Abstract
Fission yeast Spc1/StyI MAPK is activated by many environmental insults including high osmolarity, oxidative stress, and heat shock. Spc1/StyI is activated by Wis1, a MAPK kinase (MEK), which is itself activated by Wik1/Wak1/Wis4, a MEK kinase (MEKK). Spc1/StyI is inactivated by the tyrosine phosphatases Pyp1 and Pyp2. Inhibition of Pyp1 was recently reported to play a crucial role in the oxidative stress and heat shock responses. These conclusions were based on three findings: 1) osmotic, oxidative, and heat stresses activate Spc1/StyI in wis4 cells; 2) oxidative stress and heat shock activate Spc1/StyI in cells that express Wis1AA, in which MEKK consensus phosphorylation sites were replaced with alanine; and 3) Spc1/StyI is maximally activated in Δpyp1 cells. Contrary to these findings, we report: 1) Spc1/StyI activation by osmotic stress is greatly reduced in wis4 cells; 2) wis1-AA and Δwis1 cells have identical phenotypes; and 3) all forms of stress activate Spc1/StyI in Δpyp1 cells. We also report that heat shock, but not osmotic or oxidative stress, activate Spc1 in wis1-DD cells, which express Wis1 protein that has the MEKK consensus phosphorylation sites replaced with aspartic acid. Thus osmotic and oxidative stress activate Spc1/StyI by a MEKK-dependent process, whereas heat shock activates Spc1/StyI by a novel mechanism that does not require MEKK activation or Pyp1 inhibition.
INTRODUCTION
In both prokaryotes and eukaryotes, a major adaptive response to various stress conditions is to change the repertoire of gene expression. Prokaryotic cells commonly employ the two-component signal transduction systems, where a “sensor” histidine kinase, often located in the plasma membrane, mediates environmental signals to a cytoplasmic “response regulator” that controls transcription of the target gene (reviewed by Hoch and Silhavy, 1995). Although homologous mechanisms have been found also in some eukaryotic organisms (Swanson and Simon, 1994), recent studies have uncovered a pivotal role of MAPK cascades in stress signaling of yeast and vertebrate cells. The prototype of stress-response kinase cascades was first identified in budding yeast Saccharomyces cerevisiae. When exposed to high osmolarity stress, budding yeast cells increase the intracellular osmolarity by upregulating glycerol synthesis, which is induced by activation of the HOG (high osmolarity glycerol response) pathway composed of Hog1p MAPK, Pbs2p MAPK kinase (MEK), and redundant MEK kinases (MEKK) Ssk2p, Ssk22p, and Ste11 (Boguslawski, 1992; Brewster et al., 1993; Maeda et al., 1995; Posas and Saito, 1997). Subsequently, stress-activated MAPK homologs were isolated in mammalian cells as JNK/SAPK (Dérijard et al., 1994; Kyriakis et al., 1994) and p38/RK/CSBP (Han et al., 1994; Lee et al., 1994; Rouse et al., 1994). These kinases are activated by environmental stress and inflammatory factors. These protein kinases regulate gene expression upon stimuli through phosphorylation of the transcription factors c-Jun (Dérijard et al., 1994; Kyriakis et al., 1994), ATF2 (Gupta et al., 1995; Livingstone et al., 1995; Raingeaud et al., 1996), Elk-1 (Cavigelli et al., 1995; Price et al., 1996), CHOP/GADD153 (Wang and Ron, 1996), NFAT4 (Chow et al., 1997), and MEF2C (Han et al., 1997).
In the fission yeast Schizosaccharomyces pombe, a HOG1 homolog, Spc1 (also known as StyI and Phh1) was identified as a regulator of the osmotic response and cell cycle (Millar et al., 1995; Shiozaki and Russell, 1995a; Kato et al., 1996). Spc1 is activated by many different forms of stress including high osmolarity, oxidative stress, heat shock, UV irradiation, and nutritional limitation (Millar et al., 1995; Shiozaki and Russell, 1995a, 1996; Degols et al., 1996; Degols and Russell, 1997; Shieh et al., 1997; Shiozaki et al., 1997). Spc1 plays a crucial role in cell survival under these stress conditions. A key substrate of Spc1 is the bZIP transcription factor Atf1/Gad7 (Takeda et al., 1995; Kanoh et al., 1996), which is most homologous to mammalian ATF-2 (Shiozaki and Russell, 1996; Wilkinson et al., 1996). Activated Atf1 induces transcription of various stress response genes (Takeda et al., 1995; Kanoh et al., 1996; Shiozaki and Russell, 1996; Wilkinson et al., 1996). Atf1 is also responsible for stress-induced expression of the Pyp2 tyrosine phosphatase (Millar et al., 1992; Ottilie et al., 1992) and a type 2C serine/threonine phosphatase, Ptc1 (Shiozaki et al., 1994). Pyp2 dephosphorylates the activating tyrosine phosphorylation in Spc1 (Millar et al., 1995; Degols et al., 1996), and Ptc1 negatively regulates Atf1-dependent transcription of stress-response genes (Gaits et al., 1997), which constitutes dual loops of negative feedback. However, genetic data imply that Atf1 is not the sole target of Spc1 because the G2-M cell cycle regulation carried out by Spc1 is independent of Atf1 (Shiozaki and Russell, 1996; Wilkinson et al., 1996).
Wis1 (Warbrick and Fantes, 1991) is the MEK that phosphorylates and activates Spc1 (Millar et al., 1995; Shiozaki and Russell, 1995a). In Δwis1 mutant cells, activating tyrosine phosphorylation of Spc1 is not detected under any stress conditions (Millar et al., 1995; Shiozaki and Russell, 1995a; Degols et al., 1996; Degols and Russell, 1997), indicating that other MEK homologs in S. pombe (Neiman et al., 1993) are not involved in activation of Spc1. Wis1 activation of Spc1 is counteracted by Pyp1 and Pyp2 tyrosine phosphatases, with Pyp1 having the major activity (Degols et al., 1996). Therefore, the activity of Spc1 MAPK is determined by the balance between Wis1 versus Pyp1 and Pyp2 (Millar et al., 1995; Shiozaki and Russell, 1995a). Disturbing the balance by either wis1+ overexpression or simultaneous deletion of pyp1+ and pyp2+ brings about hyperactivation of Spc1, which is toxic to the cell (Millar et al., 1992; Ottilie et al., 1992; Shiozaki and Russell, 1995a). Recently, a MEKK homolog that functions upstream of Wis1 was identified as Wis4/Wik1/Wak1 (Samejima et al., 1997; Shieh et al., 1997; Shiozaki et al., 1997). It is thought that Wis4 is regulated by a two-component osmosensor (Shieh et al., 1997; Shiozaki et al., 1997), which is homologous to the budding yeast Sln1p-Ypd1p-Ssk1p phosphorelay system (Ota and Varshavsky, 1993; Maeda et al., 1994; Posas et al., 1996).
