Abstract
Up-regulation of the cAMP pathway by forskolin or α-melanocyte stimulating hormone induces melanocyte and melanoma cell differentiation characterized by stimulation of melanin synthesis and dendrite development. Here we show that forskolin-induced dendricity is associated to a disassembly of actin stress fibers. Since Rho controls actin organization, we studied the role of this guanosine triphosphate (GTP)-binding protein in cAMP-induced dendrite formation. Clostridium botulinum C3 exotransferase, which inhibits Rho, mimicked the effect of forskolin in promoting dendricity and stress fiber disruption, while the Escherichia coli toxin cytotoxic necrotizing factor-1 (CNF-1), which activates Rho and the expression of a constitutively active Rho mutant, blocked forskolin-induced dendrite outgrowth. In addition, overexpression of a constitutively active form of the Rho target p160 Rho-kinase (P160ROCK) prevented the dendritogenic effects of cAMP. Our results suggest that inhibition of Rho and of its target p160ROCK are required events for cAMP-induced dendrite outgrowth in B16 cells. Furthermore, we present evidence that Rho is involved in the regulation of melanogenesis. Indeed, Rho inactivation enhanced the cAMP stimulation of tyrosinase gene transcription and protein expression, while Rho constitutive activation impaired these cAMP-induced effects. This reveals that, in addition to controlling dendricity, Rho also participates in the regulation of melanin synthesis by cAMP.
INTRODUCTION
Melanocytes are epidermal cells that are derived from the neural crest. During embryonic development, melanocyte precursors migrate to the basal layer of the epidermis where they differentiate and acquire the capacity to synthesize and distribute melanin pigment to surrounding keratinocytes (Fitzpatrick et al., 1979; Le Douarin, 1982). Two main features characterize melanocyte differentiation: 1) an increased melanin synthesis due to the up-regulation of tyrosinase, the rate-limiting enzyme in the melanin synthesis cascade (Hearing and Jimenez, 1989; Hearing and Tsukamoto, 1991), and 2) a stimulation of dendrite outgrowth that ensures melanin distribution within the skin (Buscàet al., 1996). In melanocytes, melanin is stored in discrete subcellular organelles (melanosomes) redistributed from the perinuclear area to the growing dendrites during differentiation. Melanin pigment provides photoprotection against UV radiation damaging and carcinogenic effects. This system constitutes a clear physiological example of cell–cell interactions that result in the tanning response (reviewed by Gilchrest et al., 1996).
α-Melanocyte–stimulating hormone (α-MSH),1 which up-regulates the cAMP pathway in melanocyte cells, and other cAMP-elevating agents, such as forskolin, isobutylmethylxanthine, or cholera toxin, induce melanocyte and melanoma cell differentiation (Wong and Pawelek, 1975; Halaban et al., 1983; Hearing and Tsukamoto, 1991; Hunt et al., 1994; Englaro et al., 1995). The mechanisms of cAMP induction of melanogenesis are currently not fully elucidated. We previously reported that cAMP elevating agents are able to activate the MAP kinase pathway (Englaro et al., 1995). More recently, we showed that cAMP inhibits both phosphatidylinositol-3 kinase (PI3-K) and p70S6 kinase (p70S6K) in the same cell system. Furthermore, we also demonstrated that inhibition of PI3-K by the specific inhibitor LY294002 promotes a strong melanogenic effect and induces dendrite outgrowth in B16 cells, while inhibition of the PI3-K target p70S6K by rapamycin leads to an increased melanogenesis without dendrite formation (Buscàet al., 1996). These observations led us to conclude that cAMP might exert its melanogenic role by inhibiting the PI3-K/p70S6K pathway, but have raised new questions concerning the induction of dendrite outgrowth by PI3-K inhibition. The fact that only PI3-K inhibition but not p70S6K inhibition promotes dendricity, has suggested the existence of other PI3-K targets involved in dendrite outgrowth.
In this report, we first studied early events involved in the control of dendrite formation during cAMP-induced differentiation of B16 melanoma cells. We showed that the actin cytoskeleton becomes reorganized upon treatment with cAMP-elevating agents. These actin cytoskeleton rearrangements are characterized by the disappearance of stress fibers. Formation of stress fibers is controlled by the small GTP-binding protein Rho (Ridley and Hall, 1992) and by its downstream target, the Ser/Thr kinase P160ROCK (Ishizaki et al., 1996; Leung et al., 1996; Matsui et al., 1996). Rho-GTP binds and activates P160ROCK, which then indirectly promotes MLC phosphorylation leading to actin bundling and stress fiber formation (Amano et al., 1996; Chrzanowska-Wodnicka and Burridge 1996; Kimura et al., 1996). Since in B16 melanoma cells cAMP up-regulation induces disassembly of these actin structures, we focused our attention to the putative role of the small GTP-binding protein Rho in cAMP-induced dendrite outgrowth in this melanocyte cell system.
Several tools are now available to study Rho. Among them we find the bacterial Clostridium difficile toxin B (Just et al., 1994, 1995), which inactivates the Rho family of small GTP-binding proteins (Rho, Rac, and Cdc42) by UDP-glucosylation, the Clostridium botulinum C3 ADP-ribosyl transferase, which specifically inactivates Rho (Rubin et al., 1988), and the E. coli cytotoxic necrotizing factor-1 (CNF-1) shown recently to constitutively activate this GTP-binding protein (Flatau et al., 1997). Using these toxins and overexpression of Rho mutants in transfected cells, here we present evidence that Rho inhibition is required for dendrite development in B16 melanoma cells. Furthermore we show that P160ROCK might function downstream of Rho to regulate dendricity in this cell system. Finally we present data suggesting that Rho inhibition leads to a stimulation of tyrosinase expression, indicating a connection between Rho and melanogenesis.
