Abstract
Association of the Golgi-specific adaptor protein complex 1 (AP-1) with the membrane is a prerequisite for clathrin coat assembly on the trans-Golgi network (TGN). The AP-1 adaptor is efficiently recruited from cytosol onto the TGN by myristoylated ADP-ribosylation factor 1 (ARF1) in the presence of the poorly hydrolyzable GTP analog guanosine 5′-O-(3-thiotriphosphate) (GTPγS). Substituting GTP for GTPγS, however, results in only poor AP-1 binding. Here we show that both AP-1 and clathrin can be recruited efficiently onto the TGN in the presence of GTP when cytosol is supplemented with ARF1. Optimal recruitment occurs at 4 μM ARF1 and with 1 mM GTP. The AP-1 recruited by ARF1·GTP is released from the Golgi membrane by treatment with 1 M Tris-HCl (pH 7) or upon reincubation at 37°C, whereas AP-1 recruited with GTPγS or by a constitutively active point mutant, ARF1(Q71L), remains membrane bound after either treatment. An incubation performed with added ARF1, GTP, and AlFn, used to block ARF GTPase-activating protein activity, results in membrane-associated AP-1, which is largely insensitive to Tris extraction. Thus, ARF1·GTP hydrolysis results in lower-affinity binding of AP-1 to the TGN. Using two-stage assays in which ARF1·GTP first primes the Golgi membrane at 37°C, followed by AP-1 binding on ice, we find that the high-affinity nucleating sites generated in the priming stage are rapidly lost. In addition, the AP-1 bound to primed Golgi membranes during a second-stage incubation on ice is fully sensitive to Tris extraction, indicating that the priming stage has passed the ARF1·GTP hydrolysis point. Thus, hydrolysis of ARF1·GTP at the priming sites can occur even before AP-1 binding. Our finding that purified clathrin-coated vesicles contain little ARF1 supports the concept that ARF1 functions in the coat assembly process rather than during the vesicle-uncoating step. We conclude that ARF1 is a limiting factor in the GTP-stimulated recruitment of AP-1 in vitro and that it appears to function in a stoichiometric manner to generate high-affinity AP-1 binding sites that have a relatively short half-life.
INTRODUCTION
Movement of proteins between membrane-bound intracellular compartments is typically mediated by small transport vesicles, which bud from a donor compartment and then fuse with an appropriate acceptor compartment. The budding process appears to be facilitated by coat proteins that assemble at specific bud sites on the donor membrane. The coat is thought to perform two interrelated functions: the physical deformation of the underlying membrane into a deeply invaginated bud and, concurrently, the preferential sorting of cargo molecules for inclusion within the bud. At present, three kinds of well-defined transport vesicles have been described, the coat protein complex I (COPI)-, COPII-, and clathrin-coated vesicles (Robinson, 1994; Rothman, 1994; Schekman and Orci, 1996). COPI-1 and COPII-coated vesicles are involved in membrane traffic between the endoplasmic reticulum and the Golgi complex (Barlowe et al., 1994; Aridor et al., 1995; Bednarek et al., 1995; Gaynor and Emr, 1997), and COPI-coated vesicles also appear to participate in anterograde transport through Golgi cisternae (Orci et al., 1997).
Clathrin-containing coats assemble at two principal intracellular sites, the trans-Golgi network (TGN) and the plasma membrane (Robinson, 1994; Brodsky, 1997; Kirchhausen et al., 1997; Schmid, 1997). The coats that assemble on the TGN are involved in the sorting of newly synthesized lysosomal enzymes and lysosomal membrane proteins away from other proteins destined for the cell surface or for secretion (Kornfeld and Mellman, 1989). Clathrin coats that form on the plasma membrane play a direct role in receptor-mediated endocytosis (Davis et al., 1986; Jadot et al., 1992; Kirchhausen et al., 1997). The two clathrin-coated vesicle populations are both assembled from a single pool of cytosolic clathrin trimers, but each can be distinguished by their respective adaptor proteins. The adaptors are structurally related heterotetrameric complexes with one, termed clathrin-associated adaptor protein complex 1 (AP-1), largely restricted to the TGN-derived population, whereas the second complex, AP-2, is associated preferentially with the plasma membrane–derived vesicles (Robinson, 1987; Ahle et al., 1988).
How this remarkable spatial separation of AP-1 and AP-2 is achieved within the cell is not completely understood. Both adaptor complexes can bind to cytosol-oriented, tyrosine-based trafficking signals via the μ subunit of the heterotetramer (Ohno et al., 1995; Ohno et al., 1996). Transmembrane proteins, like the transferrin and low-density lipoprotein receptors, which cycle repetitively between the cell surface and endosomes, are efficiently internalized in AP-2–containing clathrin-coated vesicles. Endocytosis of these proteins depends largely on the trafficking signal, as mutation or deletion of the signal traps the molecule at the surface (Davis et al., 1986; Jadot et al., 1992). Why these proteins do not bind to AP-2 as they traverse the biosynthetic pathway is not clear. In fact, some proteins, like the transferrin receptor, are found predominantly within the endosomal compartment at steady state, but this pool of molecules does not appear to interact with either AP-2 or AP-1. This and the relatively low affinity of the interaction between tyrosine-based trafficking signals and μ subunits (Rapoport et al., 1997) make unregulated interaction of adaptors with these signals seem unlikely.
One way in which adaptors could be targeted to a particular membrane site with good precision is if the initial recruitment event is not governed by the trafficking signal directly. There is already evidence that this is how AP-1 is specifically targeted to the TGN (Robinson and Kreis, 1992). The relocation of AP-1 from the cytosol onto the Golgi is preceded by the recruitment of ADP-ribosylation factor (ARF), a member of the Ras-related small GTP-binding protein superfamily. The involvement of ARF was initially suggested by the observation that the fungal metabolite brefeldin A (BFA) alters the intracellular distribution of AP-1 in vivo (Robinson and Kreis, 1992; Wong and Brodsky, 1992). Direct involvement of ARF was verified in subsequent studies showing that in the presence of guanosine 5′-O-(3-thiotriphosphate) (GTPγS), a poorly hydrolyzable analog of GTP, recombinant myristoylated ARF1 promotes efficient AP-1 binding to the TGN in vitro (Stamnes and Rothman, 1993; Traub et al., 1993). The involvement of a GTP-binding protein in clathrin-coat assembly at the TGN indicates that AP-1 does not simply bind to a trafficking signal to initiate bud formation.
Small GTP-binding proteins similarly regulate COP- coat assembly, with ARF1 and Sar1p being required for COPI- and COPII-vesicle formation, respectively. Both COPI- and COPII-coated vesicle formation have been reconstituted in vitro using defined cytosolic components and, in both instances, coated vesicles can be recovered after coat assembly reactions in the presence of either GTPγS or guanylylimidodiphosphate (GMPPNP) (Serafini et al., 1991; Tanigawa et al., 1993; Barlowe et al., 1994; Bednarek et al., 1995). There have been no reports of budding of clathrin-coated vesicles from the TGN in the presence of GTPγS, however. Several factors might underlie this failure to generate clathrin-coated vesicles in the presence of GTPγS. An additional GTP-binding protein, dynamin, is necessary for the fission of AP-2-containing clathrin-coated buds from the plasma membrane (Takei et al., 1996; Schmid, 1997; Urrutia et al., 1997). A temperature-sensitive mutation in dynamin causes a block of endocytosis at the restrictive temperature. Internalization is arrested at a late stage, after the initiation of clathrin coat assembly but preceding the fission of deeply invaginated coated pits (Damke et al., 1994; Takei et al., 1996). In the presence of GTPγS, dynamin forms extensive, polymeric ring-like structures that extend abnormally between clathrin-coated buds and the underlying plasma membrane (Takei et al., 1995). Dynamin appears to be recruited into the coated bud by amphiphysin. These proteins associate directly through the interaction of an Src homology 3 (SH3) domain in amphiphysin with a proline-rich region in the carboxyl-terminal region of dynamin I. Coated vesicle fission can be arrested at the deeply invaginated stage by the addition of either the isolated SH3 region of amphiphysin or a peptide encompassing the proline-rich SH3 binding site of dynamin (Shupliakov et al., 1997; Wigge et al., 1997). Budding is obstructed because these treatments prevent the relocation of dynamin onto the coated bud. Together, these studies reveal that dynamin plays a role in the terminal stages of clathrin-coat budding and indicate that dynamin·GTPγS might arrest the fission process (Hinshaw and Schmid, 1995; Takei et al., 1995). As dynamin or dynamin-related proteins are also found on Golgi membranes (Henley and McNiven, 1996), GTP hydrolysis may then also be required for the fission of clathrin-coated vesicles from the TGN. Consequently, in vitro assays performed in the presence of GTPγS might enable AP-1 and clathrin to assemble on the TGN but halt the fission of the bud.
Defining conditions that allow for GTP-driven, AP-1-dependent clathrin coat assembly should overcome many of the limitations inherent in the use of GTPγS. In this study, we provide evidence that soluble ARF1 is a limiting factor when AP-1 and clathrin recruitment onto the TGN is examined in standard in vitro binding assays together with GTP. We describe conditions to achieve efficient and reproducible coat assembly in the presence of GTP and then use this system to further dissect the initial steps in the formation of clathrin-coated vesicles on the TGN, revealing features not previously seen with GTPγS.
