Abstract
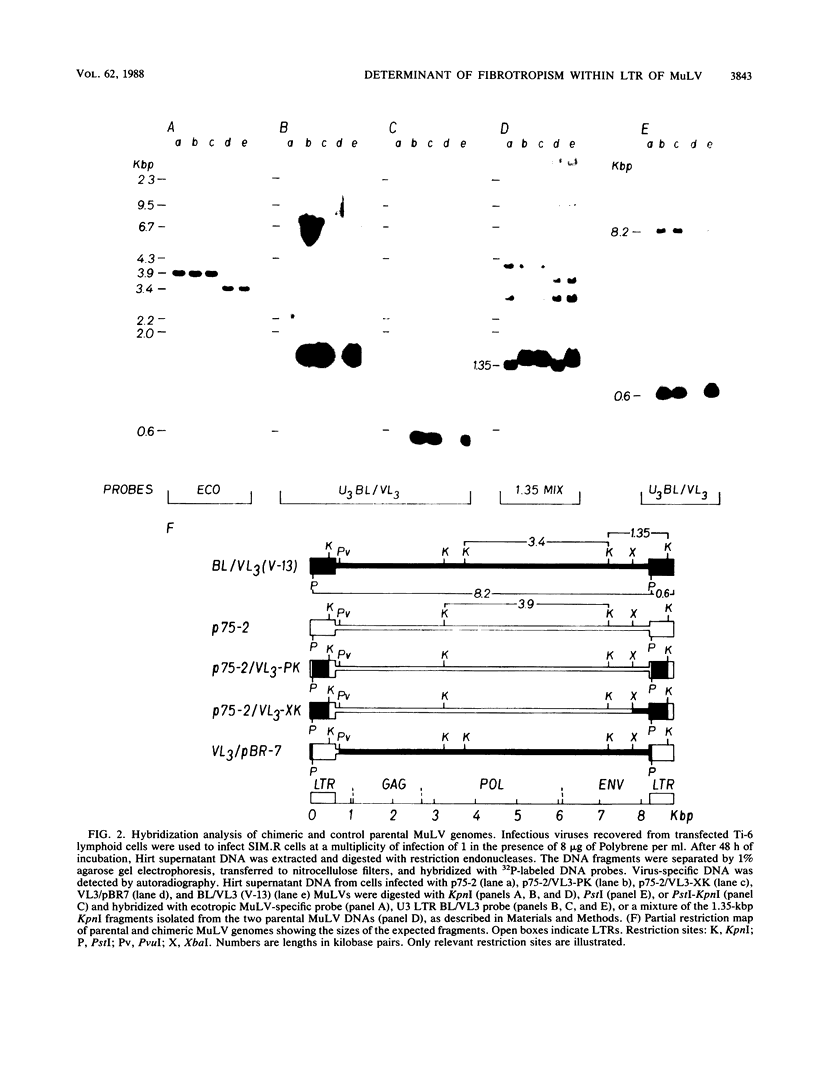
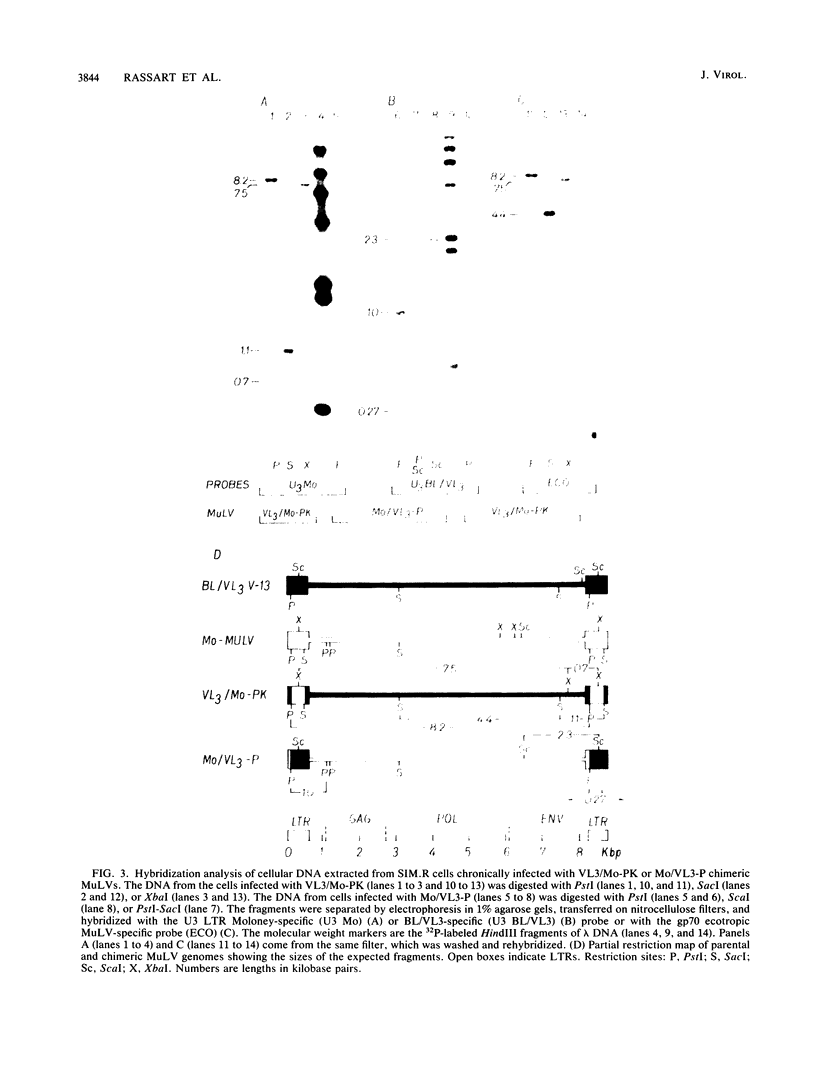
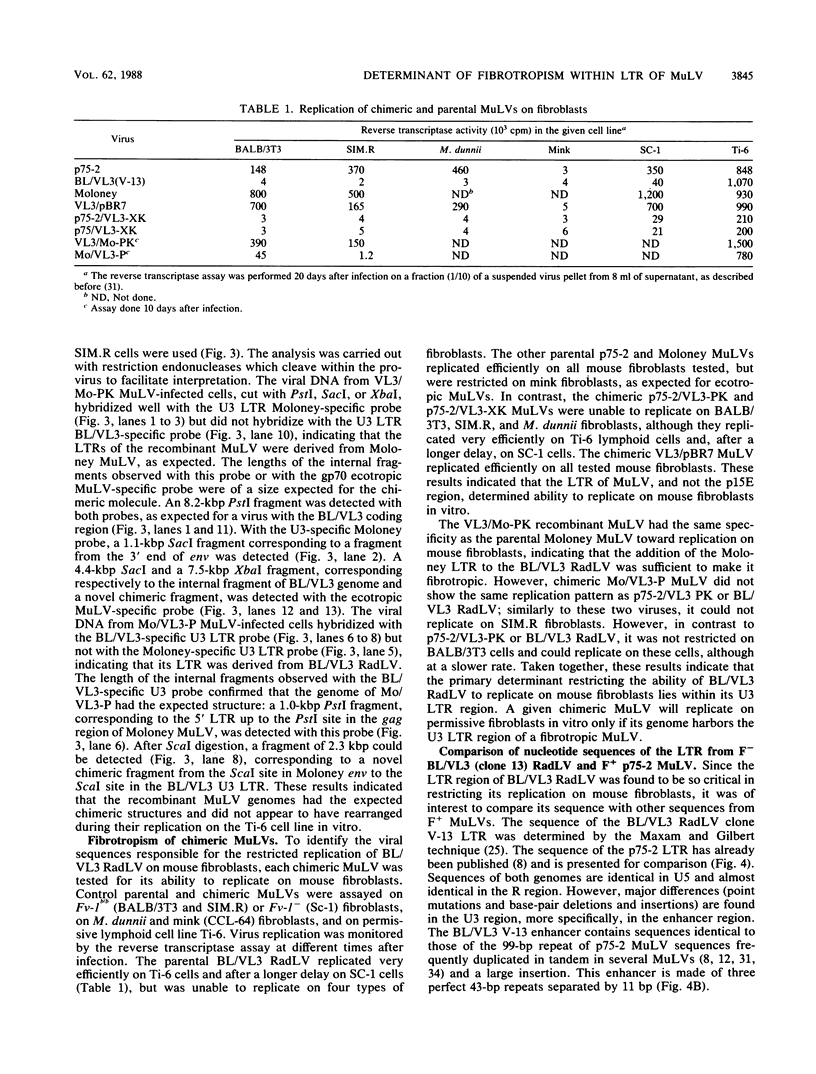
The molecularly cloned infectious Kaplan radiation leukemia virus has previously been shown to be unable to replicate on mouse fibroblasts (E. Rassart, M. Shang, Y. Boie, and P. Jolicoeur, J. Virol. 58:96-106, 1986). To map the viral sequences responsible for this, we constructed chimeric viral DNA genomes in vitro with parental cloned infectious viral DNAs from the nonfibrotropic (F-) BL/VL3 V-13 radiation leukemia virus and the fibrotropic (F+) endogenous BALB/c or Moloney murine leukemia viruses (MuLV). Infectious chimeric MuLVs, recovered after transfection of Ti-6 lymphocytes with these recombinant DNAs, were tested for capacity to replicate on mouse fibroblasts in vitro. We found that chimeric MuLVs harboring the long terminal repeat (LTR) of a fibrotropic MuLV replicated well on mouse fibroblasts. Conversely, chimeric MuLVs harboring the LTR of a nonfibrotropic MuLV were restricted on mouse fibroblasts. These results indicate that the LTR of BL/VL3 radiation leukemia virus harbors the primary determinant responsible for its inability to replicate on mouse fibroblasts in vitro. Our results also show that the primary determinant allowing F+ MuLVs (endogenous BALB/c and Moloney MuLVs) to replicate on mouse fibroblasts in vitro resides within the LTR.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declève A., Lieberman M., Ihle J. N., Rosenthal P. N., Lung M. L., Kaplan H. S. Physicochemical, biological and serological properties of a leukemogenic virus isolated from cultured RadLV-induced lymphomas of C57BL/Ka mice. Virology. 1978 Oct 1;90(1):23–35. doi: 10.1016/0042-6822(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Declève A., Sato C., Lieberman M., Kaplan H. S. Selective thymic localization of murine leukemia virus-related antigens in C57BL-Ka mice after inoculation with radiation virus. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3124–3128. doi: 10.1073/pnas.71.8.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekaban G. A., Ball J. K. Integration of type B retroviral DNA in virus-induced primary murine thymic lymphomas. J Virol. 1984 Dec;52(3):784–792. doi: 10.1128/jvi.52.3.784-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Mapping the viral sequences conferring leukemogenicity and disease specificity in Moloney and amphotropic murine leukemia viruses. J Virol. 1984 Nov;52(2):448–456. doi: 10.1128/jvi.52.2.448-456.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Jolicoeur P. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J Virol. 1983 Dec;48(3):685–696. doi: 10.1128/jvi.48.3.685-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Somatically acquired recombinant murine leukemia proviruses in thymic leukemias of AKR/J mice. J Virol. 1983 Apr;46(1):70–82. doi: 10.1128/jvi.46.1.70-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Wozney J., Chatis P. A., Hopkins N., Hartley J. W. Construction of recombinants between molecular clones of murine retrovirus MCF 247 and Akv: determinant of an in vitro host range property that maps in the long terminal repeat. J Virol. 1985 Jan;53(1):152–157. doi: 10.1128/jvi.53.1.152-157.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E. Effect of Fv-1 gene product on synthesis of linear and supercoiled viral DNA in cells infected with murine leukemia virus. J Virol. 1980 Jan;33(1):183–195. doi: 10.1128/jvi.33.1.183-195.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rassart E., Sankar-Mistry P. Strong selection for cells containing new ecotropic recombinant murine leukemia virus provirus after propagation of C57BL/6 radiation-induced thymoma cells in vitro or in vivo. Mol Cell Biol. 1983 Sep;3(9):1675–1679. doi: 10.1128/mcb.3.9.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P., Rosenberg N., Cotellessa A., Baltimore D. Leukemogenicity of clonal isolates of murine leukemia viruses. J Natl Cancer Inst. 1978 Jun;60(6):1473–1476. doi: 10.1093/jnci/60.6.1473. [DOI] [PubMed] [Google Scholar]
