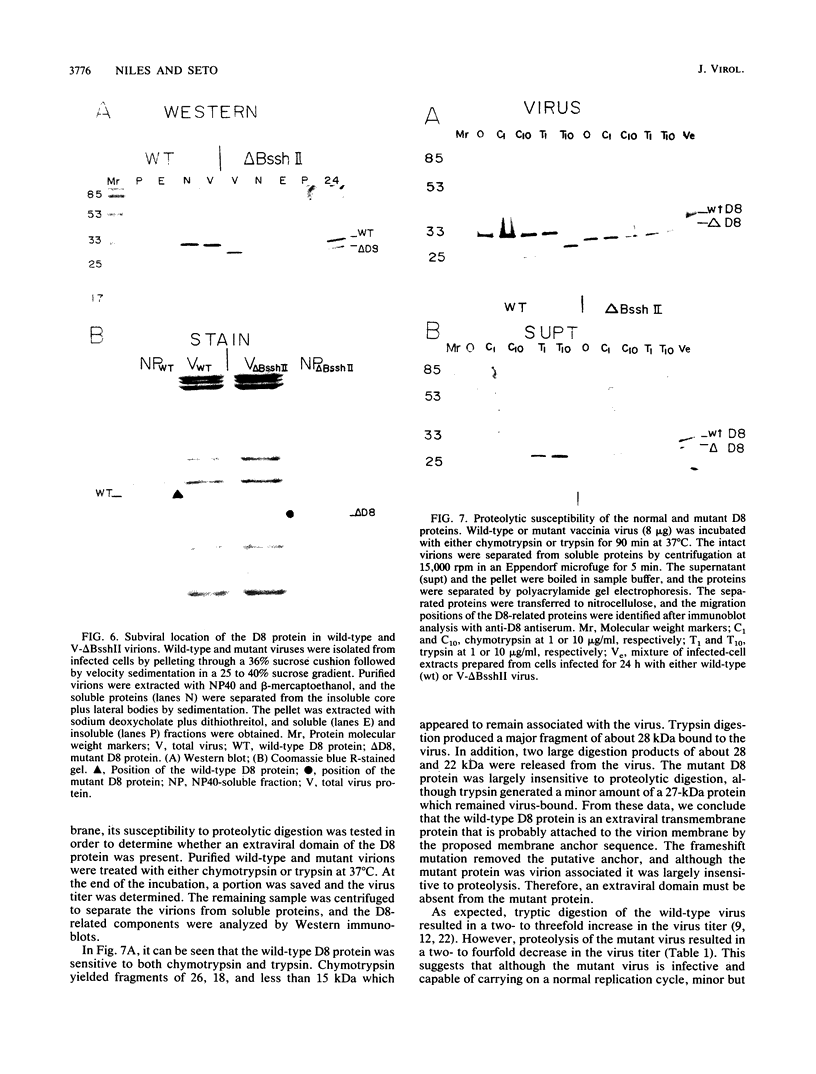
Transcription mapping studies and DNA sequence analysis of the vaccinia virus HindIII D fragment predict that gene D8 encodes a protein 304 amino acids in length, with a molecular mass of 35,426 daltons, that is expressed at late times in infection. In order to determine whether the native D8 protein is required for virus propagation, we constructed a frameshift mutation in the D8 coding sequence. Virus containing this mutation were isolated and shown to replicate in a single-step growth experiment with wild type virus growth kinetics, demonstrating that the normal-length D8 protein is not essential for virus propagation in tissue culture. In order to investigate the synthesis of the wild-type and the mutant D8 proteins in virus-infected cells, we raised polyclonal antisera to a fusion protein consisting of a portion of the D8 coding sequence linked to the Escherichia coli trpE gene. Western blot (immunoblot) analysis of the time course of D8 protein synthesis in cells infected with either wild-type or mutant virus demonstrated that D8 protein was synthesized late in infection in each case and accumulated throughout the experiment. To determine whether the D8 protein was incorporated into the mutant or wild-type virus, purified virions were fractionated into Nonidet P-40-soluble, deoxycholate-soluble, and detergent-insoluble fractions. In both the wild-type and the mutant viruses, the D8 protein was an integral viral protein. The wild-type protein partitioned into the Nonidet P-40-soluble fraction, suggesting that it was a viral membrane protein. The mutant protein fractionated into the detergent-insoluble component, demonstrating that although the altered protein was incorporated into the virus, it was found in a abnormal location. In order to determine whether the D8 protein was present on the virion surface, the susceptibility of the D8 protein to proteolysis was tested by analyzing the products of incubation of the wild-type and mutant viruses with either chymotrypsin or trypsin. These studies demonstrated that the wild-type D8 protein was a transmembrane protein with a major extraviral domain that was released largely intact from the virus by trypsin. The mutant D8 protein was relatively refractory to proteolysis, confirming the hypothesis that although it is associated with the virus, it is in a conformation different from that of the wild-type protein. Tryptic digestion of the wild-type virus increased plaque formation severalfold, concomitant with the removal of the extraviral domain of the D8 protein.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard G., Hapel A. J., Boulter E. A. An antigenic difference between intracellular and extracellular rabbitpox virus. J Gen Virol. 1971 Oct;13(1):9–17. doi: 10.1099/0022-1317-13-1-9. [DOI] [PubMed] [Google Scholar]
- Baroudy B. M., Moss B. Purification and characterization of a DNA-dependent RNA polymerase from vaccinia virions. J Biol Chem. 1980 May 10;255(9):4372–4380. [PubMed] [Google Scholar]
- Condit R. C., Motyczka A. Isolation and preliminary characterization of temperature-sensitive mutants of vaccinia virus. Virology. 1981 Aug;113(1):224–241. doi: 10.1016/0042-6822(81)90150-1. [DOI] [PubMed] [Google Scholar]
- Dales S., Pogo B. G. Biology of poxviruses. Virol Monogr. 1981;18:1–109. doi: 10.1007/978-3-7091-8625-1. [DOI] [PubMed] [Google Scholar]
- Esposito J., Condit R., Obijeski J. The preparation of orthopoxvirus DNA. J Virol Methods. 1981 Feb;2(3):175–179. doi: 10.1016/0166-0934(81)90036-7. [DOI] [PubMed] [Google Scholar]