MATERIALS AND METHODS
Materials
DMEM, penicillin, streptomycin, IBMX, forskolin, α-MSH, BSA, protein A-Sepharose, 4-(2-aminoethyl)-benzene-sulfonyl fluoride (AEBSF), aprotinin, leupeptin, paraformaldehyde, NH4Cl, and Triton X-100 were purchased from Sigma (St. Louis, MO). FCS, lipofectamine, and optimem were from GIBCO-BRL (Gaithersburg, MD). Immunofluorescence mounting medium was from ICN (Costa Mesa, CA), and the DNA prep kit for transfecting was from Qiagen (Courtaboeuf, France).
Cell Culture
B16/F10 murine melanoma cells were cultured in DMEM with 10% FCS (100 IU/50 μg/ml of penicillin and streptomycin), in a humidified atmosphere containing 5% CO2 at 37°C.
Toxins
Toxin B and CNF-1 were incubated for at least 4 h to allow the toxins to penetrate the cells and have an action on Rho. The C3 chymera toxin (named ETA-C3) was constructed by adding the membrane binding and the translocation domains of the exotoxin A of Pseudomonas aeruginosa to the C3 exotransferase from Clostridium botulinum, and it was provided by S. Contamin (INSERM U452, Nice, France). This toxin penetrates very slowly into the cells and, therefore, cells were incubated for 60 h with ETA-C3.
Antibodies
The polyclonal anti-tyrosinase antibody PEP7 was purchased from Dr. V. Hearing (NIH, Bethesda, MD) and the dilution used was 1:400 or 1:4000 for immunofluorescence and Western blot, respectively; the anti-α-tubulin antibody (Amersham, Buckinghamshire, England) was used at 1:100 for immunofluorescence. Phalloidin-rhodamine (Molecular Probes, Leiden, Holland) was used at 1:250, and anti-myc monoclonal 9E10, kindly provided by Dr. Tanti (INSERM U.145, Nice), was used at 1 μg/ml concentration for immunodetections. The HRP- and FITC-conjugated secondary antibodies were from Dakopatts (Zurich, Switzerland) and were used at 1:4000 and 1:50 dilutions, respectively.
Plasmids
pEF-C3 was kindly given by Dr. R. Treisman (London, United Kingdom) (Hill et al., 1995). pEXVRhoA-V14, pEXVRhoA-N19, and pEXVRac-V12 were purchased from Dr. M. Symons (Qiu et al., 1995). The constitutively active version of P160ROCK (pCAGGS-p160ROCK) has been previously described (Ishizaki et al., 1997).
Immunofluorescence Microscopy
For immunofluorescence studies, B16 melanoma cells growing on glass coverslips (5000 cells/cm2) were briefly rinsed in PBS and fixed with methanol (−20°C) for 2 min to determine tyrosinase localization, or in 3% paraformaldehyde and then incubated with 50 mM NH4Cl and permeabilized with 0.1% Triton X-100 for 2 min for detection of cytoskeleton proteins. The primary antibody diluted in PBS containing 1% of BSA was applied for 40 min at 37°C, after which cells were washed for 10 min in PBS and exposed to the secondary antibody. FITC-conjugated antibodies or phalloidin-rhodamine was diluted in PBS containing 1% of BSA and applied for 30 min at 37°C. The coverslips were rinsed in PBS for 10 min and were observed and photographed with a Zeiss-Axiophot fluorescence microscope (Carl Zeiss, Thornwood, NY) by using Kodak T-Max 400 Iso film.
Transfection
For immunofluorescence analysis, B16 melanoma cells on glass coverslips in 12-well dishes were transfected using the lipofectamine method with 0.8 μg of the corresponding cDNA per well. After 6 h, the transfection medium was changed, and cells were incubated with or without forskolin for 12 h before immunofluorescence analysis. To test the effect of Rho on the tyrosinase promoter activity, B16 cells in 24-well dishes (40,000 cells) were cotransfected using lipofectamine with 0.35 μg of the test plasmid (empty vector, pEXVRho-V14 or pEXVRho-N19), 0.2 μg of the luciferase reporter plasmid containing a 2.2-kb fragment of the mouse tyrosinase promoter, and 0.05 μg of pCMVβGal to control the variability of the transfection efficiency. Luciferase assays were performed 48 h after transfection.
Luciferase Assays
Cells were washed with a saline phosphate buffer and lysed with a 25 mM Tris-phosphate (pH 7.8) buffer containing 1% Triton X-100, 2 mM EDTA, and 2 mM DTT. Soluble extracts were harvested and assayed for luciferase by using the luciferase assay system from Promega France (Charbonnières, France) and for β-galactosidase activity. The luminometer used was a MicroLumat LB 96P from EG&G Instruments (Evry, France) All transfections were repeated at least five times using different plasmid preparations and gave similar results.
Western Blot Analysis
For tyrosinase immunoblot detection, cells were lysed in phosphate buffer, pH 6.8, containing 1% (wt/vol) Triton X-100, 100 IU/ml aprotinin, and 1 mM AEBSF. The solubilized proteins (25 μg) were loaded onto 10% SDS-polyacrylamide gels and transferred onto nitrocellulose (Amersham, England). The membranes were saturated with 5% powdered milk in a saline buffer, and tyrosinase was detected with the polyclonal PEP7 antibody diluted 1:3000 in the saturation buffer, and with a secondary peroxidase-conjugated anti-rabbit antibody at a 1:4000 dilution. After the antibody incubation, three 10-min washes were performed using a solution containing 0.05% Triton X-100 and 0.5% powdered milk in a saline buffer. The blot was developed using the ECL system from Amersham.