MATERIALS AND METHODS
Materials
GTPγS was purchased from Boehringer Mannheim (Indianapolis, IN). GTP, BFA, and protease inhibitors were obtained from Sigma (St. Louis, MO). Male Sprague Dawley rats were obtained from Charles River (Boston, MA). Nitrocellulose membrane was from Schleicher & Scheull (Keene, NH); siliconized microfuge tubes were obtained from Midwest Scientific (St. Louis, MO); and the reagents for ECL detection were from Amersham (Arlington Heights, IL). Recombinant myristoylated bovine ARF1, with amino acids 3–7 replaced with the corresponding residues of yeast ARF2 (Liang et al., 1997) to facilitate myristoylation, was expressed in Escherichia coli cotransfected with the yeast N-myristoyltransferase plasmid pBB131 (Duronio et al., 1990). The protein was purified by sequential diethylaminoethyl and Sephadex G-75 chromatography (Weiss et al., 1989).
Antibodies
The preparation of the affinity-purified antibodies AE/1, which recognizes the γ subunit of the AP-1 complex, GD/1, directed against the adaptor β subunits, and RY/1, directed against the carboxyl-terminal sequence of the μ1 subunit of AP-1, has been described previously (Traub et al., 1995). Affinity-purified DE/1 was prepared similarly and is directed against the 14 carboxyl-terminal residues of the ς1 sequence (DESPRSVLEEMGLA) of AP-1. The anti-clathrin heavy-chain mAb TD.1 (Nathke et al., 1992) was kindly provided by Frances Brodsky (University of California, San Francisco, CA). mAb 100/3, directed against the γ subunit of AP-1, was a generous gift of Ernst Ungewickell (Washington University, St. Louis, MO) and was used for the detection of bovine AP-1 or for affinity purification of bovine AP-1 from cytosol. A monoclonal antibody, mAb 2F7.1, which is specific for rat TGN38, was kindly provided by George Banting (University of Bristol, Bristol, United Kingdom), and mAb 53FC3, which recognizes rat α-mannosidase II, was purchased from BAbCO (Richmond, CA). The anti-ARF monoclonal 1D9 was a kind gift from Rick Kahn (University of Georgia, Atlanta, GA). Rabbit antiserum raised against ARF1 GAP was kindly provided by Dan Cassel (Technion, Haifa, Israel). Horseradish peroxidase-conjugated anti-rabbit and anti-mouse Ig antibodies were purchased from Amersham.
Preparation of Golgi Membranes, Cytosol, and Clathrin-coated Vesicles
Preparations enriched in Golgi membranes, referred to here as “Golgi membranes,” were prepared from fresh rat liver as described (Tabas and Kornfeld, 1979), except that 5 mM EDTA replaced the magnesium in the buffers. Fresh rat liver or bovine adrenal cytosol was prepared (Traub et al., 1993, 1995), and before use in binding assays, the cytosol was desalted over a PD-10 column (Pharmacia, Piscataway, NJ) equilibrated in assay buffer (25 mM HEPES-KOH, pH 7.0, 125 mM potassium acetate, 2.5 mM magnesium acetate, 1 mM DTT) and then centrifuged at 245,000 × gmax for 20 min at 4°C in a Beckman Instruments (Palo Alto, CA) TLA-100.3 rotor. The protein concentrations of Golgi membrane and cytosol preparations were determined using the Bradford assay (Bio-Rad, Hercules, CA) with BSA as a standard. Clathrin-coated vesicles were isolated from fresh rat liver (Campbell et al., 1984) and purified further by centrifugation on discontinuous sucrose gradients (Kedersha and Rome, 1986) to remove the contaminating vaults. A crude coat protein fraction was prepared from the purified coated vesicles by extraction with 1.0 M Tris-HCl (pH 7.0), and AP-1 was subsequently purified from this fraction by sequential chromatography over Superose 6 and hydroxylapatite columns (Ahle et al., 1988).
Affinity Purification of Bovine Adrenal AP-1 Adaptors
A 10-ml aliquot of bovine adrenal cytosol was centrifuged at 100,000 × gmax for 1 h at 4°C to remove insoluble material. The supernatant was mixed with 1.5 mg of anti-γ subunit antibody mAb 100/3 coupled to cyanogen bromide–activated Sepharose-4B beads (1.0 ml) and tumbled for several hours at 4°C. The mixture was then loaded into a column, and the beads were washed with 20 ml of assay buffer without DTT. Cytosolic AP-1 was then eluted by the addition of 1 ml of the epitope peptide dissolved in assay buffer without DTT to give a ∼50-fold molar excess of peptide over immobilized antibody. After 10 min at 37°C elution was repeated with a 25-fold molar excess of peptide in 1 ml and finally with an equimolar solution of peptide in 1 ml. The three elutions were combined and dialyzed overnight against 1 l of assay buffer without sucrose at 4°C, and then the purified AP-1 was clarified by centrifugation at 245,000 × gmax for 20 min at 4°C. A total of ∼40 μg of pure AP-1 can be isolated from 10 ml bovine adrenal cytosol using this procedure.
Golgi Membrane Binding Assay
Typical coat recruitment assays were performed in a final volume of 200 μl in 1.5-ml siliconized microfuge tubes in assay buffer supplemented with 250 mM sucrose. Gel-filtered cytosol, Golgi membranes, ARF1, nucleotides, and BFA were added to the concentrations noted in the figure legends. All additions were done on ice. The reaction mixtures were then incubated at 37°C for 15 min, followed by rapid cooling on ice. Two volumes of ice-cold assay buffer without sucrose were added to each tube, and then the membranes were collected by centrifugation at 16,000 × gmax for 15 min at 4°C. The supernatants were aspirated and discarded; the tubes were recentrifuged at 16,000 × gmax for 2 min, and any residual supernatant was removed. The Golgi membrane pellets were dissolved by boiling in 20 μl of 1× SDS sample buffer for 5 min and then fractionated by discontinuous SDS-PAGE as described (Traub et al., 1993, 1995). After transfer onto nitrocellulose and incubation with the indicated antibodies, labeled bands were visualized by ECL.
For the Tris-HCl extraction experiments, Golgi membrane pellets were resuspended in 20 μl of assay buffer using a pipette tip. Then 80 μl of either assay buffer as control or 1.25 M Tris-HCl (pH 7.0) were added, mixed, and incubated on ice for 10 min. The Golgi membranes were recovered by centrifugation and the supernatants precipitated with chloroform-methanol after addition of 5 μg of BSA as a carrier (Wessel and Flugge, 1984). Equivalent volumes of the supernatant and pellet fractions were then analyzed on immunoblots. To assess the stability of membrane-bound clathrin and AP-1 to reincubation at 37°C, 1.2-ml binding reactions were performed, and then the Golgi membranes were recovered, without dilution, by centrifugation at 45,000 × gmax for 15 min at 4°C in the TLA-100.3 rotor. Each pellet was resuspended in 0.6 ml of assay buffer containing 0.25 M sucrose and 100-μl aliquots mixed with 100 μl of either assay buffer or 10 mg/ml gel-filtered rat liver cytosol and then incubated at 37°C for various times as noted in the figure legends. At each time point, the tubes were rapidly cooled on ice and diluted with 200 μl of assay buffer without sucrose. After completion of the experiment, the membranes were recovered by centrifugation and then analyzed as described above.
For two-stage AP-1- and clathrin-binding assays, the Golgi membranes in assay buffer with 250 mM sucrose were primed in the first stage by incubation with 4 μM ARF1 and either 1 mM GTP or 100 μM GTPγS at 37°C for 15 min. The reactions were cooled on ice and diluted with 1 vol of ice-cold assay buffer lacking sucrose, and the membranes were recovered by centrifugation as described above. After resuspension in assay buffer, the primed membranes were mixed with buffer, gel-filtered cytosol, or purified AP-1 and incubated on ice for 15 min for the second stage. No nucleotides were present during the second-stage reaction. The membranes were then recovered by centrifugation for immunoblot analysis.
AP-1 Recruitment in Semi-intact Cells
Normal rat kidney cells, grown on glass coverslips, were permeabilized with 25 μg/ml digitonin as described previously (Traub et al., 1996). After incubation on ice for 10 min in 25 mM HEPES-KOH (pH 7.2), 125 mM potassium acetate, 5 mM magnesium acetate, 1 mM DTT and 1 mg/ml d-glucose to deplete cytosol, 5 mg/ml gel-filtered rat liver cytosol, 4 μM recombinant ARF1, 1 mM GTP, and 100 μM GTPγS were added as indicated in the figure legends. After gentle mixing, the coverslips were incubated in a 37°C water bath for 20 min, returned to ice, washed twice, and then fixed for immunofluorescence analysis. For analysis with the anti-α-mannosidase II mAb, cells were fixed in 3.7% formaldehyde while analysis with the anti-TGN38 mAb was done on cells fixed with methanol at −20°C. For staining with the anti-γ subunit AE/1 antibody, the cells were fixed in Bouin’s fixative (Traub et al., 1996).
RESULTS
ARF1-dependent Recruitment of AP-1 and Clathrin onto Golgi Membranes
Although myristoylated ARF is absolutely required for AP-1 recruitment onto the TGN, in vitro, the extent of adaptor recruitment depends on the nucleotide used. Approximately 5- to 10-fold more AP-1 is recruited from cytosol onto membranes in the presence of GTPγS than with GTP (Figure 1, lane 7 compared with lane 3) (Le Borgne et al., 1993; Stamnes and Rothman 1993; Traub et al., 1993). As ARF functions in the GTP-bound state, the poorly hydrolyzable GTPγS prevents physiological inactivation of the protein by nucleotide hydrolysis. With nucleotide exchange in the presence of GTP, however, the ARF1·GTP formed can be deactivated rapidly by nucleotide hydrolysis promoted by an ARF1-specific GTPase-activating protein (GAP) (Helms et al., 1993; Tanigawa et al., 1993; Finazzi et al., 1994; Makler et al., 1995; Randazzo, 1997). Because ARF1 GAP activity is abundant in rat liver Golgi membranes and cytosol (Makler et al., 1995; Randazzo, 1997), relatively little ARF1·GTP is expected to accumulate on the membranes at steady state. Furthermore, the cytosol concentration we routinely use (5 mg/ml) is at least 10-fold lower than the intracellular cytosol concentration and might be unable to supply sufficient ARF1 to the membrane to drive AP-1 recruitment in the presence of GTP.
