Abstract
Background
Various factors can modify the health effects of outdoor air pollution. Prior findings about modifiers are inconsistent, and most of these studies were conducted in developed countries.
Objectives
We conducted a time-series analysis to examine the modifying effect of season, sex, age, and education on the association between outdoor air pollutants [particulate matter < 10 μm in aerodynamic diameter (PM10), sulfur dioxide, nitrogen dioxide, and ozone] and daily mortality in Shanghai, China, using 4 years of daily data (2001–2004).
Methods
Using a natural spline model to analyze the data, we examined effects of air pollution for the warm season (April–September) and cool season (October–March) separately. For total mortality, we examined the association stratified by sex and age. Stratified analysis by educational attainment was conducted for total, cardiovascular, and respiratory mortality.
Results
Outdoor air pollution was associated with mortality from all causes and from cardiorespiratory diseases in Shanghai. An increase of 10 μg/m3 in a 2-day average concentration of PM10, SO2, NO2, and O3 corresponds to increases in all-cause mortality of 0.25% [95% confidence interval (CI), 0.14–0.37), 0.95% (95% CI, 0.62–1.28), 0.97% (95% CI, 0.66–1.27), and 0.31% (95% CI, 0.04–0.58), respectively. The effects of air pollutants were more evident in the cool season than in the warm season, and females and the elderly were more vulnerable to outdoor air pollution. Effects of air pollution were generally greater in residents with low educational attainment (illiterate or primary school) compared with those with high educational attainment (middle school or above).
Conclusions
Season, sex, age, and education may modify the health effects of outdoor air pollution in Shanghai. These findings provide new information about the effects of modifiers on the relationship between daily mortality and air pollution in developing countries and may have implications for local environmental and social policies.
Keywords: air pollution, modifiers, mortality, time-series studies
Epidemiologic studies have reported associations of outdoor air pollution with daily mortality and morbidity from cardiorespiratory diseases (Goldberg et al. 2003). Multicity analyses conducted in the United States, Canada, and Europe provide further evidence supporting coherence and plausibility of the associations (Burnett et al. 2000; Dominici et al. 2006; Katsouyanni et al. 1997, 2001; Samet et al. 2000a). Recently, interest has been focused on the possible modifying effect of season (Peng et al. 2005; Touloumi et al. 2006; Zeka et al. 2006), preexisting health status (Bateson and Schwartz 2004; Goldberg et al. 2001; Katsouyanni et al. 2001), and population demographic characteristics such as sex and age (Atkinson et al. 2001; Bateson and Schwartz 2004; Cakmak et al. 2006; Katsouyanni et al. 2001) on the relation between air pollution and daily mortality. It is also hypothesized that the effects of air pollution exposure on health are greater in people with lower socioeconomic status (SES) (O’Neill et al. 2003). However, prior findings about the modifying effect of SES remain inconsistent: some studies found evidence of modification (Finkelstein et al. 2003; Jerrett et al. 2004; Krewski et al. 2005; Zeka et al. 2006), but others did not (Bateson and Schwartz 2004; Cakmak et al. 2006; Samet et al. 2000b; Zanobetti and Schwartz 2000). Moreover, most of these studies were conducted in developed countries, and only a small number of studies have been conducted in Asia (Health Effects Institute 2004). The need remains for studies of cities in developing countries, where characteristics of outdoor air pollution (e.g., air pollution level and mixture, transport of pollutants), meteorological conditions, and sociodemographic patterns may differ from those in North America and Europe.
Better knowledge of these modifying factors will help in public policy making, risk assessment, and standard setting, especially in cities of developing countries with fewer existing studies. In the present study, we conducted a time-series analysis to examine the modifying effect of season, sex, age, and education on the association between outdoor air pollutants [particulate matter < 10 μm in diameter (PM10), sulfur dioxide, nitrogen dioxide, and ozone] and daily mortality in Shanghai, China. This study is a part of the joint Public Health and Air Pollution in Asia (PAPA) program supported by the Health Effects Institute (HEI).
Materials and Methods
Data
Shanghai, the most populous city in China, comprises urban/suburban districts and counties, with a total area of 6,341 km2 and had a population of 13.1 million by the end of 2004. Our study area was limited to the traditional nine urban districts of Shanghai (289 km2). The target population includes all permanent residents living in the area—around 6.3 million in 2004. In the target population, the male/female ratio was 100.9%, and the elderly (> 65 years of age) accounted for 11.9% of the total population.
Daily nonaccidental mortality data from 1 January 2001 to 31 December 2004 were collected from the database of the Shanghai Municipal Center of Disease Control and Prevention (SMCDCP). Death certificates are completed either by community doctors for deaths at home or by hospital doctors for deaths in hospitals. The information on the certificates is then sent to the SMCDCP through their internal computer network. In Shanghai, all deaths must be reported to appropriate authorities before cremation. The database for 2001 and 2002–2004 was coded according to the International Classification of Diseases, Revision 9 [ICD-9; World Health Organization (WHO) 1978] and Revision 10 (ICD-10; WHO 1993), respectively. The mortality data were classified into deaths due to all nonaccidental causes (ICD-9 codes < 800; ICD-10 codes A00–R99), cardiovascular diseases (ICD-9 codes 390–459; ICD-10 codes I00–I99), and respiratory diseases (ICD-9 codes 460–519; ICD-10 codes J00–J98). The data were also classified by sex and age (0–4, 5–44, 45–64, and ≥ 65 years) for all-cause deaths. Educational attainment has often been used as a surrogate indicator of SES in time-series studies (Cakmak et al. 2006; Jerrett et al. 2004; Zanobetti and Schwartz 2000; Zeka et al. 2006). We therefore classified all-cause, cardiovascular, and respiratory deaths by educational attainment (low, illiterate or primary school; high, middle school or above).