In contrast to the budding yeast HOG pathway, which is activated only by osmostress (Schüller et al., 1994), S. pombe Spc1 and mammalian stress-activated kinases are responsive to many different forms of stress. With the aim of understanding how fission yeast cells perceive various stress stimuli and funnel them to Spc1, we initiated a genetic dissection of the Spc1 pathway in search of stress-specific activation mechanisms. Analyses using strains that express Wis1 with mutations at the MEKK phosphorylation sites have demonstrated that osmostress and oxidative stress signals are transmitted by MEKKs. While Wis4 MEKK is mostly responsible for osmostress signaling, another unidentified MEKK is also involved in transmitting oxidative stress signals to Wis1. Unexpectedly, we have found that heat stress activates Spc1 by a pathway that is independent of MEKKs. It was recently suggested that heat stress activated Spc1 by inhibition of the tyrosine phosphatase Pyp1 (Samejima et al., 1997), but our studies reveal that heat stress activates Spc1 in a Δpyp1 strain. Thus, osmotic and oxidative stress activate Spc1 by different MEKK-dependent processes, whereas heat shock activates Spc1 by a novel mechanism that does not require MEKK activation or Pyp1 inhibition.
MATERIALS AND METHODS
Yeast Strains and General Methods
The S. pombe strains used in this study are listed in Table 1. They are all derivatives of 972h− and 975h+ (Mitchison, 1970). Standard procedures and growth media for S. pombe genetics have been followed according to Moreno et al. (1991) and Alfa et al. (1993). YES and synthetic EMM2 media were used in growing S. pombe cells.
Table 1.
S. pombe strains used in this study
| Strains | Genotype | Source or reference |
|---|---|---|
| PR109 | h− | Lab stock |
| JM535 | h+ pyp1::LEU2 | Lab stock |
| KS1376 | h− spc1:HA6H(ura4+) | (Shiozaki and Russell, 1995) |
| GD1682 | h− spc1:HA6H(ura4+) wis1::ura4+ | Lab stock |
| KS1867 | h− pyp1::LEU2 spc1:HA6H(ura4+) | This study |
| KS1875 | h+ his7-366 wis4::ura4+ | (Shiozaki et al., 1997) |
| KS1960 | h− his7-366 spc1:HA6H(ura4+) wik1::his7+ | This study |
| KS1966 | h− his7-366 wik1::his7+ | This study |
| KS2079 | h− wis1:12myc(ura4+) | This study |
| KS2080 | h− wis1AA:12myc(ura4+) | This study |
| KS2081 | h− wis1DD:12myc(ura4+) | This study |
| KS2086 | h− spc1:HA6H(ura4+) wis1AA:12myc(ura4+) | This study |
| KS2088 | h− spc1:HA6H(ura4+) wis1DD:12myc(ura4+) | This study |
| KS2096 | h− spc1:HA6H(ura4+) wis1:12myc(ura4+) | This study |
| KS2125 | h− spc1::ura4+ wis1DD:12myc(ura4+) | This study |
All strains are leu1-32 ura4-D18.
wis4+ Gene Disruption
The DNA sequences immediate upstream (−407 to +1) and downstream (+4207 to + 4723) of the wik1+ ORF were amplified by PCR using wild-type S. pombe genomic DNA as template with a pair of primers WK5 (5′-CGCGGA TCC ATC TAT AGT GAT AAC GGA AGT AAG-3′, BamHI restriction site is underlined) and WK6 (5′-CCG GAA TTC AGC AAC TGT CAT AGA AAA CAC TAG-3′, EcoRI restriction site is underlined) and another pair WK7 (5′-CCG CTC GAG TTA CAT GGT TTT AGG CGA ATG TGT-3′, XhoI restriction site is underlined) and WK8 (5′-CGG GGT ACC ATG TTC ACC ATT ACG CTG GCA CTA-3′, KpnI restriction site is underlined), respectively. Using the restriction sites at the ends, these two PCR products were cloned into the pBS-his7+, which is a pBluescript vector containing the 1.9-kilobase (kb) his7+ fragment (Apolinario et al., 1993) at the SmaI restriction site. The resultant plasmid was digested by BamHI and KpnI to release the wis4::his7+ fragment (see Figure 1A) and used to transform a his7–366 wis4::ura4+ strain (KS1875). Stable His+ ura− transformants were selected, and deletion of wik1+ was confirmed by Southern blot analyses with genomic DNA isolated from the transformants.
Figure 1.
(A) wis4+ gene disruption. A 0.4-kb region of the immediate upstream and a 0.5-kb region of immediate downstream of the wis4+ ORF were amplified by PCR and used to construct a plasmid to replace the chromosomal wis4+ ORF with the his7+ marker gene. Deletion of the whole ORF in the resultant strain was confirmed by genomic Southern blot analysis (our unpublished results). Restriction enzyme sites: Bg, BglII; Ec, EcoRI, Kp, KpnI; Nd, NdeI; Xh, XhoI. (B) Stress-induced activation of Spc1 in Δwis4 cells. Wild-type (KS1376) and Δwis4 mutant (KS1960) strains carrying a chromosomal spc1+ tagged with the HA6H sequence were grown to midlog phase in YES medium at 30°C. At time 0, 0.6 M KCl for osmostress and 0.3 mM H2O2 for oxidative stress were added to the culture or the temperature was shifted to 48°C for heat shock. Aliquots of cells were harvested by filtration at the following time points, and Spc1 was purified by Ni-NTA chromatography. Activation of Spc1 was examined by immunoblotting using anti-phosphotyrosine antibodies. The amount of Spc1 did not fluctuate during the experiments, which was confirmed by a duplicated immunoblotting with anti-HA antibodies (our unpublished results). (C) Cell harvest by centrifugation activates Spc1. KS1376 cells exponentially growing in YES medium were harvested either by filtration (lane F) or by centrifugation at 800 × g for 5 min (5′) and 10 min (10′). Tyrosine phosphorylation of Spc1 in each sample was examined as described above.
Detection of Stress-induced Activation of Spc1
Stress-induced tyrosine phosphorylation of Spc1 was examined using wild-type and mutant S. pombe strains which carry chromosomal spc1+ tagged with the HA6H sequence encoding two copies of hemagglutinin epitope and six consecutive histidine residues (Shiozaki and Russell, 1995a, 1997). Spc1HA6H protein was purified by Ni-NTA-agarose beads under denaturing conditions and subjected to immunoblotting analysis using anti-HA (12CA5) and anti-phosphotyrosine (4G10, Upstate Biotechnology, Lake Placid, NY) monoclonal antibodies following the procedures described previously (Shiozaki and Russell, 1997). Stress treatments of S. pombe cells by KCl, H2O2, and heat shock were performed as described previously (Shiozaki et al., 1997), and cells were harvested by filtration (Shiozaki and Russell, 1997) except in the experiment shown in Figure 1C.