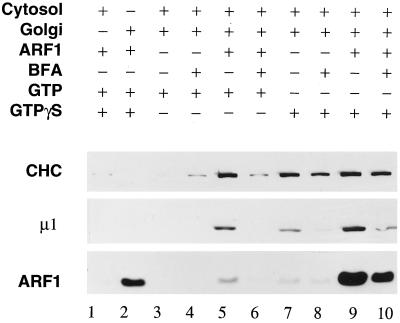
Figure 1.
Effects of exogenous ARF1 on AP-1 and clathrin recruitment. Binding assays containing 50 μg/ml Golgi membranes, 5 mg/ml gel-filtered rat liver cytosol, 4 μM ARF1, 100 μg/ml BFA, 1 mM GTP, and 100 μM GTPγS were prepared as indicated. After incubation at 37°C for 15 min, the Golgi membranes were collected by centrifugation, resolved on 12% polyacrylamide gels, and transferred onto a nitrocellulose membrane. Portions of the blot were probed with the anti-clathrin heavy-chain (CHC) mAb TD.1, the anti-AP-1 μ1 subunit (μ1) antibody RY/1, or the anti-ARF (ARF1) mAb 1D9. Lanes 1 and 2 are the cytosol and Golgi controls; lanes 3–6 are GTP-containing samples; and lanes 7–10 are GTPγS-containing samples.
Indeed, we have found that by supplementing cytosol with recombinant myristoylated ARF1, efficient clathrin-coat assembly can be obtained in the presence of GTP (Figure 1, lane 5). With ARF1 added, GTP can promote both AP-1 and clathrin recruitment as efficiently as GTPγS does in unsupplemented cytosol (Figure 1, lane 7). Addition of exogenous ARF also increases the extent of coat recruitment in the presence of GTPγS (Figure 1, lane 9) but not in proportion with the amount of ARF that binds to the membrane. Under all conditions, coat assembly is inhibited by pretreating the Golgi with brefeldin A, which specifically inhibits ARF1 GTP exchange (Donaldson et al., 1992; Helms and Rothman 1992) (Figure 1, lanes 4, 6, 8 and 10). These results suggest that on dilution of liver cytosol, ARF1 becomes a limiting factor for coat assembly. The effect of ARF1 concentration on AP-1 recruitment is shown in Figure 2A. When assayed with 5 mg/ml rat liver cytosol and 1 mM GTP, a minimum of ∼0.5 μM ARF1 is required to observe GTP-stimulated AP-1 binding, and the effect saturates at a concentration of ∼4 μM. Compared with GTPγS, which promotes maximal AP-1 recruitment at a concentration of ∼10 μM, much higher concentrations of GTP are required to saturate AP-1 binding in the presence of 4 μM ARF1 (Figure 2B), although these values may be overestimated because they are not corrected for any GTP hydrolysis that may have occurred during the course of the incubation. This confirms that the turnover of ARF1·GTP on the Golgi membrane must be rapid (Donaldson et al., 1992; Helms and Rothman, 1992; Helms et al., 1993; Tanigawa et al., 1993; Finazzi et al., 1994; Teal et al., 1994). Hydrolysis is also implied by the low amount of ARF1 that is found on the Golgi membranes in the presence of GTP (Figure 1, lane 5) compared with the substantial amount of ARF1 that translocates onto the Golgi membranes with GTPγS (lane 9). Despite this difference, similar amounts of AP-1 and clathrin are recruited in both instances. This is consistent with the finding (Helms et al., 1993) that two pools of ARF1 associate with Golgi membranes: one loosely associated pool easily extracted by lipid vesicles and another pool more stably bound, and suggests that only the pool of ARF1·GTP that associates with a saturable docking site promotes AP-1 binding (Helms et al., 1993; Traub et al., 1993).
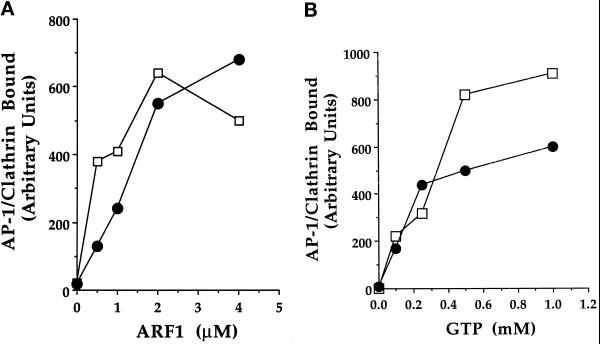
Figure 2.
Effect of ARF1 and GTP concentration on AP-1 and clathrin recruitment. (A) Binding assays containing 50 μg/ml Golgi membranes, 5 mg/ml gel-filtered rat liver cytosol, 1 mM GTP, and increasing concentrations of ARF1 were prepared and analyzed as outlined in Figure 1. The amount of AP-1 adaptors (•), measured by the presence of the μ1 subunit, and of clathrin (□), measured by the presence of the clathrin heavy chain, were determined by densitometry. For each ARF1 concentration a parallel assay containing 100 μg/ml BFA was performed, and the resultant AP-1 and clathrin signals were subtracted from the corresponding sample incubated without the BFA. (B) Binding assays were performed as in A, except that increasing concentrations of GTP were added to incubations containing 4 μM ARF1 (□, clathrin heavy chain; •, μ1 subunit of AP-1). The data shown are representative of several similar experiments.
Immunofluorescent Localization of ARF1·GTP-stimulated AP-1 Recruitment to the TGN
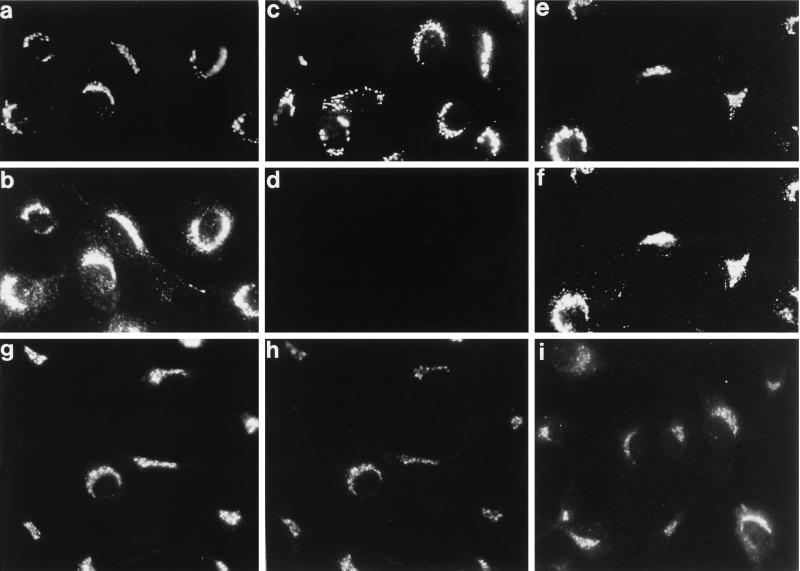
It was important to verify that AP-1 is appropriately recruited onto the TGN in the presence of GTP as different nucleotide conditions have been shown to affect the fidelity of in vitro recruitment of the AP-2 complex onto intracellular membranes (Seaman et al., 1993; Traub et al., 1996; West et al., 1997). Adaptor and clathrin recruitment can be followed morphologically in permeabilized cells (Robinson and Kreis, 1992; Wong and Brodsky, 1992; Traub et al., 1996) where, after incubation with gel-filtered cytosol and GTPγS, AP-1 localizes primarily to a perinuclear compartment (Figure 3b). Double labeling with antibodies directed against the medial Golgi marker α-mannosidase II and the β subunit of the AP-1 adaptor complex shows good colocalization (Figure 3, a and b). The additional punctate AP-1 staining in the cell periphery most probably represents AP-1 recruited onto early endosomes (Le Borgne et al., 1996). Simply substituting GTP for GTPγS markedly diminishes the AP-1 translocation into the perinuclear region (Figure 3d) unless ARF1 is added (Figure 3f). A more precise localization of AP-1, recruited in the presence of ARF·GTP, to the TGN is seen after double labeling with antibodies to TGN38 (Figure 3g) and the β subunit of AP-1 (Figure 3h). The pattern of staining of the γ subunit of AP-1 shows a similar cellular localization (Figure 3i). These experiments confirm that AP-1 is targeted correctly when 4 μM ARF1 and GTP are used to drive coat assembly.
Figure 3.
Recruitment of AP-1 onto the TGN with ARF·GTP. Digitonin-permeabilized normal rat kidney cells were mixed with 5 mg/ml gel-filtered rat liver cytosol and either 100 μM GTPγS (a and b) or 1 mM GTP (c and d) or 1 mM GTP and 4 μM ARF1 (e–i) and then incubated at 37°C for 20 min. After washing and fixation, the cells were analyzed with the anti-α-mannosidase II mAb 53FC3 (a, c, and e), affinity-purified anti-adaptor β subunit antibody GD/1 (b, d, f, and h), the anti-TGN38 mAb 2F7.1 (g), or affinity-purified anti-AP-1 γ subunit antibody AE/1 (i).