RESULTS
Dendrite Outgrowth Precedes Melanogenesis in cAMP-induced Differentiation of B16 Melanoma Cells
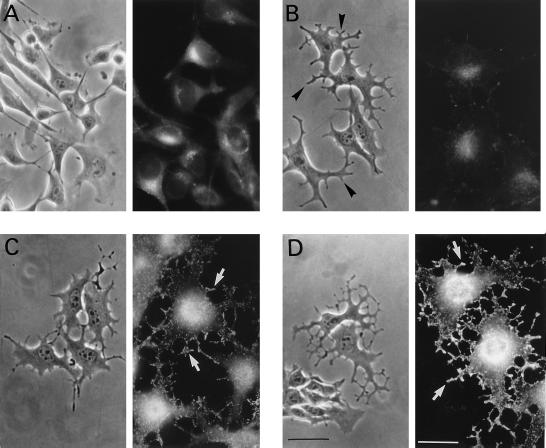
Phase contrast microscopy and immunofluorescence studies (using an anti-tyrosinase antibody) allowed us to evaluate the effects of the cAMP- elevating agent forskolin on cell morphology, tyrosinase expression, and melanosome redistribution (Figure 1). In growing conditions, B16 cells presented a flat fibroblastic appearance with basal tyrosinase levels mainly localized in the perinuclear area (Figure 1A). After a 30-min incubation with forskolin, dendricity considerably increased (see arrows in Figure 1B) while tyrosinase staining remained low. After 12 h of forskolin exposure, dendrite outgrowth was evident with increased tyrosinase expression becoming localized in melanosomes reaching the growing dendrites (Figure 1C). Forskolin incubation for 48 h induced strong cell dendricity and much higher tyrosinase expression, and melanosomes at the end of the dendrites appeared heavily labeled (Figure 1D). These observations indicate that the effect of cAMP up-regulation on dendrite formation is a quite rapid process preceding tyrosinase protein expression and melanosome relocalization that account for the late melanogenic event. It is worth mentioning that similar results were obtained using α-MSH instead of forskolin.
Figure 1.
Dendritogenesis, tyrosinase expression, and subcellular distribution upon cAMP treatment. B16 cells growing in serum-containing medium (A) were treated with 20 μM forskolin for 30 min (B), 12 h (C), and 48 h (D). Cells were visualized using phase contrast microscopy (image on the left) or fixed and labeled with the anti-tyrosinase antibody PEP7 and a FITC-conjugated secondary antibody (image on the right). Arrows in B indicate growing dendrites; arrows in C indicate tyrosinase labeling corresponding to melanosomes at the growing dendrites; arrows in D indicate strong tyrosinase-positive dendrites. Bar in phase contrast images, 80 μm; bar in immunofluorescence images, 40 μm.
cAMP Dendrite Outgrowth Is Associated with an Actin Cytoskeleton Reorganization
To study cytoskeleton modifications occurring upon dendrite outgrowth, we followed the behavior of the actin and microtubular networks during this cellular process. Immunofluorescence studies to detect F-actin and microtubules were performed. As shown in Figure 2, a–d, forskolin did not induce a major remodeling of the microtubular network in the cytosol; however, growing dendrites did contain organized microtubules. In nonstimulated cells (NS), actin appeared organized in numerous stress fibers crossing the cytoplasm (Figure 2A). In the presence of forskolin, these stress fibers were disassembled, leaving only phalloidin-labeled spots (punctate actin) (Figure 2, B–D). This event could be observed as early as 5 min after the addition of forskolin (Figure 2B) and became more evident after 1 h (Figure 2C) and 24 h (Figure 2D) of forskolin exposure. Western blot analysis to detect actin revealed that cAMP up-regulation did not affect the levels of actin protein (not shown). Thus, cAMP only promoted a rearrangement of the actin cytoskeleton.
Figure 2.
cAMP reorganizes the actin cytoskeleton in B16 melanoma cells. B16 melanoma cells growing in serum-containing medium (A and a) were stimulated with forskolin (20 μM) for 5 min (B and b), 1 h (C and c) or 24 h (D and d). F-actin was detected by using phalloidin-rhodamine (A, B, C, and D), and the microtubular network was visualized by an anti-α-tubulin antibody (a, b, c, and d). Arrow in A indicates stress fiber; arrow in B indicates early actin disorganization (punctate actin); arrows in C and c indicate growing dendrites with disorganized actin and positive tubulin labeling. Bar, 40 μm.
Rho Inhibition by C3 Transferase and Toxin B Induces Dendricity While Rho Constitutive Activation by CNF-1 Prevents cAMP Induction of the Dendritic Phenotype
Since Rho plays a crucial regulatory role in F actin reorganization, we thus studied the role of this GTP-binding protein in dendrite formation during cAMP-induced differentiation of B16 cells. First, we incubated cells with either the C. botulinum C3 exoenzyme, which specifically inhibits Rho by ADP-ribosylation (Figure 3, C and c), or with the C. difficile toxin B, which blocks Rho, Rac, and Cdc42 (Figure 3, D and d) to evaluate effects of Rho inhibition on cell shape (seen by phase-contrast microscopy) (Figure 3, A–D) and actin cytoskeleton organization (Figure 3, a–d). Both toxins induced a disorganization of the actin cytoskeleton characterized by the disruption of stress fibers (Figure 3, C and c and D and d), similar to the effects driven by forskolin (Figure 3, B and b). Furthermore, we observed membrane outgrowth resembling dendrites, indicating that Rho inhibition is sufficient to induce dendrite formation. Then CNF-1 was used to constitutively activate Rho as shown in Figure 3 (E and e). CNF-1 did not alter the overall basal shape of B16 cells, but increased stress fiber formation. When cells were pretreated with CNF-1 for 3 h and then stimulated for 30 min with forskolin (Figure 3, F and f), no dendrite formation was detected, indicating that constitutive Rho activation prevents cAMP-promoted dendrite outgrowth.