Daily air pollution data, including PM10, SO2, NO2, and O3, were retrieved from the database of the Shanghai Environmental Monitoring Center, the government agency in charge of collection of air pollution data in Shanghai. The daily concentrations for each pollutant were averaged from the available monitoring results of six fixed-site stations in the nine urban districts and covered by China National Quality Control. These stations are mandated to be located away from major roads, industrial sources, buildings, or residential sources of emissions from the burning of coal, waste, or oil; thus, our monitoring results reflect the background urban air pollution level in Shanghai rather than local sources such as traffic or industrial combustion.
We abstracted the daily 24-hr mean concentrations for PM10, SO2, and NO2, and maximal 8-hr mean concentrations for O3. The maximal 8-hr mean was used because the WHO (2000) recommended that the 8-hr mean reflects the most health-relevant exposure to O3. For the calculation of both 24-hr mean concentrations of PM10, SO2, and NO2, as well as maximal 8-hr mean O3 concentrations, at least 75% of the 1-hr values must have been available on that particular day.
To allow adjustment for the effect of weather conditions on mortality, we obtained daily mean temperature and humidity data from the Shanghai Meteorological Bureau database. The weather data were measured at a single fixed-site station in the Xuhui District of Shanghai.
All of the mortality, weather, and air pollution data were validated by an independent auditing team assigned by the HEI. The team checked a sample of the original death certificates and monitoring records and validated the generation process of mortality, weather, and air pollution data used for the time-series analysis.
Statistical methods
Our statistical analysis followed the Common Protocol of the PAPA program. We used a generalized linear model (GLM) with natural splines (ns) to analyze the data. First, we built the basic models for various mortality outcomes excluding the air pollution variables. We incorporated the ns functions of time and weather conditions, which can accommodate nonlinear and non-monotonic relationships of mortality with time and weather variables, offering a flexible modeling tool (Hastie and Tibshirani 1990). We used the partial autocorrelation function (PACF) to guide the selection of degrees of freedom (df) for time trend (Katsouyanni et al. 2001; Touloumi et al. 2004, 2006). Specifically, we used 4–6 df per year for time trend. When the absolute magnitude of the PACF plot was < 0.1 for the first two lag days, the basic model was regarded as adequate; if this criterion was not met, autoregression terms for lag up to 7 days were introduced to improve the model. In this way, 4, 4, and 5 df per year for time trend, as well as 3, 2, and 4 lag-day autoregression terms, were used in our basic models for total, cardiovascular, and respiratory mortality, respectively. In addition, we used 3 df (whole period of study) for temperature and humidity because this has been shown to control well for their effects on mortality (Dominici et al. 2006; Samet et al. 2000a). Day of the week was included as a dummy variable in the basic models. We examined residuals of the basic models to determine whether there were discernable patterns and autocorrelation by means of residual plots and PACF plots. After we established the basic models, we introduced the pollutant variables and analyzed their effects on mortality outcomes.
Briefly, we fit the following log-linear GLM to obtain the estimated pollution log-relative rate β in Shanghai:
 |
where E(Yt) represents the expected number of deaths at day t; β represents the log-relative rate of mortality associated with a unit increase of air pollutants; Zt indicates the pollutant concentrations at day t; DOW is dummy variable for day of the week; ns(time,df) is the ns function of calendar time; and ns(temperature/humidity, 3) is the ns function for temperature and humidity with 3 df. Current-day temperature and humidity (lag 0) and 2-day moving average of air pollutant concentrations (lag 01) were used in our analyses.
We assessed both total nonaccidental and cause-specific mortality. We were able to stratify by sex and age only for total mortality. We analyzed effects of air pollution separately for the warm season (April–September) and the cool season (October–March) as well as for the entire year (Peng et al. 2005; Touloumi et al. 2006). The basic models of seasonal analyses were different from those of whole-period analyses, using various dfs for time trend. Analyses by educational attainment were conducted for total, cardiovascular, and respiratory mortality. We tested the statistical significance of differences between effect estimates of the strata of a potential effect modifier (e.g., the difference between females and males) by calculating the 95% confidence interval (CI) as
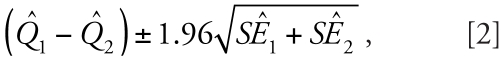 |
where Q̂1 and Q̂2 are the estimates for the two categories, and SÊ1 and SÊ2 are their respective SEs (Zeka et al. 2006). Regardless of significance, we considered modification of effect by a factor of ≥ 2 to be important and worthy of attention (Zeka et al. 2006).
As a sensitivity analysis, we also examined the impact of model specifications such as lag structure and df selection on the effects of air pollutants (Welty and Zeger 2005). We did not find substantial differences using alternative specifications.