Sensitivity of ARF1·GTP- and ARF1·GTPγS-recruited AP-1 to Tris Extraction
Both clathrin and the adaptors can be stripped off purified clathrin-coated vesicles chemically using high concentrations of Tris-HCl (pH 7.0) on ice (Keen et al., 1979). Subjecting clathrin-coat containing Golgi membranes to this Tris-extraction procedure reveals that recruited clathrin similarly detaches from the membrane (Figure 4A, lanes 3, 7, and 11) and is recovered in the supernatant fraction (Figure 4A, lanes 4, 8, and 12). Bound clathrin is quantitatively released from the membrane irrespective of the nucleotide used to promote coat assembly (Figure 4A, lanes 3 and 4, GTP, and 11 and 12, GTPγS), consistent with the previous results obtained with isolated clathrin-coated vesicles.
Figure 4.
AP-1 and clathrin stability to Tris. (A) Duplicate binding assays using ARF·GTP (lanes 1–4), ARF1(Q71L)·GTP (lanes 5–8), or ARF1·GTPγS (lanes 9–12) to promote clathrin coat assembly were performed. One of the two membrane pellets was resuspended in 100 μl of the assay buffer, and the other was resuspended in 100 μl of 1 M Tris-HCl (pH 7.0). After incubation on ice for 10 min, the membranes were again collected, and the supernatants were individually aspirated and then precipitated with methanol/chloroform. The supernatant and pellet samples were resuspended in identical volumes of sample buffer and then analyzed by immunoblotting using the anti-clathrin heavy-chain (CHC) antibody and the anti-AP-1 μ1 subunit (μ1) antibody. AP and AS indicate the pellet and supernatant fractions, respectively, after reincubation with assay buffer; TP and TS denote the pellet and supernatant fractions, respectively, after reincubation with 1 M Tris-HCl (pH 7.0). (B) Quantitation of the μ1 subunit recovery in the pellet fraction after incubation in either acetate assay buffer (AP) or 1 M Tris-HCl, pH 7.0 (TP). The data are expressed as a ratio of the μ1 subunit immunoreactivity found in the Tris pellet compared with that found in the acetate pellet and are the mean ± SEM of four independent experiments. (C) Lanes 1–4 represent standard ARF1·GTP recruitment assays that were stripped with assay buffer or with 1 M Tris-HCl (pH 7.0), and lanes 5–8 represent assays treated similarly after recruitment with 50 μM AlCl3 and 30 mM NaF also present. Bkg represents the background amount of AP-1 found in the pellet after incubation in the absence of nucleotide.
Analysis of the distribution of the recruited AP-1 adaptor after the Tris treatment yields different results. Although the majority of the AP-1 recruited by ARF1·GTP is released into the Tris supernatant (Figure 4A, lanes 3 and 4), the AP-1 bound in the presence of GTPγS is almost totally resistant to this treatment and remains with the membrane pellet (Figure 4A, lanes 11 and 12). The results from four separate experiments are summarized in Figure 4B. To exclude the possibility that another, unidentified GTP-binding protein(s) underlies the Tris stability of AP-1 in the presence of GTPγS, we also used an ARF1 point mutant, ARF1(Q71L), which is known to exhibit slowed hydrolysis of bound GTP (Tanigawa et al., 1993; Teal et al., 1994). AP-1 recruited with ARF1(Q71L) and GTP is also resistant to Tris extraction (Figure 4A, lane 7), although to a lesser extent than that seen with GTPγS (Figure 4A, lane 11). This phenomenon correlates very well with the known ARF1·GTP hydrolysis rates (ARF1·GTP > ARF1(Q71L or I)·GTP > ARF1·GTPγS), suggesting that GTP hydrolysis might confer sensitivity of the membrane-bound coat complex to Tris extraction. Importantly, in all cases the AP-1-clathrin complex on the Golgi membrane remains intact when the acetate-containing assay buffer is used (Figure 4A, lanes 1, 5 and 9) instead of 1.0 M Tris to extract the membrane pellets on ice.
The correlation between the resistance of AP-1 to Tris extraction and ARF·GTP hydrolysis rates suggested that it should be possible to obtain a Tris-resistant coat assembled with ARF1 and GTP by blocking ARF GAP activity during the assays. To do this, we made use of an earlier observation that AlFn appears to block ARF GAP activity in vitro (Finazzi et al., 1994). Addition of 50 μM AlCl3 and 30 mM NaF to assays containing 4 μM ARF1 and GTP switches the recruited AP-1 from being sensitive to Tris extraction (Figure 4C, lanes 3 and 4) to being predominantly Tris resistant (Figure 4C, lanes 7 and 8). Together, these results strongly suggest AP-1 adaptors associate more tightly with TGN membranes before nucleotide hydrolysis and that the Tris extraction procedure is a good discriminator of AP-1 binding in the presence of ARF·GTP or ARF·GDP.
Susceptibility of ARF1·GTP- and ARF1·GTPγS-recruited AP-1 to Temperature-induced Dissociation
We have demonstrated previously that isolated rat liver Golgi membranes contain associated AP-1 and clathrin, which, upon incubation in acetate assay buffer at 37°C, detach from the membrane (Traub et al., 1993). When Golgi membranes containing AP-1 and clathrin bound by prior incubation with ARF1 and either GTP or GTPγS are similarly reincubated in assay buffer at 37°C, clathrin dissociates from the membrane (Figure 5, lanes 1–6). Again, the nucleotide used to promote coat assembly does not affect clathrin release, and by 15 min, most of the clathrin has come off the Golgi. However, the bound AP-1, as seen above in the Tris extraction experiments, behaves differently. AP-1 recruited with ARF·GTP dissociates from the membrane upon reincubation, with kinetics slightly slower than clathrin (Figure 5, lanes 1–3), and consistent with the behavior of AP-1 found on isolated Golgi membranes (Traub et al., 1993). By contrast, the AP-1 recruited by ARF·GTPγS is very stably bound to the membrane, and, after 15 min of incubation at 37°C, adaptor loss is negligible (Figure 5, lanes 4–6). Together with the Tris extraction results, these data suggest that there is a significant difference in the nature of the interaction of AP-1 with the membrane when recruitment is performed with GTP or with GTPγS. Furthermore, they indicate that the interaction between the membrane and AP-1 recruited with ARF1·GTP is similar to the resident AP-1 found on the membranes immediately after purification.
Figure 5.
AP-1 and clathrin stability to reincubation at 37°C. Golgi membranes from 1.2-ml binding assays containing either GTP or GTPγS were pelleted, and the membranes were resuspended in 600 μl of assay buffer. Three aliquots of 100 μl of the resuspended membranes were transferred to new tubes, mixed with 100 μl of assay buffer, and then incubated for 0, 5, or 15 min at 37°C. Another set of three aliquots of 100 μl was mixed with 100 μl of 10 mg/ml rat liver cytosol and incubated similarly for 0, 5, or 15 min. After each time point, samples were cooled on ice, and the membranes were pelleted and analyzed for clathrin, AP-1, and ARF content by immunoblotting. The elevated level of clathrin found in the pellet when the reincubation step is performed with cytosol (lanes 7–12) reflects some nonspecific sedimentation of clathrin from the cytosolic pool. A similar amount of clathrin to that seen in lane 9 is found in the pellet fraction of cytosol incubated without added membranes (our unpublished observations). This explains why clathrin appears to dissociate more slowly than the AP-1 adaptor in lanes 7–9.
When the reincubation step is performed in nucleotide-free (gel-filtered) cytosol instead of the acetate buffer, the pattern of coat disassembly after binding with ARF1 and GTP remains similar (Figure 5, lanes 7–9 compared with lanes 1–3). We do note, however, that AP-1 dissociation is reproducibly faster when followed in the presence of cytosol. Interestingly, the coat assembled in the presence of GTPγS does not change discernibly on reincubation with cytosol at 37°C (lanes 10–12). This reveals the dynamic nature of the clathrin-coat assembly reaction in vitro. Evidently, during the initial stages of assembly, clathrin trimers are able to cycle onto and off the membrane as long as a pool of cytosolic clathrin is present. Only when clathrin becomes limiting, as in the reincubation with buffer alone (Figure 5, lanes 1–6), or when the AP-1 is released (Figure 5, lanes 7–9) does the clathrin coat disassemble. Visualization of this cyclical clathrin assembly requires AP-1 to be tightly bound to the membrane (Figure 5, lanes 10–12 compared with lanes 7–9), confirming that the clathrin cannot assemble without AP-1.
ARF1·GTP Hydrolysis Occurs during the Priming Step
It is also of note that the Golgi membranes recovered after incubation with ARF and GTP lack detectable ARF immunoreactivity, whereas membranes incubated with ARF and GTPγS contain abundant ARF (Figure 5). Prolonged exposure of the blot revealed the presence of trace amounts of ARF in the GTP-containing lanes, similar to that seen in Figure 1 (lane 5). This suggests that most of the bound ARF·GTP is rapidly hydrolyzed, allowing the GTP-binding protein to dissociate from the membrane during the incubation. The Tris sensitivity and the temperature-induced dissociation of membrane-bound AP-1 that has been recruited onto the TGN during incubation with ARF1- and GTP-supplemented cytosol suggest that the assembled coat structure still remains membrane-bound after deactivation of ARF·GTP by hydrolysis has taken place. To try to pinpoint when this nucleotide-hydrolysis event occurs, we made use of two-stage binding assays. After incubating the membranes with ARF1 and GTP at 37°C in the first stage, the Golgi membranes were pelleted, resuspended in gel-filtered cytosol, and then incubated on ice for the second-stage coat assembly reaction. In this type of experiment, AP-1 is recruited on ice onto the ARF·GTP-primed membranes, but the adaptor is almost completely extracted from the membranes by the Tris treatment (Figure 6, top panel). This agrees well with the results obtained in the standard one-stage assay (Figure 4). In two-stage binding assays in which affinity-purified AP-1 is used instead of cytosol as the source of adaptors for the second incubation on ice, the bound AP-1 remains fully extractable by the Tris wash (Figure 6, middle panel) if ARF·GTP-primed membranes are used. Given that no ARF GAP immunoreactivity is detectable in the purified AP-1 preparation (our unpublished observations) and that the second-stage incubation is performed on ice, we consider it unlikely that ARF GAP activity deactivates the ARF·GTP-primed binding sites during the second stage. When the same AP-1 preparation is added to membranes that were primed with ARF·GTPγS, the recruited adaptor is, as expected, Tris resistant (Figure 6, bottom panel). This result establishes that the Tris sensitivity of the GTP-primed membranes does not reflect a basic inability to generate the Tris-resistant state in the two-stage assay and that, in fact, high-affinity AP-1 binding can occur on ice. Our interpretation of these results, then, is that GTP is not being hydrolyzed during the second incubation step, but, rather, nucleotide hydrolysis accompanies nucleotide exchange and membrane binding of ARF·GTP very rapidly. This notion has been proposed before by others (Donaldson et al., 1992; Helms et al., 1993; Tanigawa et al., 1993; Finazzi et al., 1994; Teal et al., 1994).