Figure 3.
Toxin B and C3 induce dendricity, and CNF-1 inhibits cAMP-induced dendrite outgrowth. B16 melanoma cells growing in serum-containing medium (A and a) were treated with 20 μM forskolin for 15 min (B and b), with 10−7 M of the chimeric toxin ETA-C3 for 60 h (C and c), with 0.5 ng/ml of toxin B for 4 h, (D and d), with 10−7 M CNF-1 for 5 h (E and e), and finally simultaneously with forskolin and CNF-1 (F and f). Then cells were photographed using phase contrast microscopy (A, B, C, D, E, and F) or were labeled with rhodamine-phalloidin to detect F-actin (a, b, c, d, e, and f). Bar in phase contrast images, 80 μm; bar in actin images, 40 μm.
Rho-V14 Blocks Dendrite Induction by cAMP Elevating Agents
To further confirm the specific role of Rho in dendritogenesis, we overexpressed Rho-V14 (constitutively active) tagged with the epitope myc, by cell transfection. Transfected cells were left untreated or were treated with forskolin. Rho-V14–expressing cells were visualized with the anti-myc 9E10 antibody (Figure 4A, a and c), and the actin cytoskeleton was labeled with phalloidin-rhodamine (Figure 4A, b and d). In the absence of forskolin, RhoV14-expressing cells presented a flattened fibroblastic shape similar to nontransfected cells and, as expected, actin was organized in stress fibers (Figure 4A, a and b). When treated with forskolin, RhoV14-positive cells retained their flattened fibroblastic appearance and exhibited stress fibers (Figure 4A, c and d), while nontransfected cells showed their usual dendritic morphology and punctate actin distribution (Figure 4A, d). Therefore, the specific constitutive activation of Rho prevented forskolin induction of the dendritic phenotype. A dominant negative form of Rho, Rho-N19, was also transfected. Inhibition of Rho by overexpression of Rho-N19 promoted a dendritic-like morphology mimicking the effect of cAMP-elevating agents (data not shown).
Figure 4.
Rho-V14 overexpression, as well as Rac-V12 overexpression, prevents forskolin-induced dendricity. (A) B16 melanoma cells were transiently transfected with an expression plasmid encoding the constitutively active mutant of RhoA (pEXVRhoA-V14) tagged with the myc epitope. After transfection, cells were incubated in serum containing medium (NS; a and b) or in serum containing medium with 20 μM forskolin (FK; c and d). Cells were then labeled with the monoclonal anti c-myc antibody 9E10 to detect Rho-V14-expressing cells (a and c) or with phalloidin-rhodamine to detect the F-actin (b and d). Arrows in c and d, Rho-V14–expressing cells. Bar, 60 μm. (B) Cells were transfected with a vector encoding the constitutive active form of Rac (pEXVRac-V12) tagged with the myc epitope. Cells were then incubated in serum containing medium (NS; a and b) or in serum containing medium with 20 μM forskolin (FK; c and d). Cells were then labeled with the monoclonal anti c-myc antibody 9E10 to detect Rac-V12-expressing cells (a and c) or with phalloidin-rhodamine to detect the F-actin (b and d). Arrows in b and d indicate cortical actin in lamellipodia structures in Rac-V12–expressing cells. Bar, 40 μm.
It has been reported that Rac, a small GTP-binding protein of the Rho family, activates Rho (Nobes and Hall, 1995). We thus wanted to assess whether Rac could also be involved in cAMP-induced dendricity. Hence, we transfected B16 cells with the constitutive active mutant of Rac (Rac-V12) tagged with the myc epitope. We observed that the expression of Rac-V12 induced lamellipodia formation and blocked the dendritic phenotype and actin disorganization induced by forskolin (Figure 4B, c and d).
Taken together, these experiments show that inhibition of Rho promotes dendrite outgrowth and that cAMP-induced dendritogenesis can be prevented by the constitutive activation of Rho or Rac.
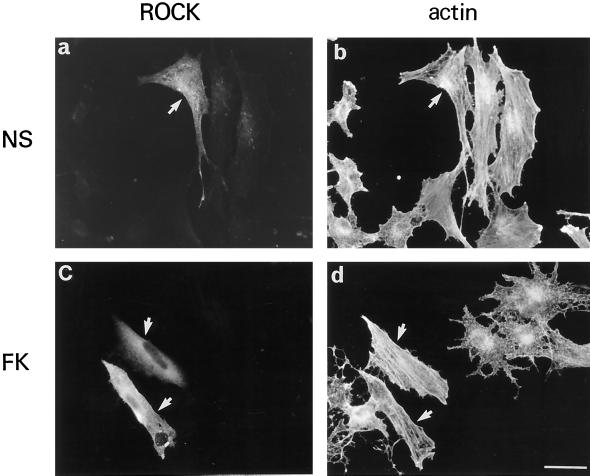
P160ROCK Is a Downstream Target of Rho Involved in B16 Cell Dendritogenesis
To investigate possible mechanisms that could mediate the action of Rho inhibition on dendrite formation upon cAMP exposure, we studied the role of the downstream Rho effector P160ROCK. Overexpression of the constitutive active mutant of P160ROCK (Ishizaki et al., 1997) resulted in elongated cells presenting numerous stress fibers (Figure 5). Upon forskolin treatment (Figure 5, b and d), P160ROCK-positive cells exhibited a nondendritic shape with stress fibers resembling control cells. The surrounding cells, which did not express P160ROCK, however, showed a dendritic morphology and the disorganization of stress fibers. This demonstrates that P160ROCK constitutive activation leads to an actin organization in stress fibers and blocks dendrite outgrowth induced by cAMP up-regulation.