All analyses were conducted in R, version 2.5.1, using the mgcv package (R Development Core Team 2007). The results are presented as the percent change in daily mortality per 10-μg/m3 increase of air pollutants.
Results
Data description
From 2001 to 2004 (1,461 days), a total of 173,911 deaths (82,597 females and 91,314 males) were registered in the study population. The percentages of total deaths by age group were 0.3% for 0–4 years, 3.2% for 5–44 years, 13.0% for 45–64 years, and 83.5% for ≥ 65 years. On average, there were approximately 119 nonaccidental deaths per day, including 44 from cardiovascular diseases and 14 from respiratory diseases (Table 1). Cardiorespiratory disease accounted for 49.1% of total nonaccidental deaths.
Table 1.
Daily deaths, air pollutant concentrations, and weather conditions (mean ± SE) in Shanghai, China, 2001–2004.
| Warm season (n = 729) | Cool season (n = 732) | Entire period (n = 1,461) | |
|---|---|---|---|
| No. of daily deaths | |||
| Total (nonaccident) | 106.1 ± 0.5 | 132.0 ± 0.8 | 119.0 ± 0.6 |
| Cardiovascular | 37.9 ± 0.3 | 50.5 ± 0.4 | 44.2 ± 0.3 |
| Respiratory | 11.4 ± 0.1 | 17.2 ± 0.3 | 14.3 ± 0.2 |
| Air pollutant concentration (μg/m3)a | |||
| PM10 | 87.4 ± 1.8 | 116.7 ± 2.8 | 102.0 ± 1.7 |
| SO2 | 39.4 ± 0.7 | 50.1 ± 1.0 | 44.7 ± 0.6 |
| NO2 | 57.3 ± 0.7 | 76.0 ± 1.0 | 66.6 ± 0.7 |
| O3 | 78.4 ± 1.5 | 48.3 ± 0.9 | 63.3 ± 1.0 |
| Meteorological measures | |||
| Temperature (°C) | 24.3 ± 0.2 | 11.2 ± 0.3 | 17.7 ± 0.2 |
| Humidity (%) | 75.1 ± 0.4 | 70.6 ± 0.5 | 72.9 ± 0.3 |
Twenty-four-hour average for PM10, SO2, and NO2; 8-hr (1000–1800 hours) average for O3.
During our study period, the mean daily average concentrations of PM10, SO2, NO2, and O3 were 102.0, 44.7, 66.6, and 63.4 μg/m3, respectively. There were two missing value days for O3 and none for the other three pollutants. The mean daily average temperature and humidity were 17.7°C and 72.9%, respectively, reflecting the subtropical climate in Shanghai.
Generally, PM10, SO2, and NO2 were relatively highly correlated with each other (Pearson correlation coefficients ranged from 0.64 to 0.73). PM10/SO2/NO2 concentrations were negatively correlated with temperature and humidity. Maximal 8-hr mean O3 was weakly correlated with PM10, SO2, and NO2 (Pearson correlation coefficients ranged from 0.01 to 0.19) and moderately correlated with temperature level (Pearson correlation coefficient, 0.48).
Effects by season
In the whole-period analyses, outdoor air pollution was associated with mortality from all causes and from cardiopulmonary diseases in Shanghai (Table 2). An increase of 10 μg/m3 of 2-day average concentrations of PM10, SO2, NO2, and O3 corresponds to 0.25% (95% CI, 0.14–0.37), 0.95% (95% CI, 0.62–1.28), 0.97% (95% CI, 0.66–1.27), and 0.31% (95% CI, 0.04–0.58) increase of all-cause mortality, respectively.
Table 2.
Percent increase [mean (95% CI)] of mortality outcomes of Shanghai residents associated with 10-μg/m3 increase in air pollutant concentrations by season, 2001–2004.a
| Mortality | Pollutant | Warm season | Cool season | Entire period |
|---|---|---|---|---|
| Total | PM10 | 0.21 (0.09 to 0.33) | 0.26 (0.22 to 0.30) | 0.25 (0.14 to 0.37) |
| SO2 | 0.57 (−0.03 to 1.18) | 1.10 (0.66 to 1.53) | 0.95 (0.62 to 1.28) | |
| NO2 | 0.46 (−0.07 to 0.98) | 1.24 (0.84 to 1.64)* | 0.97 (0.66 to 1.27) | |
| O3 | 0.22 (0.03 to 0.41) | 1.19 (0.56 to 1.83)* | 0.31 (0.04 to 0.58) | |
| Cardiovascular | PM10 | 0.22 (−0.14 to 0.58) | 0.25 (0.05 to 0.45) | 0.27 (0.10 to 0.44) |
| SO2 | 0.31 (−0.65 to 1.29) | 1.02 (0.40 to 1.65) | 0.91 (0.42 to 1.41) | |
| NO2 | 0.30 (−0.54 to 1.14) | 1.26 (0.68 to 1.84) | 1.01 (0.55 to 1.47) | |
| O3 | 0.32 (−0.05 to 0.69) | 1.42 (0.51 to 2.33) | 0.38 (−0.03 to 0.80) | |
| Respiratory | PM10 | −0.28 (−0.93 to 0.38) | 0.58 (0.25 to 0.92)* | 0.27 (−0.01 to 0.56) |
| SO2 | −1.13 (−2.86 to 0.62) | 2.47 (1.41 to 3.54)* | 1.37 (0.51 to 2.23) | |
| NO2 | −1.37 (−2.86 to 0.15) | 2.66 (1.67 to 3.65)* | 1.22 (0.42 to 2.01) | |
| O3 | 0.12 (−0.72 to 0.98) | 0.94 (−0.60 to 2.50) | 0.29 (−0.44 to 1.03) |
We used current day temperature and humidity (lag 0) and 2-day moving average of air pollutant concentrations (lag 01), and applied 3 df to temperature and humidity.