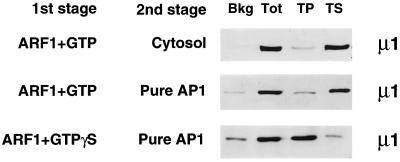
Figure 6.
Resistance of AP-1 to Tris extraction in two-stage binding assays. Golgi membranes were primed in a first-stage incubation with either ARF·GTP or ARF·GTPγS. After recovery of the membranes, either cytosol or affinity-purified AP-1 was mixed with the primed membranes on ice as indicated, followed by a further incubation on ice for the second-stage binding step. Membranes were pelleted and then extracted with 1 M Tris, and the pellets (TP) and supernatants (TS) were then separated. Unprimed Golgi membranes were used as a control to determine background (Bkg) binding of AP-1. Membranes from two-stage reactions not extracted with Tris were used to show total (Tot) AP-1 recruitment. Notice that on membranes primed with ARF·GTP and reincubated with either cytosol or pure AP-1, the bound AP-1 is fully Tris sensitive, whereas in the presence of ARF·GTPγS, the bound AP-1 is resistant to Tris extraction.
Fate of the ARF-primed Membrane Docking Sites
The fact that AP-1 adaptors can bind to Golgi membranes after nucleotide hydrolysis appears to have occurred indicates that detectable binding affinity for AP-1 persists after GTP hydrolysis. Although the affinity of this interaction is lower than that seen when ARF·GTP is present, three-stage assays, in which an additional incubation at 37°C is inserted between the priming and binding stages, can be used to follow the fate of these primed sites (Figure 7). As a control, ARF·GTPγS-primed membranes that had been incubated for up to 10 min at 37°C before adding cytosol showed no loss of AP-1 recruitment, confirming the irreversible nature of the association of AP-1 with the membrane in the presence of GTPγS. When GTP is used with ARF1 to prime the membranes, reincubating the primed membranes at 37°C for 5 min before initiating the coat-binding step only slightly diminishes the recruitment of AP-1. After a 10-min incubation at 37°C, coat recruitment has dropped to about half that observed on primed membranes, which are not reincubated at 37°C. These results indicate that the capacity of the Golgi membranes to bind AP-1 persists for a finite time after ARF·GTP hydrolysis. We believe that the best explanation of these results is that although ARF is essential to drive coat assembly, when ARF·GTP is used, nucleotide hydrolysis ensues rapidly, and consequently the bound AP-1 adaptor is not held on the membrane as it is when ARF·GTPγS is present. This difference is reflected in the Tris and temperature sensitivity of the membrane-associated adaptor complex.
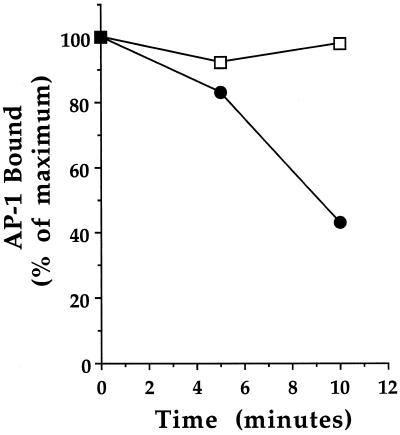
Figure 7.
Fate of the primed membrane AP-1-binding sites. Golgi membranes were primed in a first-stage incubation with ARF1 and either GTP (•) or GTPγS (□) at 37°C, recovered by centrifugation, and then resuspended in assay buffer. The membranes were then reincubated at 37°C for 0, 5, or 10 min for the second stage, chilled on ice, and again recovered by centrifugation. The resuspended membranes were then mixed with gel-filtered cytosol and incubated on ice for 15 min during the third stage of the assay. The amount of AP-1, measured by the presence of the μ1 subunit, was determined by immunoblotting and densitometry. The data are the average of two independent experiments and are expressed as the percentage of AP-1 on the membrane compared with primed membranes held on ice during the second stage, which was defined as 100%.
ARF Is a Minor Component of Isolated Clathrin-coated Vesicles
If ARF only plays a transitory role in clathrin coat assembly, then budded vesicles should not contain stoichiometric amounts of ARF. A semiquantitative estimate of the amount of ARF in purified coated vesicles was made by directly comparing the amount of the ∼20-kDa ς1 subunit of the AP-1 complex with ∼20-kDa ARF. This approach is feasible because more than ∼80% of the clathrin-coated vesicles purified from rat liver are TGN derived, which makes identification of the ς1 subunit unambiguous. First, we matched the amount of purified AP-1 (Figure 8, lane 1) with that found in 10 μg of purified liver clathrin-coated vesicles (Figure 8, lane 2), and then added purified ARF (Figure 8, lane 3) to approximate the amount of the ς1 subunit in the purified AP-1 sample (Figure 8, lane 1). Immunoblotting with an antibody against the ς1 subunit (Figure 8, top panel, lanes 4–6) confirms that the purified AP-1 and the coated vesicles have equivalent amounts of the adaptor complex. Substantially less ARF is present on the purified clathrin-coated vesicles than would be expected if the GTP-binding protein was present at a 1:1 stoichiometry with AP-1. This is seen both on immunoblots using a pan-ARF mAb, 1D9 (Figure 8, middle panel, lanes 5 and 6), and by [α-32P]-GTP overlay (Figure 8, bottom panel, lanes 5 and 6). Quantitation indicates at least a 10-fold difference in the amount of ARF in the coated vesicle sample and the purified ARF. These results argue against ARF being a stoichiometric component of purified clathrin-coated vesicle preparations and, together with our data presented above, make it unlikely that ARF plays a structural role in the coat that persists past the budding step.
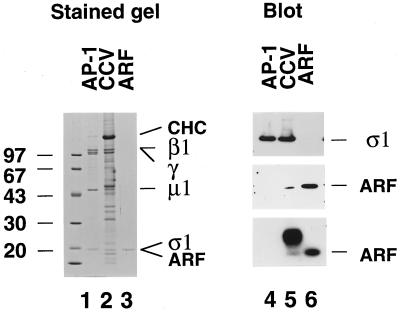
Figure 8.
Semiquantitative estimate of ARF content of clathrin-coated vesicles. Samples of 1.2 μg of hydroxylapatite-purified AP-1, 10 μg of purified rat liver clathrin-coated vesicles, and ∼80 ng of purified ARF were resolved on 10–16% polyacrylamide gradient gels and either stained with Coomassie blue (lanes 1–3) or transferred to nitrocellulose (lanes 4–6) and analyzed by immunoblotting with the anti-AP-1 ς1 subunit antibody DE/1 (top panel) or the pan-ARF mAb 1D9 (middle panel) or by [α-32P]GTP overlay (bottom panel). The position of the clathrin heavy chain (CHC) and the β1-, γ-, μ1-, and ς1 subunits of the AP-1 complex and ARF are indicated on the right. The broad band of [α-32P]GTP-binding protein(s) that migrates more slowly than ARF seen in the clathrin-coated vesicles (lane 5) is most probably a rab protein(s).
DISCUSSION
ARF has been linked to the control of membrane traffic at almost every stage along both the biosynthetic and endocytic pathways (Serafini et al., 1991; Lenhard et al., 1992; Osterman et al., 1993; Stamnes and Rothman, 1993; Traub et al., 1993; Aridor et al., 1995; Chen and Shields, 1996; Dittie et al., 1996; West et al., 1997). The ARF family of small GTP-binding proteins is composed of six members, ARF1–6. With the exception of ARF6, which does not appear to cycle on and off the membrane as do ARF1–5 (Cavenagh et al., 1996), no clear specificity for any particular member of the family has really emerged. The bulk of the effects of ARF have been attributed to ARF1 and can be reconstituted using recombinant ARF1. In fact, we have found that once on the membrane, ARF1, ARF5, and ARF6 are capable of provoking coat assembly (Liang and Kornfeld, 1997). If most trafficking pathways do not each use different ARFs with exquisite specificity, how is any degree of control over coat assembly reactions achieved within the cell? This issue is particularly problematic at the TGN, where BFA treatment blocks all protein exit from this compartment. The blockade is not due to simple retrograde redistribution of the Golgi compartment back into the ER, as cargo accumulated in the TGN at 20°C also fails to exit on warming if BFA is present (Traub and Kornfeld, 1997). The BFA sensitivity suggested the involvement of ARF, and ARF has now been shown to regulate traffic or coat assembly at the TGN in several systems (Stamnes and Rothman, 1993; Traub et al., 1993; Chen and Shields, 1996; Dittie et al., 1996; Simon et al., 1996). Unfortunately, not many downstream effectors of ARF have been found, so it remains difficult at present to explain at a mechanistic level how a single molecule might control differentially all the traffic leaving the TGN. One possibility might be phospholipid remodeling. Because ARF activates phospholipase D (Brown et al., 1993; Cockcroft et al., 1994; Liang et al., 1997), the generation of phosphatidic acid might be a common and necessary feature of many coat-mediated vesicular transport steps (Roth and Sternweis, 1997). Our data, however, argue against ARF activating an enzyme and rather point toward ARF playing an essential and presumably stoichiometric role in the generation of a high-affinity binding site for AP-1 on the TGN. This conclusion is in excellent agreement with a recent study, which found no evidence for the involvement of phospholipase D in the process of AP-1 binding to the TGN (West et al., 1997).