Figure 5.
Constitutively active P160ROCK overexpression prevents dendrite outgrowth. Growing B16 cells were transiently transfected with the pCAGGS vector containing the cDNA encoding a constitutively active form of P160ROCK tagged with the myc epitope. After transfection, cells were incubated in fresh medium (NS; a and b) or fresh medium containing 20 μM forskolin (FK; c and d) for 12 h. Then cells were stained with the anti-myc antibody 9E10 to detect P160ROCKexpressing cells (a and c) or with phalloidin-rhodamine to detect F-actin (b and d). Arrows indicate active P160ROCK expressing cells. Bar, 30 μm.
Rho Inhibition Is Not Only Required for Dendricity but Plays a Role in Melanogenesis Regulation
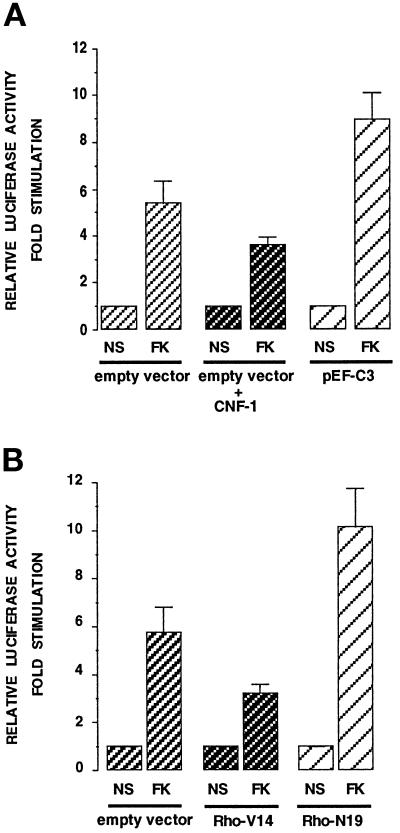
There is accumulating evidence that Rho family proteins do not only regulate cell morphology and actin organization but can also regulate gene expression (Hill et al., 1995). We therefore studied whether Rho could be involved in melanogenesis regulation, which is controlled by the tyrosinase enzyme. Effects of toxins on Rho mutants on the activity of the tyrosinase promoter were measured (Figure 6). An empty vector or an exoenzyme C3-expressing plasmid were cotransfected with a reporter plasmid containing a 2.2-kb fragment 5′ of the transcriptional start site of the mouse tyrosinase gene cloned upstream of the luciferase gene (2.2 MT-luc). Forskolin increased the luciferase activity reflecting a stimulation of the tyrosinase promoter activity, as described (Bertolotto et al., 1996). Cotransfection with the C3 exoenzyme encoding plasmid enhanced significantly the stimulation induced by forskolin (Figure 6A). Conversely, CNF-1-cell treatment decreased the stimulation of the promoter activity induced by forskolin (Figure 6A). Consistent results were obtained when the reporter plasmid 2.2 MT-luc was cotransfected with Rho mutants (Figure 6B). The constitutively active mutant of Rho (Rho-V14) reduced the stimulation evoked by forskolin, while the dominant negative mutant, Rho-N19, significantly increased the promoter activity induced by the cAMP. These results indicate that Rho is involved in the transcriptional regulation of tyrosinase.
Figure 6.
Bacterial toxins that act on Rho and Rho mutants modulate the activity of the tyrosinase promoter. (A) B16 cells were cotransfected with the 2.2 MT-luc reporter plasmid together with an empty expression vector or a plasmid encoding the C3 exoenzyme (pEF-C3) and then incubated in the absence (NS) or in the presence of 20 μM forskolin for 48 h (FK). When indicated, CNF-1 was added. (B) B16 cells were cotransfected with either an empty expression vector or with a constitutive active version of Rho (Rho-V14) or a dominant negative Rho mutant (Rho-N19); in each case cells were left in growing medium (NS) or treated with forskolin (FK) for 48 h. Forty-eight hours after transfection, soluble extracts were harvested and assayed for luciferase activity. Data are expressed in fold stimulation of the basal tyrosinase activity in each transfection case. Data are means ± SE of five experiments performed in triplicate.
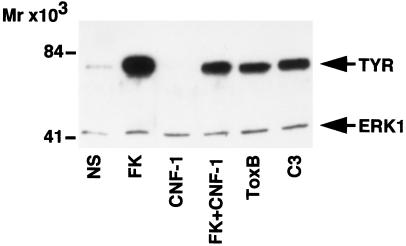
To provide more direct evidence that Rho plays a role in the regulation of melanogenesis, we tested the effect of toxins acting on Rho, on the expression of the endogenous tyrosinase protein in B16 cells (Figure 7). Western blot experiments showed that toxin B and C3 increased the amount of tyrosinase protein, thereby mimicking the effect of forskolin. In contrast, CNF-1 diminished the basal and the forskolin-induced increase of tyrosinase. These observations reveal that modulation of Rho activity can affect tyrosinase expression and therefore melanin synthesis.