Construction of wis1AA and wis1DD Mutants
wis1AA and wis1DD mutant genes were created by the overlap extension method using PCR (Higuchi et al., 1988; Ho et al., 1989). For wis1AA, the sequences encoding residues 1–477 and residues 465–605 of Wis1AA were separately amplified by PCR with the wild-type wis1+ genomic clone as template using a first pair of primers ND-WIS (5′-CCA TAT GTC TTC TCC AAA TAA TCA ACC-3′) and 3SATA (5′-ACA TCC AAT GTT AGC TTT GGA TAT AGC AGC CAC AAG ATT-3′, introduced mutations for S469A and T473A are underlined) and a second pair of primers 5SATA (5′-AAT CTT GTG GCT GCT ATA TCC AAA GCT AAC ATT GGA TGT-3′, introduced mutations for S469A and T473A are underlined) and WIS-KP (5′-CGG GGT ACC TTG CTT CTT TTT TCA CCT TTC TCT TTA AGA GCG-3′, KpnI restriction site is underlined), respectively. These PCR products were purified by agarose gel electrophoresis, mixed at a 1:1 ratio, and then subjected to another PCR using the primers ND-WIS and WIS-KP, the product of which is a full length wis1AA gene. The same procedure was used to create wis1DD mutant using primers 3SDTD (5′-ACA TCC AAT GTT ATC TTT GGA TAT ATC AGC CAC AAG ATT-3′, introduced mutations for S469D and T743D are underlined) and 5SDTD (5′-AAT CTT GTG GCT GAT ATA TCC AAA GAT AAC ATT GGA TGT-3′, introduced mutations for S469D and T473D are underlined) as well as ND-WIS and WIS-KP. The wild-type, wis1AA, and wis1DD gene fragments were cleaved at the internal DdeI site, and the 3′-end KpnI site and the 0.8-kb DdeI-KpnI fragments were cloned into p12 myc. p12 myc plasmid contains the ura4+ marker gene and a sequence encoding 12 copies of myc epitope (Evan et al., 1985) following a unique KpnI site (Degols and Russell, unpublished result). The resultant plasmids were linearized by MscI digestion and used to transform wild-type cells (PR109). Stable Ura+ transformants were selected and integration of the plasmid constructs at the wis1+ locus was confirmed by Southern hybridization analysis. Furthermore, the wis1 sequence was amplified by PCR using the genomic DNA from the transformants, and introduced mutations were confirmed by DNA sequencing.
RESULTS
Wis4-Independent Activation of Spc1 by Oxidative Stress and Heat Shock
In an attempt to evaluate the role of Wis4 MEKK in the response to various forms of stress, activation of Spc1 in the wis4− strain was monitored under osmostress, oxidative stress, and heat shock conditions. Previously, we constructed a wis4 disruption allele (wis4::ura4+) by inserting the ura4+ marker gene in the chromosomal region encoding the catalytic domain of Wis4 (Shiozaki et al., 1997). However, in this strain a C-terminal truncated form of Wis4 protein might be still expressed and disturb activation of the Spc1 pathway. Therefore, we constructed a wis4 deletion strain in which the entire open reading frame of wis4+ was replaced with the his7+ marker gene (Δwis4, Figure 1A). This Δwis4 strain showed a growth defect in high osmolarity media and a cell elongation phenotype that were indistinguishable from the wis4::ura4+ strains (our unpublished results).
To examine activation of Spc1 in the Δwis4 cells, the Δwis4 mutation was introduced into a strain carrying a chromosomal copy of the spc1+ gene tagged with the HA6H sequence encoding two copies of hemagglutinin epitope and six consecutive histidine residues. In this strain, a wild-type level of Spc1 is expressed using the spc1+ promoter and can be purified by Ni-NTA-agarose chromatography (Shiozaki and Russell, 1997). Wild-type and Δwis4 strains expressing the HA6H-tagged Spc1 were exposed to 0.6 M KCl, and Spc1 purified from these strains was subjected to antiphosphotyrosine immunoblotting to monitor the activation state. In wild-type cells, strong activation of Spc1 was observed within 5 min, but only a small increase of Spc1 tyrosine phosphorylation was detected in Δwis4 cells (Figure 1B). This result is consistent with our previous observation with the wis4::ura4+ strains, confirming that Wis4 plays an important role in osmostress signaling to Spc1 (Shiozaki et al., 1997).
It was recently reported that osmotic stress strongly activates Spc1 in Δwis4 cells (Samejima et al., 1997), a finding that contradicts our previous study and the data shown in Figure 1B. The contradictory findings cannot be attributed to strain differences because we have observed that activation of Spc1 by osmotic stress is also severely impaired in the Δwis4 mutant strain constructed by Samejima et al. (our unpublished observations). One key difference in the experimental protocols is the method of harvesting cells. We harvest cells by rapid filtration (Shiozaki and Russell, 1997), whereas Samejima et al. harvested cells by centrifugation (P. Fantes, personal communication). We explored whether this methodological difference might account for the discordant findings. When compared with filtration, we found that centrifugation caused a large increase in Spc1 tyrosine phosphorylation (Figure 1C). Thus, the findings of Samejima et al. are complicated by the fact that cells were being stressed during harvest. The implications of these findings are considered in greater detail in the DISCUSSION.
Activation of Spc1 in Δwis4 cells was also examined after oxidative stress and heat shock. Wild-type and Δwis4 cells were exposed to hydrogen peroxide or incubated at 48°C, and the tyrosine phosphorylation of Spc1 was followed by anti-phosphotyrosine antibodies. In contrast to osmostress, oxidative stress and heat shock induced strong Spc1 activation, although the kinetics of maximal Spc1 activation were significantly delayed relative to wild-type, particularly for oxidative stress (Figure 1B). These results indicate that Wis4 contributes to Spc1 activation in response to oxidative and heat stress, but that these signals can also be transmitted to Spc1 independently of Wis4.
Wis1 Activity Is Regulated through the Conserved MEKK Phosphorylation Sites
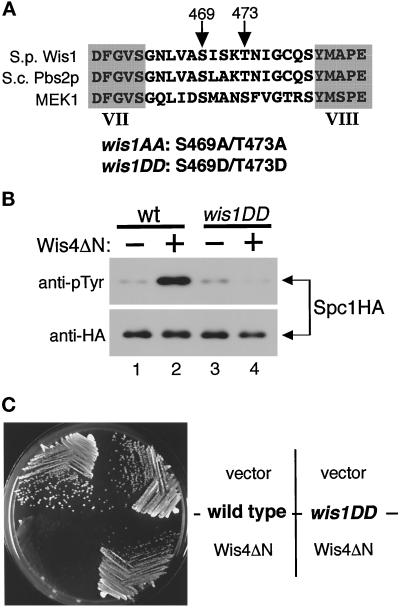
A number of protein kinases, including MEKs and MAPKs, are activated by phosphorylation between the kinase subdomains VII and VIII (Johnson et al., 1996). Human MEK1 is activated by c-raf and MEKK through the phosphorylation of the serine 218 and 222 residues (Alessi et al., 1994; Pagès et al., 1994; Zheng and Guan, 1994). These two phosphorylation sites are also conserved in the MEKs of the yeast stress-sensing pathways: S. pombe Wis1 and S. cerevisiae Pbs2p (Figure 2A [Warbrick and Fantes, 1991; Boguslawski, 1992]). To test whether the conserved MEKK phosphorylation sites in Wis1 are involved in stress signaling, we constructed two kinds of wis1 mutants. In wis1AA, Ser 469 and Thr 473 codons were substituted with codons encoding unphosphorylatable alanine residues. In wis1DD the same sites were changed to encode aspartic acid residues to mimic phosphorylation. wis1+, wis1AA, and wis1DD genes were tagged with a sequence encoding the myc epitope just before the termination codon so that expressed mutant proteins could be detected by anti-myc epitope antibodies (Evan et al., 1985). These constructs were used to replace the chromosomal wis1+ gene; therefore they are expressed from the endogenous wis1+ promoter and are the only wis1 genes in the genome. Immunoblotting using anti-myc antibodies against the crude lysates of the wild-type, wis1AA, and wis1DD strains showed that proteins of the expected molecular weight were expressed (our unpublished results).