Using GTPγS, we show that GTP-bound ARF generates a high-affinity binding site for AP-1, and in the absence of nucleotide hydrolysis, the association of the adaptor with the TGN is essentially irreversible. This interaction is stable to reincubation at 37°C and resistant to chemical extraction with Tris. Direct involvement of ARF·GTP in the generation of the high-affinity binding site for AP-1 is implied by the results we obtained using the constitutively activated ARF1(Q71L) mutant and ARF1·GTP in the presence of AlFn. Although it was initially suggested that AlFn slows the hydrolysis of ARF·GTP via an indirect mechanism involving a heterotrimeric GTP-binding protein that modulates ARF GAP activity (Finazzi et al., 1994), recent evidence suggests that the AlFn acts directly on the ARF·ARF GAP complex to slow GTP hydrolysis. It is now known that heterotrimeric GTP-binding proteins contain an invariant arginine residue within the GTP-binding domain that is required to effect GTP hydrolysis and that the equivalent arginine is absent from the small GTP-binding proteins, such as ARF. This accounts for their low rate of intrinsic GTP hydrolysis. The critical arginine residue is provided by GAP via the so-called arginine finger, which inserts into the active site of the small GTP-binding protein and facilitates nucleotide hydrolysis. In the presence of this GAP-supplied residue, GDP-bound small GTP-binding proteins can coordinate AlFn (Mittal et al., 1996; Rittinger et al., 1997). In fact, Ras has now been crystalized with GDP·AlF3 (Scheffzek et al., 1997), but the complex can only form in the presence of ras GAP (Mittal et al., 1996; Scheffzek et al., 1997). Although the molecular interactions of GDP·AlFn with Ras mimic the transition state, they are not identical to those formed with GTP and do not favor dissociation (Scheffzek et al., 1997). In light of these findings, the most likely explanation for our experiments using AlFn in the presence of both ARF·GTP and ARF GAP is that a stable ARF·GDP·AlFn·GAP complex is formed, thereby neutralizing available ARF GAP activity. Any additional ARF·GTP delivered onto the membrane surface during the assay through the action of the ARF guanine nucleotide exchange factor would not be susceptible to GAP-mediated hydrolysis. We find that under these conditions, AP-1 bound to the Golgi membrane is largely resistant to extraction by Tris.
Somewhat surprisingly, clathrin coat formation at the TGN cannot be reconstituted in vitro by simply supplementing cytosol with millimolar concentrations of GTP (Figure 1; Robinson and Kreis, 1992; Stamnes and Rothman, 1993; Traub et al., 1993). Similarly, ARF-dependent assembly of COPI coats from the coatomer within cytosol is very difficult to demonstrate in the presence of GTP (Osterman et al., 1993; Finazzi et al., 1994). Reproducible ARF·GTP-driven COPI coat assembly requires partial purification of the coatomer complex from cytosol and is further improved by salt washing the Golgi membranes before use in binding assays (Osterman et al., 1993; Finazzi et al., 1994). That nonhydrolyzable analogs of GTP are necessary to promote the stable association of coat proteins with the membrane implies either that, in crude systems using whole cytosol, GTP is consumed too rapidly to promote efficient ARF recruitment, or that hydrolysis of ARF·GTP results in the detachment of coat proteins from the membrane.
In practice, both phenomena probably occur in our assays and influence coat assembly in the presence of GTP. Certainly a variety of soluble and organelle-associated GTP-binding proteins are likely to bind to and hydrolyze a significant fraction of the added GTP, and purification of coat components conceivably removes some of these factors. Nevertheless, the effect of hydrolysis of ARF-bound GTP on coat assembly is clear. Rapid deactivation of membrane-bound ARF·GTP necessitates that substantially higher concentrations of ARF are required to drive coat assembly at 37°C. Artificially raising the concentration of ARF1 in the in vitro binding reactions should circumvent both of these limitations to coat recruitment in the presence of GTP and, by adding up to 4 μM of near quantitatively myristoylated ARF1 to our assays, we show that we are able to follow efficient binding of both AP-1 and clathrin to Golgi membranes.
Using these conditions to perform recruitment assays in which GTP hydrolysis can occur reveals the dynamic nature of the coat assembly process. When AP-1 is recruited onto the TGN with ARF1·GTP, and the membranes are then reincubated at 37°C in assay buffer, the bound adaptor (and clathrin) detaches from the Golgi. Similar results were obtained on incubating freshly isolated Golgi membranes at 37°C (Traub et al., 1993). The release of AP-1 from the membrane does not appear to be the result of GTP hydrolysis occurring during the reincubation step for two reasons. First, at the end of a 15-min one-stage assay containing added ARF1 and GTP, all the bound AP-1 is sensitive to Tris extraction. Given the good correlation we have found between nucleotide hydrolysis and Tris sensitivity, we believe this to indicate that the ARF1·GTP hydrolysis event has already passed. Our finding that very little ARF is recovered on the membrane fraction under these conditions is consistent with this interpretation. Second, no change in sensitivity to Tris extraction is seen when the adaptor binding step is performed on ice with either cytosol or pure AP-1, devoid of ARF GAP immunoreactivity, again indicating that nucleotide hydrolysis seems to occur during the priming stage, before AP-1 binding. Three-stage incubations, in which membranes primed with ARF·GTP are incubated in buffer at 37°C before the addition of cytosol in the third stage, show that significant AP-1 and clathrin recruitment does remain after a 10-min second-stage incubation. Others have shown that membrane-associated ARF GAP activity hydrolyses in excess of 90% of bound GTP under these conditions (Helms et al., 1993; Teal et al., 1994). It is also unlikely that what we are following is the activity of an ARF·GDP·Pi complex, which, although having passed the hydrolysis stage, still behaves like ARF·GTP. Pi is released very rapidly from Ras after GAP-catalyzed hydrolysis of GTP and is consistent with a conformational change back to the deactivated GDP-bound state either before or simultaneous with nucleotide cleavage (Nixon et al., 1995). Our interpretation of the data, then, is that although ARF·GTP is necessary to generate the high-affinity docking site, detectable binding affinity is still manifest after the rapid conversion of bound GTP to GDP.
Several possible mechanisms could explain the change in affinity for AP-1 that accompanies GTP hydrolysis. Activated ARF could cause a conformational change in a docking protein(s), which demarcates the membrane attachment site for AP-1, and which, as we have speculated previously (Traub et al., 1993), generates the high-affinity site. After hydrolysis, reversion to the ground state might take a finite period, and the drop in affinity we note could reflect the decay of the activated state after the dissociation of ARF. Alternatively, the high-affinity binding site could be the result of ARF-driven association of one or more subunits of the putative docking complex. Again, after being driven together by ARF·GTP, this protein–protein interaction might be preserved for a short period after hydrolysis. Either model is consistent with the more rapid decay of the AP-1 binding sites that occurs when ARF·GTP-primed Golgi membranes are incubated at 37°C compared with membranes held on ice.
Our experiments lead us to propose a refined model for the early stages of clathrin coat assembly at the TGN (Figure 9). The interaction of cytosolic, GDP-bound ARF1 with a guanine nucleotide exchange factor leads to GDP–GTP exchange and association of the GTP-bound ARF with the membrane. This initiates coat assembly, as activated ARF·GTP will interact rapidly with the membrane binding site for AP-1, the putative docking protein(s), to generate a binding site that is able to bind AP-1 with very high affinity. Although ARF1·GTP is required to maintain this Tris-insensitive binding site, our two-stage assays indicate that ARF·GTP hydrolysis occurs constantly, whether AP-1 binds to the membrane or not. If the adaptor complex does bind, recruitment of clathrin trimers, via the β1 subunit of the immobilized adaptor (Traub et al., 1995), follows. Then, through lateral association of the membrane-bound clathrin molecules, the characteristic clathrin lattice would begin to form. It should be pointed out that several aspects of this model are similar to the scheme proposed for COPII-mediated cargo sorting and vesicle budding (Schekman and Orci, 1996).
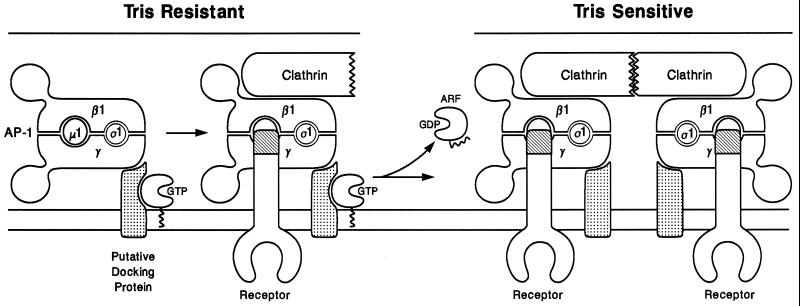
Figure 9.