- KAPLAN H. S. THE ROLE OF RADIATION ON EXPERIMENTAL LEUKEMOGENESIS. Natl Cancer Inst Monogr. 1964 May;14:207–220. [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Lander M. R., Chattopadhyay S. K. A Mus dunni cell line that lacks sequences closely related to endogenous murine leukemia viruses and can be infected by ectropic, amphotropic, xenotropic, and mink cell focus-forming viruses. J Virol. 1984 Nov;52(2):695–698. doi: 10.1128/jvi.52.2.695-698.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Declève A., Kaplan H. S. Rapid in vitro assay for thymotropic, leukemogenic murine C-type RNA viruses. Virology. 1978 Oct 15;90(2):274–278. doi: 10.1016/0042-6822(78)90311-2. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Declève A., Ricciardi-Castagnoli P., Boniver J., Finn O. J., Kaplan H. S. Establishment, characterization and virus expression of cell lines derived from radiation- and virus-induced lymphomas of C57BL/Ka mice. Int J Cancer. 1979 Aug;24(2):168–177. doi: 10.1002/ijc.2910240208. [DOI] [PubMed] [Google Scholar]
- Lieberman M., Niwa O., Declève A., Kaplan H. S. Continuous propagation of radiation leukemia virus on a C57BL mouse-embryo fibroblast line, with attenuation of leukemogenic activity. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1250–1253. doi: 10.1073/pnas.70.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Potter H., Weir L., Leder P. Enhancer-dependent expression of human kappa immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., DesGroseillers L., Jolicoeur P. Molecular cloning of B- and N-tropic endogenous BALB/c murine leukemia virus circular DNA intermediates: isolation and characterization of infectious recombinant clones. J Virol. 1981 Jul;39(1):162–171. doi: 10.1128/jvi.39.1.162-171.