Figure 7.
Toxins that act on Rho modify tyrosinase protein expression. Solubilized proteins (20 μg) from nonstimulated (NS) growing cells and cells treated for 84 h with forskolin (FK), CNF-1, forskolin together with CNF-1, toxin B (toxB), and with the C3 exoenzyme (ETA-C3) were subjected to Western blot analysis using the PEP7 antibody for detection of tyrosinase (TYR). The blot was simultaneously incubated with an anti-ERK1 antibody to show that each electrophoretic lane was loaded with the same amount of protein. Molecular masses indicated on the left are expressed in kilodaltons.
DISCUSSION
During cAMP-induced differentiation of B16 melanoma cells we observed rapid cell shape alterations characterized by the development of numerous dendrites. To characterize the nature of such changes, we analyzed the behavior of the actin cytoskeleton and the microtubular network upon differentiation. Experimental evidence revealed that microtubules are not significantly altered by cAMP-elevating agents but they invade new growing dendrites, suggesting their active role in the development of these cellular structures. Examination of the actin cytoskeleton showed that dendrite outgrowth is accompanied by a strong disorganization of the actin cytoskeleton leading to stress fiber disruption and appearance of a punctate form of actin. The striking effect of cAMP on actin organization has led us to propose that the retraction of actin stress fibers could be a required early event that, together with a role of microtubules, would be essential to construct new dendritic structures. Indeed, an alternation of actin- and tubulin polymerization-dependent processes in neurite development (Joshi et al., 1985; Dennerl et al., 1988; Gordon-Weeks, 1991) and in the polarity of retinal photoreceptors (Madreperla and Adler, 1989) have already been described.
Stress fiber formation has been shown to be controlled by the small GTP-binding protein Rho (Ridley and Hall, 1992); we thus studied the role of Rho in the dendritogenic process of B16 melanoma cells. Inhibition of Rho activity, either by toxin B, by the C3 exoenzyme, or by a dominant negative mutant of Rho, promoted dendrite development and actin disorganization, mimicking effects of cAMP-elevating agents. Furthermore, CNF-1, which activates Rho, or the overexpression of a constitutively active form of Rho, Rho-V14, were able to prevent dendritogenesis and actin disorganization induced by forskolin. Studies made in N1E-115 neuroblastoma and PC12 cells have shown consistently that LPA and thrombin, which activate Rho, as well as Rho-V14, cause neurite retraction and cell rounding, while C3 treatment inhibits thrombin- and LPA-induced generation of actomyosin-based contractile forces and therefore induces neurite expansion (Rubin et al., 1988; Gebbink et al., 1997). Therefore, our results, in agreement with those data suggest that Rho inhibition is a required event in the development of dendrite/neurite structures. Further, the small GTP-binding protein Rac has been shown to promote stress fiber formation through Rho activation (Nobes and Hall., 1995). In B16 cells, overexpression of a constitutive active mutant of Rac blocked forskolin-induced dendricity, suggesting that cAMP could inhibit Rac, thus leading to Rho inhibition and stress fiber disorganization.
It is clear from our study that inhibition of Rho leading to dendritogenesis can be induced by a physiological stimulus such as α-MSH, which acts on the cAMP pathway. Little information is available concerning the cAMP/PKA action on the actin cytoskeleton and cell shape changes. According to Ridley and Hall (1994), the increase of the intracellular cAMP levels did not prevent formation of new stress fibers in Swiss 3T3 cells; however, our results are in agreement with those of Lamb et al. (1988) who previously described that cAMP promotes the disassembly of these preexisting actin structures.
To further dissect steps connecting Rho and stress fiber disruption, we focused our attention on the recently described Rho-kinase P160ROCK (Amano et al., 1996; Ishizaki et al., 1996). Overexpression of a constitutive active form of P160ROCK was able to revert the cAMP-induced dendritic phenotype, indicating that inhibition of the kinase, due to Rho inactivation by cAMP, could play a role in dendrite formation. Inactivation of P160ROCK would decrease myosin light chain phosphorylation, leading to a decrease in actin bundling and consequently to stress fiber disassembly.
Some other targets of Rho have been identified, among them PKN, Citron, Rhotekin, and Rhophilin (reviewed by Tapon and Hall, 1997). We cannot eliminate the possibility that some of these Rho targets could also participate in the cAMP and Rho action to induce dendrite outgrowth in the melanocyte cell system.
Together with dendrite formation, melanin synthesis is the second feature characterizing melanocyte and melanoma cell differentiation. Using toxins and Rho mutants, we show that Rho inhibition by itself induces tyrosinase protein expression and enhances the effect of cAMP on the mouse tyrosinase promoter activity. Rho constitutive activation, on the other hand, decreases the melanogenic effect promoted by the cAMP signaling. The mechanisms by which Rho regulates tyrosinase expression remain puzzling. P160ROCK does not seem to affect tyrosinase expression (our unpublished observations), suggesting that some other Rho targets mentioned above could mediate the effect of Rho on melanogenesis. Up to date, a clear role of Rho in cell differentiation has not been stated; however, Rho has been recently implicated in early thymic development (Henning et al., 1997) and Hill et al. (1995) have shown that it plays a role in growth control and mitogenesis. Hence, from our results, we may propose that Rho inhibition would result in a decreased cell growth and would commit the melanocyte cell in its differentiation program, thereby leading to the up-regulation of melanogenesis. It is worth mentioning that the involvement of Rho in tyrosinase regulation is less pronounced compared with its role on cytoskeleton rearrangements during B16 cell differentiation. While CNF or Rho-V14 overexpression completely block the dendritic effect of cAMP-elevating agents, Rho constitutive activation modestly decreases the tyrosinase stimulation promoted by these agents. This result suggests that Rho can participate in the mechanisms activated by cAMP to induce melanogenesis. The regulation of this molecular event, however, is probably constituted by a complex system involving several other signaling pathways, such as the PI3-K/p70S6K pathway and/or the MAP kinase cascade, as we have previously reported (Englaro et al., 1995; Buscàet al., 1996).