Figure 2.
(A) wis1AA and wis1DD mutants. Serine 469 and threonine 473 between the protein kinase subdomains VII and VIII were substituted with alanine (wis1AA) or aspartic acid (wis1DD) residues. These sites correspond to Ser 514 and Thr 518 in S. cerevisiae Pbs2p and Ser 218 and Ser 222 in human MEK1, which are phosphorylated by c-raf and MEKK. (B) Spc1 is not responsive to Wis4 MEKK in the wis1DD mutant strain. Wild-type (KS2096) and wis1DD mutant (KS2088) strains carrying a chromosomal spc1+ tagged with the HA6H sequence were transformed with either the pREP1 vector alone (−) or pREP1-wis4ΔN plasmid (+), which expresses Wis4ΔN from the thiamine-repressible nmt1+ promoter (Maundrell, 1990). Spc1 was purified by Ni-NTA-chromatography from the cells grown in EMM2 medium without vitamin B1 for 14 h at 30°C. Immunoblotting by anti-phosphotyrosine antibodies indicated that expression of Wis4ΔN induced activation of Spc1 in wild-type cells but not in wis1DD mutant cells. (C) Strains used in panel B were streaked on an EMM2 agar plate without thiamine to induce expression of Wis4ΔN from the nmt1+ promoter. The plate was incubated for 3 d at 30°C and photographed. wis1DD mutant cells are insensitive to the toxicity of Wis4ΔN expression.
If Wis4 MEKK functions by phosphorylating Ser 469 and Thr 473 of Wis1, then wis1AA and wis1DD mutants should be unresponsive to Wis4 activity. A mutant Wis4 protein lacking the N-terminal noncatalytic domain (Wis4ΔN) is constitutively active, and even in the absence of stress stimuli, expression of Wis4ΔN induces activation of Spc1 in a wis1+-dependent manner (Samejima et al., 1997; Shiozaki et al., 1997). We found that Wis4ΔN did not stimulate Spc1 activation in the wis1DD (Figure 2B) and wis1AA (our unpublished results) strains. Consistent with this observation, the wis1DD mutation rescued the growth defect by Wis4ΔN expression (Figure 2C). While Wis4ΔN causes a swollen cell morphology and frequent cell lysis in wild-type cells (Samejima et al., 1997; Shiozaki et al., 1997), wis1DD cells expressing Wis4ΔN showed no apparent difference from the same strain carrying the empty vector as a control. These results confirmed that Ser 469 and Thr 473 are essential for the regulation of Wis1 by Wis4 MEKK.
Osmostress and Oxidative Stress Signals Are Transmitted through the Ser 469 and Thr 473 of Wis1
Figure 3 shows the cell morphology of wild-type, wis1AA, and wis1DD strains. In comparison to wild-type cells (Figure 3A), wis1AA mutant cells are more elongated (Figure 3B), exhibiting a phenotype that is indistinguishable from the Δwis1 cells (Warbrick and Fantes, 1991). As was the case with Δwis1 cells (Millar et al., 1995; Shiozaki and Russell, 1995b), wis1AA cells exhibited an osmosensitive growth phenotype (our unpublished results), implying that Wis1AA protein is not functional. In contrast, wis1DD mutant cells have a significantly shorter cell length than wild type (Figure 3C), which resembles the cell morphology of the Δpyp1 mutants (Figure 3D) (Millar et al., 1992; Ottilie et al., 1992). Pyp1 tyrosine phosphatase dephosphorylates and inhibits Spc1, and the Δpyp1 mutation results in an elevated level of Spc1 activity (Shiozaki and Russell, 1995a). Therefore, it is likely that Wis1DD has a higher activity than the wild-type Wis1 and activates Spc1 even in the absence of stress. This was confirmed by comparing the level of Spc1 tyrosine phosphorylation between wild-type and the wis1 mutant strains (Figure 4A). In wis1DD cells, Spc1 tyrosine phosphorylation was higher than in wild-type cells in the absence of stress (compare lanes 2 and 8), which is consistent with the idea that the wis1DD mutation stimulates Wis1 activity by mimicking the phosphorylation at positions 469 and 473. (Note that the experiment shown in Figure 2B was performed with cells grown in minimal EMM2 media, which causes moderate stress [Shiozaki and Russell, 1995a], accounting for the similar level of Spc1 tyrosine phosphorylation in wild-type and Wis1DD cells in lanes 1 and 3). On the other hand, as in Δwis1 cells (lane 1), Spc1 tyrosine phosphorylation was not detectable in wis1AA cells before and after osmostress, suggesting that substitution of the Ser 469 and Thr 473 with the nonphosphorylatable residue abolishes Wis1 activity (lanes 5–7). These results indicate that conserved MEKK phosphorylation sites, Ser 469 and Thr 473, are essential for Wis1 activation.
Figure 3.
Cell morphology of wild-type (A), wis1AA (B), wis1DD (C), and Δpyp1 (D) mutant strains. Wild-type (KS2079), wis1AA (KS2080), wis1DD (KS2081), and Δpyp1 (JM535) cells were grown to midlog phase in YES medium at 30°C and photographed by DIC microscopy. In comparison to wild type, wis1AA cells are elongated and, like Δpyp1 cells, wis1DD strain has a shorter cell length, which reflects the Spc1 activity in the cell.
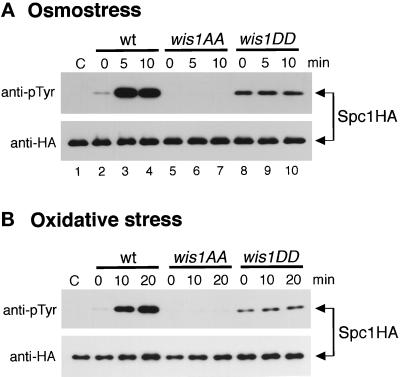
Figure 4.