Proposed scheme for the early events in clathrin coat assembly on the TGN. After ARF guanine–nucleotide exchange factor catalyzes exchange of GTP for bound GDP, ARF1·GTP is tethered to the Golgi membrane via the exposed N-terminal myristic acid moiety. The membrane-bound ARF·GTP associates with a putative AP-1 docking protein (or protein complex) to form a high-affinity AP-1 binding site. In the absence of nucleotide hydrolysis, AP-1 binds tightly in a Tris-resistant manner. Clathrin assembly begins to form a coated pit into which transmembrane cargo receptors can move. Upon stimulation of GTP hydrolysis by ARF·GAP activity, ARF·GDP is released from the docking protein, resulting in a lowered binding affinity for AP-1, which is manifest as Tris sensitivity. As the interaction of ARF·GDP with the docking site decays, the coordinated association of the adaptor complex with polymerizing clathrin triskelia and with transmembrane cargo receptors keeps the assembling clathrin coat on the Golgi membrane. Additional AP-1 molecules would be recruited into the coated pit region at the periphery by ARF-driven activation of new docking sites.
If AP-1 does not bind to the docking site promptly, and the ARF·GTP becomes deactivated by ARF GAP activity, the activated state of the docking site decays, more rapidly at 37°C than on ice. If the docking site decays quickly, how is a coat ever assembled in vivo? Despite the constant nucleotide hydrolysis that occurs in in vitro assays, we cannot rule out that ARF GAP activity might be more tightly regulated in vivo. Two recent publications support the idea that ARF GAP activity is subject to regulation (Antonny et al., 1997; Aoe et al., 1997). Alternatively, in the cell, the transient activation of the membrane binding site and AP-1 recruitment must be tightly coupled. Rapid lateral expansion of the new lattice, by the sustained recruitment of adaptors and clathrin onto activated docking sites at the periphery of the lattice, would form a new clathrin-coated bud, which, in turn, would concentrate transmembrane proteins and receptors conveying cargo. The high local density of AP-1 within the growing lattice ensures that even weak interactions between trafficking signals and the adaptor μ1 subunit result in preferential inclusion of the transmembrane proteins within the emerging bud. This is actually seen in our standard one-stage assays, in which, despite rapid hydrolysis of ARF·GTP, a membrane-associated clathrin coat is formed and recovered on the membrane at the end of the assay. In the cell, then, the rapid and sequential interaction of AP-1 with clathrin and transmembrane molecules must hold the clathrin coat on the membrane and ensure the vectorial nature of the process (Figure 9). Mannose 6-phosphate receptors (MPRs) are important and perhaps the major transmembrane proteins sorted into AP-1-containing clathrin coats assembling at the TGN. The role that these receptors play in the initiation and growth of the clathrin lattice remains controversial. One model (Ludwig et al., 1995), based on several published studies (Le Borgne et al., 1993, 1996; Alconada et al., 1996; Mauxion et al., 1996; Salamero et al., 1996; Le Borgne and Hoflack, 1997) emphasizes that sorted molecules, particularly the MPRs, play an active role in the AP-1 recruitment process at the TGN. In our system, however, the MPRs do not appear to play a major part in bringing AP-1 to the TGN membrane. By comparing MPR-positive and MPR-negative fibroblasts, we have been unable to find any differences in either the steady-state distribution of AP-1 in the intact cells or in the ability of membranes prepared from either cell type to recruit AP-1 in the presence of GTP or GTPγS (our unpublished observations).
Finally, we have found that ARF does not appear to be a stoichiometric component of the coat of clathrin-coated vesicles purified from rat liver. In fact, although the pan-ARF mAb 1D9 detects a protein with the appropriate molecular weight in the clathrin-coated vesicles, isoform-specific antibodies directed against each member of the ARF family indicate that this protein is not ARF1, -3, -4, -5, or -6 (Cavenagh et al., 1996). Our estimation of the amount of ARF in coated vesicles might then be an overestimation. Other groups have identified this GTP-binding protein within preparations of clathrin-coated vesicles previously (Lenhard et al., 1992; Cavenagh et al., 1996), but no quantitative estimates were made. The paucity of ARF on purified clathrin-coated vesicles is fully consistent with the fact that vesicle-associated adaptors are efficiently liberated by Tris extraction. This further supports the notion that ARF has already performed its physiological function in clathrin coat assembly before budding. The data also indicate that ARF is unlikely to play a role in vesicle uncoating, as has been proposed for the COPI coat (Tanigawa et al., 1993; Rothman, 1994; Antonny et al., 1997).
ACKNOWLEDGMENTS
We thank our colleagues for readily providing us with antibodies, which were essential for this study. We also thank J. Liang for supplying the purified ARF1(Q71L) and Rosalind Kornfeld and members of the Kornfeld laboratory for helpful comments on the manuscript. This work was supported in part by National Institutes of Health (NIH) Grant R01 CA08759 to S. Kornfeld, by NIH Training Grant HL0708823, and by an Edward Mallinckrodt Jr. Foundation Research Grant to L.M.T. Y.Z. and L.M.T. contributed equally to this study.
Footnotes
Abbreviations used: AP, clathrin-associated adaptor protein complex; ARF, ADP-ribosylation factor; BFA, brefeldin A; COP, coat protein complex; GAP, GTPase-activating protein; GTPγS, guanosine 5′-O-(3-thiotriphosphate); MPR, mannose 6-phosphate receptor; SH3, Src homology 3; TGN, trans-Golgi network.
REFERENCES
- Ahle S, Mann A, Eichelsbacher U, Ungewickell U. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localised in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- Antonny B, Huber I, Paris S, Chabre M, Cassel D. Activation of ADP-ribosylation factor 1 GTPase-activating protein by phosphatydylcholine-derived diacylglycerols. J Biol Chem. 1997;272:30848–30851. doi: 10.1074/jbc.272.49.30848. [DOI] [PubMed] [Google Scholar]
- Aoe T, Cukierman E, Lee A, Cassel D, Peters PJ, Hsu VW. The KDEL receptor, ERD2, regulates intracellular traffic by recruiting a GTPase-activating protein for ARF1. EMBO J. 1997;16:7305–7316. doi: 10.1093/emboj/16.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Bannykh SI, Rowe T, Balch WE. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Bednarek SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COPI- and COPII-coated vesicles bud directly from the endoplasmic reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Brodsky FM. New fashions in vesicle coats. Trends Cell Biol. 1997;7:175–179. doi: 10.1016/S0962-8924(97)01038-6. [DOI] [PubMed] [Google Scholar]
- Brown HA, Gutowski S, Moomaw CR, Slaughter C, Sternweis PC. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell. 1993;75:1137–1144. doi: 10.1016/0092-8674(93)90323-i. [DOI] [PubMed] [Google Scholar]
- Campbell C, Squicciarini J, Shia M, Pilch PF, Fine RE. Identification of a protein kinase as an intrinsic component of rat liver coated vesicles. Biochemistry. 1984;23:4420–4426. doi: 10.1021/bi00314a028. [DOI] [PubMed] [Google Scholar]
- Cavenagh MM, Whitney JA, Carroll K, Zhang C, Boman AL, Rosenwald AG, Mellman I, Kahn RA. Intracellular distribution of Arf proteins in mammalian cells: Arf6 is uniquely localized to the plasma membrane. J Biol Chem. 1996;271:21767–21774. doi: 10.1074/jbc.271.36.21767. [DOI] [PubMed] [Google Scholar]
- Chen Y-G, Shields D. ADP-ribosylation factor-1 stimulates formation of nascent secretory vesicles form the trans-Golgi network of endocrine cells. J Biol Chem. 1996;271:5297–5300. doi: 10.1074/jbc.271.10.5297. [DOI] [PubMed] [Google Scholar]
- Cockcroft S, Thomas GM, Fensome A, Geny B, Cunningham E, Gout I, Hiles I, Totty NF, Truong O, Hsuan JJ. Phospholipase D: a downstream effector of ARF in granulocytes. Science. 1994;263:523–526. doi: 10.1126/science.8290961. [DOI] [PubMed] [Google Scholar]
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CG, Lehrman MA, Russell DW, Anderson RG, Brown MS, Goldstein JL. The J.D. mutation in familial hypercholesterolemia: amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell. 1986;45:15–24. doi: 10.1016/0092-8674(86)90533-7. [DOI] [PubMed] [Google Scholar]
- Dittie AS, Hajibagheri N, Tooze SA. The AP-1 adaptor complex binds to immature secretory granules from PC12 cells, and is regulated by ADP-ribosylation factor. J Cell Biol. 1996;132:523–536. doi: 10.1083/jcb.132.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Duronio RJ, Jackson-Machelski E, Heuckeroth RO, Olins PO, Devine CS, Yonemoto W, Slice LW, Taylor SS, Gordon JI. Protein N-myristoylation in Escherichia coli: reconstitution of a eukaryotic protein modification in bacteria. Proc Natl Acad Sci USA. 1990;87:1506–1510. doi: 10.1073/pnas.87.4.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finazzi D, Cassel D, Donaldson JG, Klausner RD. Aluminum fluoride acts on the reversibility of ARF1-dependent coat protein binding to Golgi membranes. J Biol Chem. 1994;269:13325–13330. [PubMed] [Google Scholar]
- Gaynor EC, Emr SD. COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J Cell Biol. 1997;136:789–802. doi: 10.1083/jcb.136.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JB, Palmer DJ, Rothman JE. Two distinct populations of ARF bound to Golgi membranes. J Cell Biol. 1993;121:751–760. doi: 10.1083/jcb.121.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JB, Rothman JE. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- Henley JR, McNiven MA. Association of a dynamin-like protein with the Golgi apparatus in mammalian cells. J Cell Biol. 1996;133:761–775. doi: 10.1083/jcb.133.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw JE, Schmid SL. Dynamin self-assembles into rings suggesting a mechanism for coated vesicle budding. Nature. 1995;374:190–192. doi: 10.1038/374190a0. [DOI] [PubMed] [Google Scholar]