Significantly different from the warm season (p < 0.05).
There were more deaths, higher concentrations of pollutants (except for O3, which had higher concentrations in the warm season), and drier weather conditions in the cool season than in the warm season (Table 1).
The effect estimates of PM10 on total mortality were similar in both seasons. Effect estimates were approximately 2–3 times higher for SO2 and NO2 in the cool season compared with the warm season. The effect estimate of O3 was significant in both cool and warm seasons, and the magnitude of the O3-associated increase in total mortality was approximately 5-fold higher in the cool season than in the warm season. Between-season differences in total mortality were significant for NO2 and O3 but not for PM10 or SO2 (Table 2).
For cardiovascular mortality, the effect estimate of PM10 was similar in both seasons. For SO2, NO2, and O3, the effect estimate in the cool season were approximately 3–4 times higher than in the warm season. Between-season differences in cardiovascular mortality were insignificant for all four pollutants.
For the smaller category of respiratory mortality, the effect estimates of PM10, SO2, and NO2 were significant only in the cool season, and their between-season differences were significant. The effect effect estimate of O3 on respiratory mortality was insignificant in either season.
Effects by sex and age
The percent increase associated with higher concentration levels of air pollutants varied by sex or age group (Table 3). The effect estimates of PM10 and O3 among females were approximately twice those among males, although their between-sex differences were insignificant. The effect estimates of SO2 and NO2 on total mortality in females were slightly higher than in males.
Table 3.
Percent increase [mean (95% CI)] in total mortality of Shanghai residents associated with a 10-μg/m3 increase in air pollutant concentrations by sex and age.a
| Pollutant
|
|||||
|---|---|---|---|---|---|
| Mean daily deaths (n) | PM10 | SO2 | NO2 | O3 | |
| Sex | |||||
| Female | 56.5 | 0.33 (0.18 to 0.48) | 1.06 (0.62 to 1.51) | 1.10 (0.69 to 1.51) | 0.40 (0.03 to 0.76) |
| Male | 62.5 | 0.17 (0.03 to 0.32) | 0.85 (0.43 to 1.28) | 0.88 (0.49 to 1.28) | 0.19 (−0.16 to 0.55) |
| Age (years) | |||||
| 5–44 | 3.7 | 0.04 (−0.52 to 0.59) | 1.21 (−0.47 to 2.91) | 0.52 (−1.01 to 2.08) | −0.08 (−1.38 to 1.25) |
| 45–64 | 15.5 | 0.17 (−0.11 to 0.45) | 0.22 (−0.60 to 1.04) | 0.64 (−0.11 to 1.40) | 0.47 (−0.19 to 1.12) |
| ≥65 | 99.6 | 0.26 (0.15 to 0.38) | 1.01 (0.65 to 1.36) | 1.01 (0.69 to 1.34) | 0.32 (0.03 to 0.61) |
We used current day temperature and humidity (lag 0) and 2-day moving average of air pollutant concentrations (lag 01), and applied 3 df to temperature and humidity.
The number of deaths for residents under 5 years of age was very low and therefore was excluded from our analysis. We did not observe significant effects of air pollution in residents 5–44 years of age or 45–64 years of age. Among those ≥ 65 years of age, the effect estimates of all four pollutants were significant, and approximately 2–5 times higher than among people 5–44 years of age or 45–64 years of age, although the between-age differences among all three groups were insignificant.
Effects by education
Generally, residents with low educational attainment (illiterate or primary school) had a higher number of deaths from air pollution–related effects than those with high educational attainment (middle school or above) (Table 4).
Table 4.
Percent increase in number of deaths due to total, cardiovascular, and respiratory causes associated with a 10-μg/m3 increase in air pollutants by educational attainment.a
| Pollutant
|
||||||
|---|---|---|---|---|---|---|
| Mortality | Educational attainment | Mean daily deaths (n) | PM10 | SO2 | NO2 | O3 |
| Total | Low | 67.3 | 0.33 (0.19 to 0.47) | 1.19 (0.77 to 1.61) | 1.27* (0.89 to 1.66) | 0.26 (−0.09 to 0.60) |
| High | 42.1 | 0.18 (0.01 to 0.36) | 0.66 (0.16 to 1.17) | 0.62 (0.15 to 1.09) | 0.30 (−0.11 to 0.71) | |
| Cardiovascular | Low | 27.8 | 0.30 (0.10 to 0.51) | 1.08 (0.47 to 1.69) | 1.15 (0.58 to 1.72) | 0.39 (−0.13 to 0.90) |
| High | 16.4 | 0.23 (−0.03 to 0.50) | 0.57 (−0.20 to 1.35) | 0.73 (0.01 to 1.45) | 0.26 (−0.38 to 0.91) | |
| Respiratory | Low | 8.9 | 0.36 (0.00 to 0.72) | 1.54 (0.43 to 2.66) | 1.59 (0.57 to 2.62) | 0.20 (−0.74 to 1.16) |
| High | 5.4 | 0.02 (−0.43 to 0.47) | 0.73 (−0.61 to 2.09) | 0.34 (−0.89 to 1.60) | 0.27 (−0.86 to 1.41) | |
We used current day temperature and humidity (lag 0) and 2-day moving average of air pollutants concentrations (lag 01) and we applied 3 df to temperature and humidity.