Osmostress and oxidative stress response of Spc1 in wild-type, wis1AA, and wis1DD cells. Wild-type (KS2096), wis1AA (KS2086), and wis1DD (KS2088) strains carrying a chromosomal spc1+ tagged with the HA6H sequence were grown in YES medium, and 0.6 M KCl (A) and 0.3 mM H2O2 (B) were added to the cultures at time 0. Spc1HA6H protein was purified on Ni-NTA-agarose beads from cells harvested at each time point and subjected to immunoblotting analyses with anti-phosphotyrosine (pTyr) and anti-HA epitope antibodies. Spc1HA6H was also purified from Δwis1 strain (GD1682) and used as a negative control for anti-pTyr antibodies (lanes C).
If Wis4 phosphorylates Ser 469 and Thr 473 of Wis1 in response to osmotic stress and activates the kinase cascade, mutations at these sites should make Spc1 unresponsive to osmotic stress. As expected, the level of Spc1 tyrosine phosphorylation was unchanged in wis1DD cells after exposure to osmotic stress (Figure 4A). In wild-type cells, Spc1 was robustly activated within 5 min after osmostress by 0.6 M KCl (lanes 2–4). However, the activation level of Spc1 showed no change in wis1DD cells during the experiment (lanes 8–10). No Spc1 tyrosine phosphorylation was detected in wis1AA cells exposed to osmotic stress.
Very similar results were obtained when wis1AA and wis1DD cells were exposed to oxidative stress generated by hydrogen peroxide (Figure 4B). Spc1 was strongly activated in wild-type cells after exposure to oxidative stress, while no change in Spc1 tyrosine phosphorylation was detected in the wis1DD strain. Hence, these data strongly suggest that osmotic and oxidative stress signals are transmitted by phosphorylation of Ser 469 and Thr 473 of Wis1 to activate Spc1, and Spc1 is unresponsive to this stress in the strains carrying nonphosphorylatable residues at these sites. It is noteworthy that the level of active Spc1 in wis1DD cells was much lower than in wild-type cells stimulated by osmotic stress and oxidative stress. Presumably, aspartic acid residues at positions 469 and 473 do not activate Wis1 as well as phosphoserine and phosphothreonine at those sites.
Heat Shock Can Induce Spc1 Activation Independently of MEKK Activity
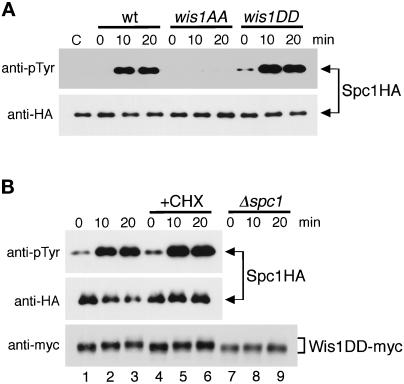
We also examined Spc1 activation in the wis1AA and wis1DD strains in response to heat shock. Surprisingly, a large increase of Spc1 tyrosine phosphorylation was observed in wis1DD cells after the shift from 30°C to 48°C, as was the case in wild-type cells. These results suggest that while osmotic and oxidative stress signals are transmitted to Wis1 through Ser 469 and Thr 548 phosphorylation, heat shock stimuli can be transmitted to Spc1 independently of the conserved MEKK phosphorylation sites of Wis1. However, this heat shock-specific activation of Spc1 is still dependent on a basal level of Wis1 activity, because no tyrosine phosphorylation of Spc1 was observed in Δwis1 (Degols et al., 1996) or wis1AA (Figure 5A) strains after heat shock.
Figure 5.
Heat shock induces Spc1 activation in wis1DD cells. (A) Wild-type (KS2096), wis1AA (KS2086), and wis1DD (KS2088) strains expressing Spc1 tagged with HA6H were grown in YES medium at 30°C, and the cultures were shifted to 48°C at time 0. Spc1 was purified on Ni-NTA-beads from cells harvested every 10 min and analyzed by anti-phosphotyrosine (pTyr) and anti-HA immunoblotting as indicated. Lane C is Spc1HA6H protein purified from Δwis1 cells (GD1682) for a negative control. (B) wis1DD cells (KS2088) exponentially growing in YES medium at 30°C were pretreated with 0.1 mg/ml cycloheximide for 30 min (+CHX) and then subjected to heat shock at 48°C. Spc1HA6H protein was purified and analyzed as in panel A, showing that cycloheximide does not affect heat shock-induced Spc1 activation in wis1DD cells. To monitor Wis1DD protein during the experiment, immunoblotting analysis with anti-myc antibodies was performed using the crude cell lysates in the presence and absence of cycloheximide treatment and in a Δspc1 background (KS2125).
Heat shock also induces a set of gene expression through activation of the heat shock factor (HSF) (Wu, 1995). To test whether heat shock-specific activation of Spc1 in wis1DD cells is dependent on de novo protein synthesis induced by the HSF pathway or other distinct pathways, activation of Spc1 upon heat shock was also examined in wis1DD cells in the presence of cycloheximide, a protein synthesis inhibitor. As shown in Figure 5B, pretreatment of wis1DD cells with cycloheximide did not affect the activation of Spc1 after heat shock, indicating that heat shock-induced activation of Spc1 does not require de novo protein synthesis. Interestingly, we observed that heat shock induced a mobility shift of Wis1DD protein in SDS-PAGE (Figure 5B, bottom panel). Wis1DD migrated with a reduced mobility after heat shock both in the presence (lanes 1–3) and absence (lanes 4–6) of cycloheximide, which correlated well with activation of Spc1. Indeed, no apparent shift of Wis1DD was detected in Δspc1 cells (lanes 7–9), suggesting that the observed mobility shift is dependent on Spc1. The simplest explanation of these findings is that activated Spc1 phosphorylates Wis1.
Heat Shock Activates Spc1 in a Δpyp1 Strain
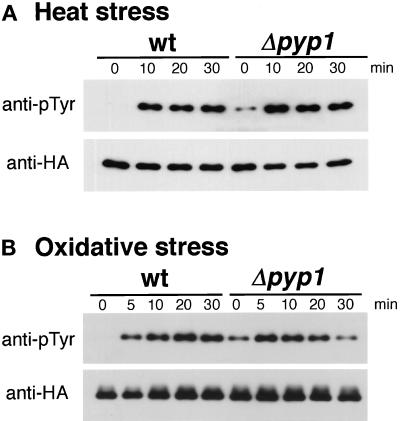
It was recently proposed that arsenite activates JNK by inhibiting a phosphatase that dephosphorylates and inactivates JNK (Cavigelli et al., 1996). Arsenite is known to bring about cellular responses similar to those induced by heat shock (Johnston et al., 1980; Tanguay et al., 1983; Welch, 1985). Therefore, we tested the hypothesis that heat shock activates Spc1 by inhibiting the phosphatases that dephosphorylate Spc1 rather than by activating the kinases that phosphorylate Spc1. In S. pombe, there are two tyrosine phosphatases, Pyp1 and Pyp2, which negatively regulate Spc1 activity (Millar et al., 1995; Shiozaki and Russell, 1995a). Pyp1 accounts for major cellular activity that dephosphorylates Spc1, whereas Pyp2 has only a very minor effect on the phosphorylation state of Spc1 in a wild-type background (Degols et al., 1996; Samejima et al., 1997). In comparison with wild-type cells, Δpyp1 cells have a higher level of Spc1 tyrosine phosphorylation in the absence of stress (Figure 6, time 0) (Shiozaki and Russell, 1995a). If heat shock activates Spc1 by inhibiting the Spc1 phosphatases, there would be little increase in Spc1 tyrosine phosphorylation in Δpyp1 cells after heat shock. However, a large activation of Spc1 was observed in Δpyp1 as well as in wild-type cells (Figure 6A). Similar results were obtained in oxidative stress (Figure 6B) and osmotic stress experiments (our unpublished observations). This experiment excludes the possibility that these forms of stress activate Spc1 by inhibiting Pyp1 tyrosine phosphatase.