- Essani K., Dales S. Biogenesis of vaccinia: evidence for more than 100 polypeptides in the virion. Virology. 1979 Jun;95(2):385–394. doi: 10.1016/0042-6822(79)90493-8. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Matsumoto S., Dales S. Biogenesis of poxviruses: role of A-type inclusions and host cell membranes in virus dissemination. Virology. 1971 Dec;46(3):507–532. doi: 10.1016/0042-6822(71)90056-0. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y., Tsuruhara T., Oie M. The effect of proteolytic enzymes on the infectivity of vaccinia virus. Virology. 1982 Oct 30;122(2):279–289. doi: 10.1016/0042-6822(82)90227-6. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y. Unit Complex of vaccinia polypeptides linked by disulfide bridges. Virology. 1981 Aug;113(1):277–284. doi: 10.1016/0042-6822(81)90154-9. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Kim K. S., Dalton D., Sharp D. G. Effect of some proteolytic enzymes on the plaque titer of vaccinia and rabbitpox viruses. Virology. 1969 Aug;38(4):503–511. doi: 10.1016/0042-6822(69)90170-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee-Chen G. J., Bourgeois N., Davidson K., Condit R. C., Niles E. G. Structure of the transcription initiation and termination sequences of seven early genes in the vaccinia virus HindIII D fragment. Virology. 1988 Mar;163(1):64–79. doi: 10.1016/0042-6822(88)90234-6. [DOI] [PubMed] [Google Scholar]
- Lee-Chen G. J., Niles E. G. Map positions of the 5' ends of eight mRNAs synthesized from the late genes in the vaccinia virus HindIII D fragment. Virology. 1988 Mar;163(1):80–92. doi: 10.1016/0042-6822(88)90235-8. [DOI] [PubMed] [Google Scholar]
- Lee-Chen G. J., Niles E. G. Transcription and translation mapping of the 13 genes in the vaccinia virus HindIII D fragment. Virology. 1988 Mar;163(1):52–63. doi: 10.1016/0042-6822(88)90233-4. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Niles E. G., Condit R. C., Caro P., Davidson K., Matusick L., Seto J. Nucleotide sequence and genetic map of the 16-kb vaccinia virus HindIII D fragment. Virology. 1986 Aug;153(1):96–112. doi: 10.1016/0042-6822(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Oie M., Ichihashi Y. Characterization of vaccinia polypeptides. Virology. 1981 Aug;113(1):263–276. doi: 10.1016/0042-6822(81)90153-7. [DOI] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Adsorption and penetration of enveloped and naked vaccinia virus particles. J Virol. 1978 Jul;27(1):19–27. doi: 10.1128/jvi.27.1.19-27.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G., Norrby E. Presence of haemagglutinin in the envelope of extracellular vaccinia virus particles. J Gen Virol. 1976 Jul;32(1):63–72. doi: 10.1099/0022-1317-32-1-63. [DOI] [PubMed] [Google Scholar]
- Payne L. Polypeptide composition of extracellular enveloped vaccinia virus. J Virol. 1978 Jul;27(1):28–37. doi: 10.1128/jvi.27.1.28-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov I., Joklik W. K. Studies on the nature and location of the capsid polypeptides of vaccinia virions. Virology. 1972 Nov;50(2):579–592. doi: 10.1016/0042-6822(72)90409-6. [DOI] [PubMed] [Google Scholar]
- Seto J., Celenza L. M., Condit R. C., Niles E. G. Genetic map of the vaccinia virus HindIII D Fragment. Virology. 1987 Sep;160(1):110–119. doi: 10.1016/0042-6822(87)90051-1. [DOI] [PubMed] [Google Scholar]
- Thompson C. L., Condit R. C. Marker rescue mapping of vaccinia virus temperature-sensitive mutants using overlapping cosmid clones representing the entire virus genome. Virology. 1986 Apr 15;150(1):10–20. doi: 10.1016/0042-6822(86)90261-8. [DOI] [PubMed] [Google Scholar]