Significantly different from high educational attainment (p < 0.05).
For total mortality, the effect estimates of PM10, SO2, and NO2 were significant in both education groups. The effect estimates of these three pollutants were 1–2 times larger among the low-education group compared with the high-education group, although the educational differences were significant only for NO2 for total mortality. The effect estimate of O3 of total mortality were similar and insignificant in both groups.
For cardiovascular mortality, the effect estimates of PM10 and NO2 were significant or marginally significant in both education groups; the effect estimate of SO2 was significant only in the low-education group; no significant effect of O3 was seen in either group. The effect estimates of all four pollutants were 1–2 times larger among the low-education group compared with the high-education group. The educational differences in cardiovascular mortality were not significant for any pollutants.
For respiratory mortality, the effect estimates of PM10, SO2, and NO2 were significant only among those with low education, whereas the effect estimate of O3 on respiratory mortality was not significant in either group. The effect estimates of PM10, SO2, and NO2 were several times larger among the low-education group compared with the high-education group. The educational differences in respiratory mortality were not significant for any pollutants.
Discussion
Although the associations between outdoor air pollution and daily mortality have been well established in developed countries, the question of the potential modifiers remains inconclusive. As the U.S. National Research Council (1998) pointed out, it is important to understand the characteristics of individuals who are at increased risk of adverse events due to outdoor air pollution. Our results suggest that season and individual sociodemographic factors (e.g., sex, age, SES) may modify the health effects of air pollution in Shanghai. Specifically, the association between air pollution and daily mortality was generally more evident for the cool season than the warm season; females and the elderly (≥65 years of age) appeared to be more vulnerable to air pollution than males and younger people; and disadvantaged SES may intensify the adverse health effects of outdoor air pollution.
Our finding of a stronger association between air pollution and daily mortality in the cool season is consistent with several prior studies in Hong Kong (Wong et al. 1999, 2001) and Athens, Greece (Touloumi et al. 1996), but in contrast with others reporting greater effects in the warm season (Anderson et al. 1996; Bell et al. 2005; Nawrot et al. 2007). In Shanghai, the concentrations of PM10, SO2, and NO2 were higher and more variable in the cool season than in the warm season (Table 1). Because these three pollutants were highly correlated, greater effects observed during the cool season may also be due to other pollutants that were also at higher levels during that season. In contrast, the O3 level was higher in the warm season than in the cool season, and our exposure–response relationship also revealed a flatter slope at higher concentrations of O3 for both sexes (data not shown). At higher concentrations, the risks of death could be reduced because vulnerable subjects may have died before the concentration reached the maximum level (Wong et al. 2001).
Exposure patterns may contribute to our season-specific observation. During the warm season, Shanghai residents tend to use air conditioning more frequently because of the relatively higher temperature and humidity, thus reducing their exposure. For example, in a survey of 1,106 families in Shanghai, 32.7% of the families never turn on air conditioners in the winter compared with 3.7% in the summer (Long et al. 2007). Heavy rain in the warm season may reduce time outdoors, thus reducing personal exposure. In contrast, the cool season in Shanghai is drier and less variable, so people are more likely to go outdoors and open the windows. Nevertheless, the fact that a consistently significant health effect of air pollution was observed only in the cool season in two subtropical Asian cities [Shanghai (present study) and Hong Kong (Wong et al. 1999, 2001)] suggests that the interaction of air pollution exposure and season may vary by location.
Unlike the gaseous pollutants, the constituents of the complex mix of PM10 may vary by season. Therefore, another potential explanation for the seasonal difference in the effects of PM10 is that the most toxic particles may have a cool-season maximum in Shanghai.
We found a greater effect of ambient air pollution on total mortality in females than in males. Results of prior studies on sex-specific acute effects of outdoor air pollution were discordant. For example, Ito and Thurston (1996) found the highest risk of mortality related with air pollution exposure among black women. Hong et al. (2002) found that elderly women were most susceptible to the adverse effects of PM10 on the risk of acute mortality from stroke. However, Cakmak et al. (2006) found that sex did not modify the hospitalization risk of cardiac diseases due to air pollution exposure.