Figure 6.
Heat shock and oxidative stress cause activation of Spc1 in Δpyp1 cells. (A) Wild-type (KS1376) and Δpyp1 mutant (KS1867) cells expressing HA6H-tagged Spc1 were grown in YES medium at 30°C and shifted to 48°C at time 0. Cells were harvested every 10 min, and Spc1 was purified on Ni-NTA-beads for immunoblotting analyses with anti-phosphotyrosine (pTyr) and anti-HA epitope antibodies. Before the exposure to stress (0 min), Δpyp1 cells have a higher level of Spc1 tyrosine phosphorylation than wild-type cells; however, heat shock induces further activation of Spc1 also in Δpyp1 cells. (B) The same experiment was performed with exposure to oxidative stress (0.3 mM H2O2) at 30°C.
DISCUSSION
Homologous MAPK pathways in S. pombe and mammalian cells are activated by a variety of stress conditions such as osmotic stress, oxidative stress, and heat shock. How are many different stress stimuli transmitted to a single MAPK? Two simple possibilities may be considered: 1) all stress conditions are sensed by the same receptor(s) connected to the MAPK cascade or 2) multiple receptors with different stress specificity funnel different stress stimuli into the MAPK. We have attempted to distinquish these two possibilities through the genetic dissection of the Spc1 pathway.
In mammalian cells deleted for Sek1(JNKK/MKK4), SAPK/JNKs are activated by only a subset of the stress stimuli that normally activate SAPK/JNKs, suggesting that different stress signals are mediated by more than one MEK (Nishina et al., 1997). On the other hand, no tyrosine phosphorylation of Spc1 is detected in Δwis1 cells under any stress conditions tested, showing that Wis1 is the only MEK for Spc1. Therefore, we examined whether all the stress signals are transmitted through the conserved MEKK phosphorylation sites, Ser 469 and Thr 473, in Wis1. We constructed wis1AA and wis1DD mutants that have unphosphorylatable residues at Ser 469 and Thr 473, which abolished signaling from the Wis4 MEKK. Wis1DD behaved as a constitutively active kinase, and the level of Spc1 tyrosine phosphorylation did not change in wis1DD cells before and after exposure to osmotic and oxidative stress. These observations strongly suggest that osmotic and oxidative stress signals are transmitted to Wis1 through the phosphorylations carried out by MEKK. In addition to Wis4, another unidentified MEKK seems to be involved in oxidative stress signaling, because hydrogen peroxide can induce significant activation of Spc1 in Δwis4 cells, although the response is delayed and dampened relative to wild-type cells. The gene win1+ may encode the other MEKK that activates Wis1 (Samejima et al., 1997). On the other hand, Δwis4 mutants are highly defective in osmotic stress-induced activation of Spc1, showing that transmission of the osmotic stress signal is largely dependent on Wis4.
In contrast to our recent study and new experiments described here, Samejima et al. (1997) proposed that Wis4 is not important for osmotic stress signaling because a similar level of Spc1 activation was observed in wild-type and wis4 mutant cells upon exposure to osmotic stress. However, Samejima et al. harvested cells by centrifugation, a method that can cause potent activation of Spc1. Therefore, the findings of Samejima et al. may be explained by synergistic effects of osmotic and centrifugal stress in wis4 mutant cells.
We have found that heat shock induces activation of Spc1 in wis1DD mutant cells. Although this result does not exclude a possibility that heat stress may also activate MEKK(s), it does show that heat stress can induce Spc1 activation through another pathway distinct from MEKK. However, it should be noted that heat shock-induced activation of Spc1 still requires active Wis1—even after heat shock, no tyrosine phosphorylation of Spc1 is detected in Δwis1 and wis1AA mutant cells. Thus, a basal level of Wis1 activity is required for the activation of Spc1 induced by heat shock.
In our studies we have found that substitution of a chromosomal copy of wis1+ with the wis1AA allele yields mutant phenotypes that are identical to Δwis1 cells. Identical findings have been obtained with the equivalent mutations of the S. cerevisiae kinase Pbs2p, the homolog of Wis1 (Maeda et al., 1995). These findings strongly suggest that Wis1AA protein is inactive, a conclusion that is supported by our observation that Spc1 tyrosine phosphorylation is undetectable in wis1AA cells. In contrast to these results, Samejima et al. (1997) observed that Spc1 was tyrosine phosphorylated in Δwis1 cells that expressed Wis1AA from a strong promoter on a multicopy plasmid. These findings suggest that Wis1AA protein may have very weak activity, and expression of large amounts of this weakly active kinase may be sufficient to activate Spc1 in certain conditions.
How does heat stress induce Spc1 activation by a mechanism that does not require elevated phosphorylation of Wis1 on sites that are typically phosphorylated by MEKKs? One attractive possibility is that heat stress leads to inhibition of the tyrosine phosphatases that dephosphorylate Spc1, as proposed by Samejima et al. (1997). Likewise, arsenite (As3+), a heat stress mimetic, was proposed to bring about activation of mammalian JNK by inhibiting a JNK phosphatase rather than activating JNK kinase (Cavigelli et al., 1996). In fact, mutational inactivation of the major Spc1 phosphatase, Pyp1, results in hyperactivation of Spc1 in the presence of the basal level of Wis1 activity (Shiozaki and Russell, 1995a). It was recently reported that heat shock failed to activate Spc1 in a Δpyp1 strain expressing an extremely low level of wis1+ from the weakest nmt1 promoter under the repressing condition (Samejima et al., 1997), a finding consistent with the idea that Pyp1 may be inhibited by heat shock. However, we have observed strong activation of Spc1 following heat shock in Δpyp1 cells with a wild-type wis1+ background. In the same experiment carried out with the identical Δpyp1 strain, Samejima et al. (1997) found that Spc1 was maximally tyrosine phosphorylated before and during heat stress. The same authors also reported that osmotic and oxidative stress failed to cause an increase of Spc1 tyrosine phosphorylation in Δpyp1 cells, a finding that reinforced their conclusion that Spc1 was maximally activated in Δpyp1 cells. In contrast, we found that both osmotic and oxidative stress induced a large activation of Spc1. Previous studies have shown that hyperactivation of Spc1, achieved either by overproducing Wis1 or Wis4ΔN (Shiozaki and Russell, 1995a; Samejima et al., 1997; Shiozaki et al., 1997), or by creating Δpyp1 Δpyp2 double mutant cells (Millar et al., 1992; Ottilie et al., 1992), is highly toxic. These observations show that Spc1 cannot be maximally active in Δpyp1 cells, because if it were, the cells would be inviable. Samejima et al. collected cells by centrifugation, a method that induces stress. Spc1 tyrosine phosphorylation will be hypersensitive to stress in Δpyp1 cells; therefore, it is very likely that Samejima et al. were misled because of their method of harvesting cells.