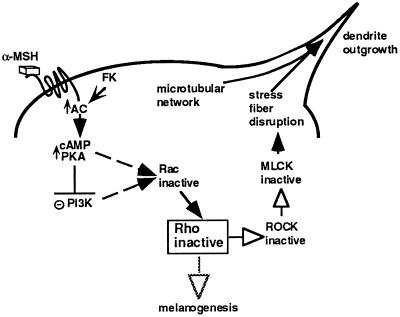
According to our results, the cAMP pathway inhibits Rho to promote stress fiber disruption and dendrite formation in the melanocyte cell system. However, the molecular events connecting cAMP up-regulation to Rho remain to be elucidated. We have previously demonstrated that cAMP inhibits PI3-K and that PI3-K inhibition induces dendrite formation in these cells. Even though there is currently no demonstration that PI3-K activates Rho, we have to take into account that PI3-K can activate Rac (Hawkins et al., 1995). In addition, Rac has been shown to activate Rho (Nobes and Hall, 1995). Considering these elements, we can propose a model to explain possible mechanisms of cAMP induction of melanocyte differentiation (Figure 8) in which cAMP would trigger a sequential inhibition of PI3-K, Rac, Rho, and P160ROCK that would lead to stress fiber disassembly. The relaxation of the actin cytoskeleton would allow other cytoskeleton rearrangements involving the microtubular network to induce dendrite outgrowth. Concomitantly, Rho inhibition by cAMP might be partially responsible for the increased tyrosinase expression and melanogenesis induction. The fine coordination of both events would cooperate to promote the distribution of melanin.
Figure 8.
Model of cAMP action on Rho to induce dendrite outgrowth and melanogenesis in B16 melanoma cells. According to our results we propose a model to explain possible mechanisms of cAMP-induced differentiation in the melanocyte cell system. Up-regulation of the cAMP pathway by α-MSH binding to its receptor or by cAMP-elevating agents would lead to the sequential inactivation of PI3-K, Rac, Rho, and its target P160ROCK. Inhibition of P160ROCK results in MLCK inactivation. Inactive MLCK does not phosphorylate MLC, thus impairing myosin binding to actin and preventing actin bundling in stress fibers. The retraction of stress fibers would lead to other cellular events, depending on the microtubular network, to promote the final dendrite outgrowth in B16 melanoma cells. At the same time, inactive Rho would cooperate with other molecular mechanisms in the induction of tyrosinase expression leading to melanogenesis. AC, adenylyl cyclase; FK, forskolin; PKA, protein kinase A; PI3-K, phosphatidylinositol 3-kinase; MLCK, myosin light chain kinase.
In the absence of a reliable technique to examine the nucleotide state of Rho, we could not demonstrate the inhibition of this small GTP-binding protein by cAMP. Thus, we cannot definitively rule out the possibility that the effects of cAMP on actin organization are mediated by parallel pathways and that Rho constitutive activation overrides these effects. Nevertheless, in this report we have gathered compelling results that strongly suggest that cAMP inhibits stress fibers through Rho inactivation. Which molecules act between cAMP and Rho as well as which other cAMP targets are involved in B16 cell dendricity constitute major questions that will require further and accurate research.
Acknowledgments
We thank Stephanette Contamin for providing the C3 exoenzyme, Dr. V. Hearing for the antibody anti-tyrosinase PEP7, Dr. M. Symons for the Rho mutants, and Dr. R. Treisman for the C3 plasmid. We are grateful to C. Minghelli for his excellent work with the illustrations, and we thank Dr. Kim Boulukos for critical reading of this manuscript. This work was supported by the Center de Recherche et d’Investigations Epidermiques et Sensorielles (C.E.R.I.E.S.), the Association pour la Recherche sur le Cancer (grant 9402), La Ligue Contre le Cancer, Fondation de France, Fondation pour la Recherche Medicale, Institut National de la Santé et de la Recherche Médicale and Université de Nice Sophia-Antipolis. R. Buscà was supported by a postdoctoral fellowship from the Spanish government.
Footnotes
Abbreviations used: AEBSF, 4-(2-aminoethyl)benzenesulphonyl fluoride; α-MSH, α-melanocyte–stimulating hormone; CNF-1, cytotoxin necrotizing factor-1; ERK1, extracellular signal regulated kinase 1; MLC, myosin light chain; MLCK, myosin light chain kinase; p70S6K, p70S6-kinase; p160ROCK, Rho-associated coiled-coil containing protein kinase; PI3-K, phosphatidylinositol 3-kinase.