The reasons for our sex-specific observations are unclear and deserve further investigation. In Shanghai, females have a much lower smoking rate than males (0.6% in females vs. 50.6% in males) (Xu 2005). One study suggested that effects of air pollution may be stronger in nonsmokers than in smokers (Künzli et al. 2005). Oxidative and inflammatory effects of smoking may dominate to such an extent that the additional exposure to air pollutants may not further enhance effects along the same pathways in males. In addition, females have slightly greater airway reactivity than males, as well as smaller airways (Yunginger et al. 1992); therefore, dose–response relations might be detected more easily in females than in males. Deposition of particles in the lung varies by sex, with greater lung deposition fractions of 1-μM particles in all regions for females (Kim and Hu 1998; Kohlhaufl et al. 1999). Sunyer et al. (2000) suggested that differing particulate deposition patterns between females and males may partly explain the difference between the sexes. Moreover, compared with males, females in Shanghai had a lower education level (73.9% in females vs. 41.0% in males); thus, lower SES might contribute to the observed larger effects of air pollution in females.
As in a few other studies (Gouveia and Fletcher 2000; Katsouyanni et al. 2001), we found the elderly were most vulnerable to the effects of air pollution. Low numbers of deaths in the 0- to 4-year age group limited our power to detect the effects of air pollution on mortality, even if they exist. Two groups, the elderly and the very young, are presumed to be at greater risk for air pollution–related effects (Gouveia and Fletcher 2000; Schwartz 2004). For the elderly, preexisting respiratory or cardiovascular conditions are more prevalent than in younger age groups; thus, there is some overlap between potentially susceptible groups of older adults and people with heart or lung diseases.
It has long been known that SES can affect health indicators such as mortality (Mackenbach et al. 1997). Recently, studies have started to examine the role of SES in the vulnerability of subpopulations to outdoor air pollution, especially for particles and O3, although the results remain inconsistent (O’Neill et al. 2003). For example, Zeka et al. (2006) found that individual-level education was inversely related to the risk of mortality associated with PM 10 .Another cohort study with small-area measures of SES in Hamilton, Ontario, Canada, found important modification of the particle effects by social class (Finkelstein et al. 2003; Jerrett et al. 2004). In contrast, Gouveia and Fletcher (2000) observed a larger effect of air pollution in areas of higher SES level; Bateson and Schwartz (2004) found no indication that susceptibility to air pollution varied by group-level SES measures. In the present study, using individual-level education as a measure of SES, we found that residents with low educational attainment were more sensitive to air pollution exposure than those with high educational attainment. Our results provide the first evidence in Mainland China that lower SES may compose a risk factor for air pollution–related health effects.
SES factors such as educational attainment may modify the health effects of outdoor air pollution in several pathways. People with lower SES may be more sensitive to air pollution–related health hazards because they have a higher prevalence of preexisting diseases that confer a greater risk of dying associated with air pollution exposure, and they may also receive inferior medical treatment for preexisting diseases. Disadvantaged living conditions may contribute to the modification effect; people with lower SES may have more limited access to fish, fresh fruits, and vegetables, resulting in reduced intake of antioxidant polyunsaturated fatty acids and vitamins that may protect against adverse consequences of particle exposure (Romieu et al. 2005). Additionally, exposure patterns may contribute to effect modification by SES. Persons with lower SES are less likely to have air conditioning (Long et al. 2007) and more likely to live near busy roadways and have coexposures due to either poor housing or occupation. For example, disadvantaged groups have been found to be more highly exposed to some air pollutants (Sexton et al. 1993). Scandinavian studies have shown differential personal exposures to particles and other pollutants by education and occupation (Rotko et al. 2000, 2001), and a study in the U.S. Great Lakes region indicates differences in exposure to gaseous pollutants by occupation and education, minority status, and income (Pellizzari et al. 1999). Finally, as Jerrett et al. (2004) pointed out, persons with lower education are less mobile and experience less exposure measurement error, thereby reducing bias toward the null.
The limitations of our analysis should be noted. As in other studies in this field, we used available outdoor monitoring data to represent the population exposure to air pollutants. Our assessment of weather conditions was derived entirely from one monitoring station. Measurement error may have substantial implications for interpreting epidemiologic studies on air pollution, particularly for the time-series design (Zeger et al. 2000). It is possible that this type of error may introduce bias to the results of our analysis; however, because of lack of available information on personal exposure to air pollutants, we could not quantify such a bias. Compared with other studies in Europe and North America, the data we collected were limited in being only one city, in sample size, and in duration. In addition, high correlation between particulate matter and gaseous pollutants in Shanghai limited our ability to separate the independent effect for each pollutant.
In summary, in this time-series analysis, we found that outdoor air pollution was associated with mortality from all causes and from cardiopulmonary diseases in Shanghai during 2001–2004. Furthermore, our results suggest that season and sociodemographic factors (e.g., sex, age, SES) may modify the acute health effects of air pollution. These findings provide new information about the effects of modifiers on the relationship between daily mortality and air pollution in developing countries and may have implications for local environmental and social policies.
Footnotes
This study was funded by the Health Effects Institute through grant 4717-RFIQ03-3/04-13. The research was also supported by the Division of Intramural Research, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services.
The views expressed in this article are those of the authors and do not necessarily reflect the views of the Health Effects Institute or its sponsors.