Our findings show that heat shock activates Spc1 by a novel mechanism that does not require increased MEKK-dependent phosphorylation of Wis1 on Ser 469 and Thr 473 or inhibition of Pyp1. Moreover, this mechanism appears to be completely independent of MEKKs, at least of Wis4 MEKK, because constitutively active Wis4ΔN does not stimulate Spc1 phosphorylation in wis1DD cells. We have observed that heat shock induces a mobility shift of Wis1DD protein, implying that Wis1 is subjected to a modification such as phosphorylation at a site or sites other than the Ser 469 and Thr 473 MEKK consensus phosphorylation sites. However, this mobility shift of Wis1 appears to be dependent on active Spc1 rather than on an upstream event evoked by heat shock. The role of this modification on Wis1 activity is not clear at present, although studies in vertebrate cells have suggested that MEKs may be inhibited by phosphorylations that are catalyzed by MAPKs (Brunet et al., 1994; Gotoh et al., 1994; Saito et al., 1994). We are currently constructing an in vitro assay for Wis1 to test whether heat shock causes an increase in the specific activity of Wis1DD. MEK is a distinctive subfamily of protein kinases that is conserved among different MAPK pathways as well as through evolution. The only described mechanism of MEK activation is phosphorylation by MEKKs; therefore, continued study of heat shock-induced activation of Spc1 by Wis1 promises to reveal a novel mechanism of MEK activation.
In summary, the data presented in this paper have demonstrated that osmotic stress, oxidative stress, and heat shock signals utilize different pathways to activate Spc1, although there may also be some overlap between the pathways. Our studies have revealed that heat stress induces a large increase of Spc1 tyrosine phosphorylation in Δpyp1 cells, disproving the hypothesis that Pyp1 inhibition is required for transmission of the heat stress signal. Based on this model, we speculate that S. pombe cells have multiple stress receptors with different specificity rather than an omnipotent receptor that is activated by all the stress stimuli.
ACKNOWLEDGMENTS
We are grateful to members of the cell cycle group at Scripps for technical advise and support, particularly Odile Mondesert and Geneviève Degols for the p12 myc plasmid and Frédérique Gaits and Junko Kanoh for helpful discussions. Ian Wilson kindly provided anti-HA epitope antibodies. Peter Fantes provided yeast strains and details of his cell harvesting method. K.S. was supported by California Division-American Cancer Society, Fellowship 1–6-95. This research was supported by National Institutes of Health grant GM-41281 awarded to P.R.
REFERENCES
- Alessi DR, Saito Y, Campbell DG, Cohen P, Sithanandam G, Rapp U, Ashworth A, Marshall CJ, Cowley S. Identification of the sites in MAP kinase kinase-1 phosphorylated by p74raf-1. EMBO J. 1994;13:1610–1619. doi: 10.1002/j.1460-2075.1994.tb06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfa C, Fantes P, Hyams J, McLeod M, Warbrick E. Experiments with Fission Yeast. A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1993. [Google Scholar]
- Apolinario E, Nocero M, Jin M, Hoffman CS. Cloning and manipulation of the Schizosaccaharomyces pombe his7+ gene as a new selectable marker for molecular genetic studies. Curr Genet. 1993;24:491–495. doi: 10.1007/BF00351711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslawski G. PBS2, a yeast gene encoding a putative protein kinase, interacts with the RAS2 pathway and affects osmotic sensitivity of Saccharomyces cerevisiae. J Gen Microbiol. 1992;138:2425–2432. doi: 10.1099/00221287-138-11-2425. [DOI] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Brunet A, Pages G, Pouyssegur J. Growth factor-stimulated MAP kinase induces rapid retrophosphorylation and inhibition of MAP kinase kinase (MEK1) FEBS Lett. 1994;346:299–303. doi: 10.1016/0014-5793(94)00475-7. [DOI] [PubMed] [Google Scholar]
- Cavigelli M, Dolfi F, Claret F-X, Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk1 phosphorylation. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli M, Li WW, Lin A, Su B, Yoshioka K, Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J. 1996;15:6269–6279. [PMC free article] [PubMed] [Google Scholar]
- Chow CW, Rincon M, Cavanagh J, Dickens M, Davis R. Nuclear accumulation of NFAT4 opposed by the JNK signal transduction pathway. Science. 1997;278:1638–1641. doi: 10.1126/science.278.5343.1638. [DOI] [PubMed] [Google Scholar]
- Degols G, Russell P. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol Cell Biol. 1997;17:3356–3363. doi: 10.1128/mcb.17.6.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dérijard B, Hibi M, Wu I-H, Barret T, Su B, Karin M, Davis R. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;74:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaits F, Shiozaki K, Russell P. Protein phosphatase 2C acts independently of stress-activated kinase cascade to regulate the stress response in fission yeast. J Biol Chem. 1997;272:17873–17879. doi: 10.1074/jbc.272.28.17873. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Matsuda S, Takenaka K, Hattori S, Iwamatsu A, Ishikawa M, Kosako H, Nishida E. Characterization of recombinant Xenopus MAP kinase kinases mutated at potential phosphorylation sites. Oncogene. 1994;9:1891–1898. [PubMed] [Google Scholar]
- Gupta S, Campbell D, Dérijard B, Davis RJ. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- Han J, Jiang Y, Li Z, Kravchenko VV, Ulevitch RJ. Activation of the transcription factor MEF2C by the MAP kinase p38 in inflammation. Nature. 1997;386:296–299. doi: 10.1038/386296a0. [DOI] [PubMed] [Google Scholar]
- Han J, Lee J-D, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hoch JA, Silhavy TJ. Two-Component Signal Transduction. Washington, DC: American Society of Microbiology; 1995. [Google Scholar]
- Johnson LN, Noble MEM, Owen DJ. Active and inactive protein kinases: structural basis for regulation. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- Johnston D, Oppermann H, Jackson J, Levinson W. Induction of four proteins in chick embryo cells by sodium arsenite. J Biol Chem. 1980;255:6975–6980. [PubMed] [Google Scholar]
- Kanoh J, Watanabe Y, Ohsugi M, Iino Y, Yamamoto M. Schizosaccharomyces pombe gad7+ encodes a phosphoprotein with a bZIP domain, which is required for proper G1 arrest and gene expression under nitrogen starvation. Genes Cells. 1996;1:391–408. doi: 10.1046/j.1365-2443.1996.d01-247.x. [DOI] [PubMed] [Google Scholar]