- Jadot M, Canfield WM, Gregory W, Kornfeld S. Characterization of the signal for rapid internalization of the bovine mannose 6-phosphate/insulin-like growth factor-II receptor. J Biol Chem. 1992;267:11069–11077. [PubMed] [Google Scholar]
- Kedersha NL, Rome LH. Isolation and characterization of a novel ribonucleoprotein: large structures contain a single species of small RNA. J Cell Biol. 1986;103:699–709. doi: 10.1083/jcb.103.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen JH, Willingham MC, Pastan IH. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979;16:303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Bonifacino JS, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Griffiths G, Hoflack B. Mannose 6-phosphate receptors and ADP-ribosylation factors cooperate for high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2162–2170. doi: 10.1074/jbc.271.4.2162. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Hoflack B. Mannose 6-phosphate receptors regulate the formation of clathrin-coated vesicles in the TGN. J Cell Biol. 1997;137:335–345. doi: 10.1083/jcb.137.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Schmidt A, Mauxion F, Griffiths G, Hoflack B. Binding of AP-1 Golgi adaptors to membranes requires phosphorylated cytoplasmic domains of the mannose 6-phosphate/insulin-like growth factor II receptor. J Biol Chem. 1993;268:22552–22556. [PubMed] [Google Scholar]
- Lenhard JM, Kahn RA, Stahl PD. Evidence for ADP-ribosylation factor (ARF) as a regulator of in vitro endosome-endosome fusion. J Biol Chem. 1992;267:13047–13052. [PubMed] [Google Scholar]
- Liang JO, Kornfeld S. Comparative activity of ADP-ribosylation factor family members in the early steps of coated vesicle formation on rat liver Golgi membranes. J Biol Chem. 1997;272:4141–4148. doi: 10.1074/jbc.272.7.4141. [DOI] [PubMed] [Google Scholar]
- Liang JO, Sung T-C, Morris AJ, Frihman MA, Kornfeld S. Different domains of mammalian ADP-ribosylation factor 1 mediate interaction with selected target proteins. J Biol Chem. 1997;272:33001–33008. doi: 10.1074/jbc.272.52.33001. [DOI] [PubMed] [Google Scholar]
- Ludwig T, Le Borgne R, Hoflack B. Roles for mannose 6-phosphate receptors in lysosomal enzyme sorting, IGF-II binding and clathrin-coat assembly. Trends Cell Biol. 1995;5:202–206. doi: 10.1016/s0962-8924(00)89000-5. [DOI] [PubMed] [Google Scholar]
- Makler V, Cukierman EMR, Admon A, Cassel D. ADP-ribosylation factor-directed GTPase-activating protein: purification and partial characterization. J Biol Chem. 1995;270:5232–5237. doi: 10.1074/jbc.270.10.5232. [DOI] [PubMed] [Google Scholar]
- Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor determines the high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- Mittal R, Ahmadian MR, Goody RS, Wittinghofer A. Formation of a transition-state analog of the Ras GTPase reaction by Ras·GDP, tetrafluoroaluminate and GTPase-activating proteins. Science. 1996;273:115–117. doi: 10.1126/science.273.5271.115. [DOI] [PubMed] [Google Scholar]
- Nathke IS, Heuser J, Lupas A, Stock J, Turck CW, Brodsky FM. Folding and trimerization of clathrin subunits at the triskelion hub. Cell. 1992;68:899–910. doi: 10.1016/0092-8674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Nixon AE, Brune M, Lowe PN, Webb MR. Kinetics of inorganic phosphate release during the interaction of p21ras with the GTPase-activating proteins, p120GAP and neurofibromin. Biochemistry. 1995;34:15592–15598. doi: 10.1021/bi00047a026. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J BIol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Ohno HJS, Fournier M-C, Bosshart H, Rhee I, Miyatake S, Saito T, Galluser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Sollner TH, Rothman JE. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Osterman J, Orci L, Tani K, Amhert M, Ravazzola M, Elazar Z, Rothman JE. Stepwise assembly of functionally active transport vesicles. Cell. 1993;75:1015–1025. doi: 10.1016/0092-8674(93)90545-2. [DOI] [PubMed] [Google Scholar]
- Randazzo PA. Resolution of two ADP-ribosylation factor 1 GTPase-activating proteins from rat liver. Biochem J. 1997;324:413–419. doi: 10.1042/bj3240413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport I, Miyazaki M, Boll W, Duckworth B, Cantley LC, Shoelson S, Kirchhausen T. Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J. 1997;16:2240–2250. doi: 10.1093/emboj/16.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittinger K, Walker PA, Eccleston JF, Smerdon SJ, Gamblin SJ. Structure at1.65 Angstrom of rhoA and its GTPase-activating protein in complex with a transition-state analogue. Nature. 1997;389:758–762. doi: 10.1038/39651. [DOI] [PubMed] [Google Scholar]
- Robinson MS. 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J Cell Biol. 1987;104:887–895. doi: 10.1083/jcb.104.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Robinson MS, Kreis TE. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992;69:129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Roth MG, Sternweis PC. The role of lipid signaling in constitutive membrane traffic. Curr Opin Cell Biol. 1997;9:519–526. doi: 10.1016/s0955-0674(97)80028-2. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Salamero J, Le Borgne R, Saudrais C, Goud B, Hoflack B. Expression of major histocompatibility complex class II molecules in HeLa cells promotes the recruitment of AP-1 Golgi-specific assembly proteins on Golgi membranes. J Biol Chem. 1996;271:30318–30321. doi: 10.1074/jbc.271.48.30318. [DOI] [PubMed] [Google Scholar]
- Scheffzek K, Ahmadian MR, Kabsch W, Wiesmuller L, Lautwein A, Schmitz F, Wittinghofer A. The Ras-Ras GAP complex: structural basis for GTPase activation and ist loss in oncogenic mutants. Science. 1997;277:333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Seaman MNJ, Ball CL, Robinson MS. Targeting and mistargeting of plasma membrane adaptors in vitro. J Cell Biol. 1993;123:1093–1105. doi: 10.1083/jcb.123.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T, Orci L, Amherdt M, Brunner M, Kahn RA, Rothman JE. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991;67:239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Shupliakov O, Low P, Grabs D, Gad H, Chen H, David C, Takei K, De Camilli P, Brodin L. Synaptic vesicle endocytosis impaired by disruption of dynamin-SH3 domain interactions. Science. 1997;276:259–263. doi: 10.1126/science.276.5310.259. [DOI] [PubMed] [Google Scholar]
- Simon JP, Ivanov IE, Shopsin B, Hersh D, Adesnik M, Sabatini DD. The in vitro generation of post-Golgi vesicles carrying viral envelope glycoproteins requires an ARF-like GTP-binding protein and protein kinase C associated with the Golgi apparatus. J Biol Chem. 1996;271:16952–16961. doi: 10.1074/jbc.271.28.16952. [DOI] [PubMed] [Google Scholar]
- Stamnes MA, Rothman JE. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- Tabas I, Kornfeld S. Purification and characterization of a rat liver Golgi alpha-mannosidase capable of processing asparagine-linked oligosaccharides. J Biol Chem. 1979;254:11655–11663. [PubMed] [Google Scholar]
- Takei K, McPherson PS, Schmid SL, De Camilli P. Tubular membrane invaginations coated by dynamin rings are induced by GTP-γS in nerve terminals. Nature. 1995;374:186–190. doi: 10.1038/374186a0. [DOI] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. The synaptic vesicle cycle: a single budding step involving clathrin and dynamin. J Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigawa G, Orci L, Amherdt M, Ravazzola M, Helms JB, Rothman JE. Hydrolysis of bound GTP by ARF protein triggers uncoating of Golgi-derived COP-coated vesicles. J Cell Biol. 1993;123:1365–1371. doi: 10.1083/jcb.123.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teal SB, Hsu VW, Peters PJ, Klausner RD, Donaldson JG. An activating mutation in ARF1 stabilizes coatomer binding to Golgi membranes. J Biol Chem. 1994;269:3135–3138. [PubMed] [Google Scholar]
- Traub LM, Bannykh SI, Rodel JE, Aridor M, Balch WE, Kornfeld S. AP-2-containing clathrin coats assemble on mature lysosomes. J Cell Biol. 1996;135:1801–1804. doi: 10.1083/jcb.135.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Kornfeld S. The trans-Golgi network: a late secretory sorting station. Curr Opin Cell Biol. 1997;9:527–533. doi: 10.1016/s0955-0674(97)80029-4. [DOI] [PubMed] [Google Scholar]
- Traub LM, Kornfeld S, Ungewickell E. Different domains of the AP-1 adaptor complex are required for Golgi membrane binding and clathrin recruitment. J Biol Chem. 1995;270:4933–4942. doi: 10.1074/jbc.270.9.4933. [DOI] [PubMed] [Google Scholar]
- Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urrutia R, Henley JR, Cook T, McNiven MA. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci USA. 1997;94:377–384. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss O, Holden J, Rulka C, Kahn RA. Nucleotide binding and cofactor activities of purified bovine brain and bacterially expressed ADP-ribosylation factor. J Biol Chem. 1989;264:21066–21072. [PubMed] [Google Scholar]
- Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- West MA, Bright NA, Robinson MS. The role of ADP-ribosylation factor and phospholipase D in adaptor recruitment. J Cell Biol. 1997;138:1239–1254. doi: 10.1083/jcb.138.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigge P, Vallis Y, Mcmahon HT. Inhibition of receptor-mediated endocytosis by the amphiphysin SH3 domain. Curr Biol. 1997;7:554–560. doi: 10.1016/s0960-9822(06)00254-5. [DOI] [PubMed] [Google Scholar]
- Wong DH, Brodsky FM. 100-kD proteins of Golgi- and trans-Golgi network-associated coated vesicles have related but distinct membrane binding properties. J Cell Biol. 1992;117:1171–1179. doi: 10.1083/jcb.117.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]