References
- Anderson HR, Ponce de Leon A, Bland JM, Bower JS, Strachan DP. Air pollution and daily mortality in London: 1987–92. BMJ. 1996;312(7032):665–669. doi: 10.1136/bmj.312.7032.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RW, Anderson HR, Sunyer J, Ayres J, Baccini M, Vonk JM, et al. Acute effects of particulate air pollution on respiratory admissions: results from APHEA 2 project. Air Pollution and Health: a European Approach. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1860–1866. doi: 10.1164/ajrccm.164.10.2010138. [DOI] [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Who is sensitive to the effects of particulate air pollution on mortality? A case-crossover analysis of effect modifiers. Epidemiology. 2004;15(2):143–149. doi: 10.1097/01.ede.0000112210.68754.fa. [DOI] [PubMed] [Google Scholar]
- Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the National Morbidity, Mortality, and Air Pollution Study. Epidemiology. 2005;16(4):436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett RT, Brook J, Dann T, Delocla C, Philips O, Cakmak S, et al. Association between particulate- and gas-phase components of urban air pollution and daily mortality in eight Canadian cities. Inhal Toxicol. 2000;12(suppl 4):15–39. doi: 10.1080/08958370050164851. [DOI] [PubMed] [Google Scholar]
- Cakmak S, Dales RE, Judek S. Do gender, education, and income modify the effect of air pollution gases on cardiac disease? J Occup Environ Med. 2006;48(1):89–94. doi: 10.1097/01.jom.0000184878.11956.4b. [DOI] [PubMed] [Google Scholar]
- Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein MM, Jerrett M, DeLuca P, Finkelstein N, Verma DK, Chapman K, et al. Relation between income, air pollution and mortality: a cohort study. CMAJ. 2003;169(5):397–402. [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Burnett RT, Bailar JC, III, Tamblyn R, Ernst P, Flegel K, et al. Identification of persons with cardiorespiratory conditions who are at risk of dying from the acute effects of ambient air particles. Environ Health Perspect. 2001;(suppl 4):487–494. doi: 10.1289/ehp.01109s4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg MS, Burnett RT, Stieb D. A review of time-series studies used to evaluate the short-term effects of air pollution on human health. Rev Environ Health. 2003;18(4):269–303. doi: 10.1515/reveh.2003.18.4.269. [DOI] [PubMed] [Google Scholar]
- Gouveia N, Fletcher T. Time series analysis of air pollution and mortality: effects by cause, age and socioeconomic status. J Epidemiol Community Health. 2000;54(10):750–755. doi: 10.1136/jech.54.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie TJ, Tibshirani RJ. Generalized Additive Models. London: Chapman and Hall; 1990. [Google Scholar]
- Health Effects Institute. Health Effects of Outdoor Air Pollution in Developing Countries of Asia: A Literature Review. Boston, MA: Health Effects Institute; 2004. [[accessed 23 July 2008]]. Special Report 15. Available: http://pubs.healtheffects.org/getfile.php?u=13. [Google Scholar]
- Hong YC, Lee JT, Kim H, Ha EH, Schwartz J, Christiani DC. Effects of air pollutants on acute stroke mortality. Environ Health Perspect. 2002;110:187–191. doi: 10.1289/ehp.02110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Thurston GD. Daily PM10/mortality associations: an investigations of at-risk subpopulations. J Expo Anal Environ Epidemiol. 1996;6(1):79–95. [PubMed] [Google Scholar]
- Jerrett M, Burnett RT, Brook J, Kanaroglou P, Giovis C, Finkelstein N, et al. Do socioeconomic characteristics modify the short term association between air pollution and mortality? Evidence from a zonal time series in Hamilton, Canada. J Epidemiol Community Health. 2004;58(1):31–40. doi: 10.1136/jech.58.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouyanni K, Touloumi G, Samoli E, Gryparis A, Le Tertre A, Monopolis Y, et al. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology. 2001;12(5):521–531. doi: 10.1097/00001648-200109000-00011. [DOI] [PubMed] [Google Scholar]
- Katsouyanni K, Touloumi G, Spix C, Schwartz J, Balducci F, Medina S, et al. Short-term effects of ambient sulphur dioxide and particulate matter on mortality in 12 European cities: results from time series data from the APHEA project. Air Pollution and Health: a European Approach. BMJ. 1997;314(7095):1658–1663. doi: 10.1136/bmj.314.7095.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Hu SC. Regional deposition of inhaled particles in human lungs: comparison between men and women. J Appl Physiol. 1998;84(6):1834–1844. doi: 10.1152/jappl.1998.84.6.1834. [DOI] [PubMed] [Google Scholar]
- Kohlhaufl M, Brand P, Scheuch G, Meyer TS, Schulz H, Haussinger K, et al. Increased fine particle deposition in women with asymptomatic nonspecific airway hyper-responsiveness. Am J Respir Crit Care Med. 1999;159(3):902–906. doi: 10.1164/ajrccm.159.3.9805036. [DOI] [PubMed] [Google Scholar]
- Krewski D, Burnett RT, Goldberg M, Hoover K, Siemiatycki J, Abrahamowicz M, et al. Reanalysis of the Harvard Six Cities Study, part II: sensitivity analysis. Inhal Toxicol. 2005;17(7–8):343–353. doi: 10.1080/08958370590929439. [DOI] [PubMed] [Google Scholar]
- Künzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, et al. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect. 2005;113:201–206. doi: 10.1289/ehp.7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenbach JP, Kunst AE, Cavelaars AE, Groenhof F, Geurts JJ. Socioeconomic inequalities in morbidity and mortality in western Europe. The EU Working Group on Socioeconomic Inequalities in Health. Lancet. 1997;349(9066):1655–1659. doi: 10.1016/s0140-6736(96)07226-1. [DOI] [PubMed] [Google Scholar]
- Long W, Zhong T, Zhang B. China: The Issue of Residential Air Conditioning. 2007. [[accessed 15 November 2007]]. Available: http://www.iifiir.org/en/doc/1056.pdf.