- Kato TJ, Okazaki K, Murakami H, Stettler S, Fantes PA, Okayama H. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- Kyriakis J, Banerjee P, Nikolakaki E, Dai T, Rubie E, Ahmad M, Avruch J, Woodgett J. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Lee J, Laydon J, McDonnell P, Gallaghe rT, Kumar S, Green D, McNulty D, Blumenthal M, Heys J, Landvatter S. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Livingstone C, Patel G, Jones N. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 1995;14:1785–1797. doi: 10.1002/j.1460-2075.1995.tb07167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Maundrell K. nmt1 of fission yeast. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- Millar JBA, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Millar JBA, Russell P, Dixon JE, Guan KL. Negative regulation of mitosis by two functionally overlapping PTPases in fission yeast. EMBO J. 1992;11:4943–4952. doi: 10.1002/j.1460-2075.1992.tb05601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison JM. Physiological and cytological methods for Schizosaccharomyces pombe. Methods Cell Physiol. 1970;4:131–146. [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Neiman AM, Stevenson BJ, Xu H-P, Sprague GF, Jr, Herskowitz I, Wigler M, Marcus S. Functional homology of protein kinases required for sexual differentiation in Schizosaccharomyces pombe and Saccharomyces cerevisiae suggests a conserved signal transduction module in eukaryotic organisms. Mol Biol Cell. 1993;4:107–120. doi: 10.1091/mbc.4.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina H, Fischer KD, Radvanyi L, Shahinian A, Hakem R, Rubie EA, Bernstein A, Mak TW, Woodgett JR, Penninger JM. Stress-signalling kinase Sek1 protects thymocytes from apoptosis mediated by CD95 and CD3. Nature. 1997;385:350–353. doi: 10.1038/385350a0. [DOI] [PubMed] [Google Scholar]
- Ota IM, Varshavsky A. A yeast protein similar to bacterial two-component regulators. Science. 1993;262:566–569. doi: 10.1126/science.8211183. [DOI] [PubMed] [Google Scholar]
- Ottilie S, Chernoff J, Hannig G, Hoffman CS, Erikson RL. The fission yeast genes pyp1+ and pyp2+ encode protein tyrosine phosphatases that negatively regulate mitosis. Mol Cell Biol. 1992;12:5571–5580. doi: 10.1128/mcb.12.12.5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès G, Brunet A, L’Allemain G, Pouysségur J. Constitutive mutant and putative regulatory serine phosphorylation site of mammalian MAP kinase kinase (MEK1) EMBO J. 1994;13:3003–3010. doi: 10.1002/j.1460-2075.1994.tb06599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posas F, Saito H. Osmotic activation of the HOG MAPK pathway via Ste11p MAPKKK: Scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multiple phosphorelay mechanism in the SLN1-YPD1-SSK1 ‘two-component’ osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Price MA, Cruzalegui FH, Treisman R. The p38 and ERK MAP kinase pathways cooperate to activate ternary complex factors and c-fos transcription in response to UV light. EMBO J. 1996;15:6552–6563. [PMC free article] [PubMed] [Google Scholar]
- Raingeaud J, Whitmarsh AJ, Barrett T, Dérijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange MA-L, A, Zamanillo D, Hunt T, Nebreda A. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Saito Y, Gomez N, Campbell DG, Ashworth A, Marshall CJ, Cohen P. The threonine residues in MAP kinase kinase 1 phosphorylated by MAP kinase in vitro are also phosphorylated in nerve growth factor-stimulated rat phaeochromocytoma (PC12) cells. FEBS Lett. 1994;341:119–124. doi: 10.1016/0014-5793(94)80252-1. [DOI] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Fantes PA. Multiple modes of activation of the stress-responsive MAP kinase pathway in fission yeast. EMBO J. 1997;16:6162–6170. doi: 10.1093/emboj/16.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüller C, Brewster JL, Alexander MR, Gustin MC, Ruis H. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 1994;13:4382–4389. doi: 10.1002/j.1460-2075.1994.tb06758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh J-C, Wilkinson MG, Buck V, Morgan BA, Makino K, Millar JBA. The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Akhavan-Niaki H, McGowan CH, Russell P. Protein phosphatase 2C encoded by ptc1+ is important in the heat shock response of fission yeast. Mol Cell Biol. 1994;14:3743–3751. doi: 10.1128/mcb.14.6.3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell-cycle control linked to the extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995a;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Counteractive roles of protein phosphatase 2C and a MAP kinase kinase homolog in the osmoregulation of fission yeast. EMBO J. 1995b;14:492–502. doi: 10.1002/j.1460-2075.1995.tb07025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Conjugation, meiosis and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Stress-activated protein kinase pathway in cell cycle control of fission yeast. Methods Enzymol. 1997;283:506–520. doi: 10.1016/s0076-6879(97)83040-6. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol Biol Cell. 1997;8:409–419. doi: 10.1091/mbc.8.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RV, Simon MI. Bringing the eukaryotes up to speed. Curr Biol. 1994;4:234–237. doi: 10.1016/s0960-9822(00)00052-x. [DOI] [PubMed] [Google Scholar]
- Takeda T, Toda T, Kominami K, Kohnosu A, Yanagida M, Jones N. Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J. 1995;14:6193–6208. doi: 10.1002/j.1460-2075.1995.tb00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanguay RM, Camato R, Lettre F, Vincent M. Expression of histone genes during heat shock and in arsenite-treated Drosophila Kc cells. Can J Biochem Cell Biol. 1983;61:414–420. doi: 10.1139/o83-056. [DOI] [PubMed] [Google Scholar]
- Wang X-Z, Ron D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP kinase. Science. 1996;272:1347–1349. doi: 10.1126/science.272.5266.1347. [DOI] [PubMed] [Google Scholar]
- Warbrick E, Fantes PA. The wis1 protein is a dosage-dependent regulator of mitosis in Schizosaccharomyces pombe. EMBO J. 1991;10:4291–4299. doi: 10.1002/j.1460-2075.1991.tb05007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch WJ. Phorbol ester, calcium ionophore, or serum added to quiescent rat embryo fibroblast cells all result in the elevated phosphorylation of two 28,000-Dalton mammalian stress proteins. J Biol Chem. 1985;260:3058–3062. [PubMed] [Google Scholar]
- Wilkinson MG, Samuels M, Takeda T, Toone WM, Shieh J-C, Toda T, Millar JBA, Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- Zheng C-F, Guan K-L. Activation of MEK family kinases requires phosphorylation of two conserved Ser/Thr residues. EMBO J. 1994;13:1123–1131. doi: 10.1002/j.1460-2075.1994.tb06361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]