REFERENCES
- Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as the serine-threonine kinase protein kinase N. Science. 1996;271:648–650. doi: 10.1126/science.271.5249.648. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20240. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Bertolotto C, Bille K, Ortonne J-P, Ballotti R. Regulation of tyrosinase gene expression by cAMP in B16 melanoma cells involves two CATGTG motifs surrounding the TATA box: implication of the microphtalmia gene product. J Cell Biol. 1996;134:747–755. doi: 10.1083/jcb.134.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscà R, Bertolotto C, Ortonne J-P, Ballotti R. Inhibition of the phosphatidylinositol 3-kinase/p70S6-kinase pathway induces B16 melanoma cell differentiation. J Biol Chem. 1996;271:31824–31830. doi: 10.1074/jbc.271.50.31824. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennerl TJ, Joshi HC, Steel VL, Buxbaum RE, Heidemann SR. Tension and compression in the cytoskeleton of PC-12 neurites. II. Quantitative measurements. J Cell Biol. 1988;107:665–674. doi: 10.1083/jcb.107.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englaro W, Rezzonico R, Durand-Clément M, Lallemand D, Ortonne J-P, Ballotti R. Mitogen-activated protein kinase pathway and AP-1 are activated during cAMP-induced melanogenesis in B-16 melanoma cells. J Biol Chem. 1995;270:24315–24320. doi: 10.1074/jbc.270.41.24315. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick TD, Szabo G, Seiji M, Quevedo WC. Biology of the Melanin Pigmentary System. 1979. [Google Scholar]
- Flatau G, Lemichez E, Gauthier M, Chardin P, Paris S, Fiorentini C, Boquet P. Toxin-induced activation of the G protein Rho by deamidation of glutamine. Nature. 1997;387:729–733. doi: 10.1038/42743. [DOI] [PubMed] [Google Scholar]
- Gebbink M, Kranenburg O, Poland M, van Horck F, Houssa B, Moolenaar WH. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J Cell Biol. 1997;137:1603–1613. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrest BA, Park H-Y, Eller MS, Yaar M. Mechanisms of ultraviolet light-induced pigmentation. Photochem Photobiol. 1996;63:1–10. doi: 10.1111/j.1751-1097.1996.tb02988.x. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks P. Evidence for microtubule capture by filopodial actin filaments in growth cones. Neuroreport. 1991;2:573–576. doi: 10.1097/00001756-199110000-00005. [DOI] [PubMed] [Google Scholar]
- Halaban R, Pomerantz SH, Marshall S, Lambert DT, Lerner AB. Regulation of tyrosinase in human melanocytes grown in culture. J Cell Biol. 1983;97:480–488. doi: 10.1083/jcb.97.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P, Eguinoa A, Qiu R-G, Stokoe D, Cooke F-T, Walters R, Wennström S, Claesson-Welsh L, Evans T, Symons M, Stephens PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- Hearing VJ, Jimenez M. Analysis of mammalian pigmentation at the molecular level. Pigm Cell Res. 1989;2:75–85. doi: 10.1111/j.1600-0749.1989.tb00166.x. [DOI] [PubMed] [Google Scholar]
- Hearing VJ, Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991;5:2902–2909. [PubMed] [Google Scholar]
- Henning S-W, Galandrini R, Hall A, Cantrell DA. The GTPase Rho has a critical regulatory role in thymus development. EMBO J. 1997;16:2397–2407. doi: 10.1093/emboj/16.9.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CS, Wynne J, Treisman R. The Rho Family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Hunt G, Todd C, Cresswell JE, Thody AJ. α-MSH and its analog Nle4DPhe7α-MSH affect morphology, tyrosinase activity and melanogenesis in cultured human melanocytes. J Cell Sci. 1994;107:205–211. doi: 10.1242/jcs.107.1.205. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Joshi HC, Chu D, Buxbaum RE, Heidemann SR. Tension and compression in the cytoskeleton of PC-12 neurites. J Cell Biol. 1985;101:697–705. doi: 10.1083/jcb.101.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just I, Fritz G, Aktories K, Giry M, Popoff MR, Boquet P, Hegenbarth S, Von Eichel-Streiber C. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J Biol Chem. 1994;269:10706–10712. [PubMed] [Google Scholar]
- Just I, Selzer J, Wilm M, Von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho-associated kinase (Rho kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Lamb NJC, Fernandez A, Conti MA, Adelstein DB, Glass DB, Welch WJ, Feramisco JR. Regulation of actin microfilament integrity in living nonmuscle cells by cAMP-dependent protein kinase and the myosin light chain kinase. J Cell Biol. 1988;106:1955–1971. doi: 10.1083/jcb.106.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. The Neural Crest. Cambridge, United Kingdom: Cambridge University Press; 1982. [Google Scholar]
- Leung T, Chen X-Q, Manser E, Lim L. The p160 RhoA-binding kinase ROKα is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madreperla SA, Adler R. Opposing microtubule- and actin-dependent forces in the development and maintenance of structural polarity in the retinal photorecepteur. Dev Biol. 1989;131:149–160. doi: 10.1016/s0012-1606(89)80046-6. [DOI] [PubMed] [Google Scholar]
- Matsui Y, Amano M, Yamamoto T, Chihara K, Nakafu M, Ito M, Nakano K, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for the small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Qiu R-G, Chen J, Mc Cormick F, Symons M. A role for Rho in Ras transformation. Proc Natl Acad Sci USA. 1995;92:11781–11785. doi: 10.1073/pnas.92.25.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A, Hall A. The small GTP-binding protein Rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. Signal transduction pathways regulating Rho-mediated stress fiber formation: requirement for a tyrosine kinase. EMBO J. 1994;13:2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin EJ, Gill M, Boquet P, Popoff MR. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988;8:418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Wong G, Pawelek J. Melanocyte-stimulating hormone promotes activation of pre-existing tyrosinase molecules in Cloudman S91 melanoma cells. Nature. 1975;255:644–645. doi: 10.1038/255644a0. [DOI] [PubMed] [Google Scholar]