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., Sankar-Mistry P., Lemay G., DesGroseillers L., Jolicoeur P. New class of leukemogenic ecotropic recombinant murine leukemia virus isolated from radiation-induced thymomas of C57BL/6 mice. J Virol. 1983 Feb;45(2):565–575. doi: 10.1128/jvi.45.2.565-575.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassart E., Shang M., Boie Y., Jolicoeur P. Studies on emerging radiation leukemia virus variants in C57BL/Ka mice. J Virol. 1986 Apr;58(1):96–106. doi: 10.1128/jvi.58.1.96-106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen C. A., Haseltine W. A., Lenz J., Ruprecht R., Cloyd M. W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985 Sep;55(3):862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard P., DesGroseillers L., Rassart E., Poirier Y., Jolicoeur P. Important role of the long terminal repeat of the helper Moloney murine leukemia virus in Abelson virus-induced lymphoma. J Virol. 1987 Oct;61(10):3266–3275. doi: 10.1128/jvi.61.10.3266-3275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Shoemaker C., Goff S., Gilboa E., Paskind M., Mitra S. W., Baltimore D. Structure of a cloned circular Moloney murine leukemia virus DNA molecule containing an inverted segment: implications for retrovirus integration. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3932–3936. doi: 10.1073/pnas.77.7.3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stocking C., Kollek R., Bergholz U., Ostertag W. Point mutations in the U3 region of the long terminal repeat of Moloney murine leukemia virus determine disease specificity of the myeloproliferative sarcoma virus. Virology. 1986 Aug;153(1):145–149. doi: 10.1016/0042-6822(86)90015-2. [DOI] [PubMed] [Google Scholar]
- Vogt M., Haggblom C., Swift S., Haas M. Envelope gene and long terminal repeat determine the different biological properties of Rauscher, Friend, and Moloney mink cell focus-inducing viruses. J Virol. 1985 Jul;55(1):184–192. doi: 10.1128/jvi.55.1.184-192.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]