- National Research Council. Research Priorities for Airborne Particulate Matter. Washington, DC: National Academy Press; 1998. [Google Scholar]
- Nawrot TS, Torfs R, Fierens F, De Henauw S, Hoet PH, Van Kersschaever G, et al. Stronger associations between daily mortality and fine particulate air pollution in summer than in winter: evidence from a heavily polluted region in western Europe. J Epidemiol Community Health. 2007;61(2):146–149. doi: 10.1136/jech.2005.044263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MS, Jerrett M, Kawachi I, Levy JI, Cohen AJ, Gouveia N, et al. Health, wealth, and air pollution: advancing theory and methods. Environ Health Perspect. 2003;111:1861–1870. doi: 10.1289/ehp.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzari ED, Perritt RL, Clayton CA. National Human Exposure Assessment Survey (NHEXAS): exploratory survey of exposure among population subgroups in EPA Region V. J Expo Anal Environ Epidemiol. 1999;9(1):49–55. doi: 10.1038/sj.jea.7500025. [DOI] [PubMed] [Google Scholar]
- Peng RD, Dominici F, Pastor-Barriuso R, Zeger SL, Samet JM. Seasonal analyses of air pollution and mortality in 100 US cities. Am J Epidemiol. 2005;161(6):585–594. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2007. [[accessed 22 July 2008]]. Available: http://cran.r-project.org/doc/manuals/refman.pdf. [Google Scholar]
- Romieu I, Tellez-Rojo MM, Lazo M, Manzano-Patino A, Cortez-Lugo M, Julien P, et al. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med. 2005;172(12):1534–1540. doi: 10.1164/rccm.200503-372OC. [DOI] [PubMed] [Google Scholar]
- Rotko T, Koistinen K, Hanninen O, Jantunen M. Sociodemographic descriptors of personal exposure to fine particles (PM2.5) in EXPOLIS Helsinki. J Expo Anal Environ Epidemiol. 2000;10(4):385–393. doi: 10.1038/sj.jea.7500104. [DOI] [PubMed] [Google Scholar]
- Rotko T, Kousa A, Alm S, Jantunen M. Exposures to nitrogen dioxide in EXPOLIS-Helsinki: microenvironment, behavioral and sociodemographic factors. J Expo Anal Environ Epidemiol. 2001;11(3):216–223. doi: 10.1038/sj.jea.7500162. [DOI] [PubMed] [Google Scholar]
- Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000a;343(24):1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- Samet JM, Zeger SL, Dominici F, Curriero F, Coursac I, Dockery DW, et al. The National Morbidity, Mortality, and Air Pollution Study. Part II: Morbidity and mortality from air pollution in the United States. Res Rep Health Eff Inst. 2000b;94(Pt 2):5–70. [PubMed] [Google Scholar]
- Schwartz J. Air pollution and children’s health. Pediatrics. 2004;113(suppl 4):1037–1043. [PubMed] [Google Scholar]
- Sexton K, Gong H, Jr, Bailar JC, III, Ford JG, Gold DR, Lambert WE, et al. Air pollution health risks: do class and race matter? Toxicol Ind Health. 1993;9(5):843–878. doi: 10.1177/074823379300900509. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Schwartz J, Tobias A, Macfarlane D, Garcia J, Anto JM. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysis. Am J Epidemiol. 2000;151(1):50–56. doi: 10.1093/oxfordjournals.aje.a010121. [DOI] [PubMed] [Google Scholar]
- Touloumi G, Atkinson R, Tertre AL, Samoli E, Schwartz J, Schindler C, et al. Analysis of health outcome time series data in epidemiological studies. Environmetrics. 2004;15(2):101–117. [Google Scholar]
- Touloumi G, Samoli E, Katsouyanni K. Daily mortality and “winter type” air pollution in Athens, Greece—a time series analysis within the APHEA project. J Epidemiol Community Health. 1996;50(suppl 1):s47–s51. doi: 10.1136/jech.50.suppl_1.s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touloumi G, Samoli E, Pipikou M, Le Tertre A, Atkinson R, Katsouyanni K. Seasonal confounding in air pollution and health time-series studies: effect on air pollution effect estimates. Stat Med. 2006;25(24):4164–4178. doi: 10.1002/sim.2681. [DOI] [PubMed] [Google Scholar]
- Welty LJ, Zeger SL. Are the acute effects of particulate matter on mortality in the National Morbidity, Mortality, and Air Pollution Study the result of inadequate control for weather and season? A sensitivity analysis using flexible distributed lag models. Am J Epidemiol. 2005;162(1):80–88. doi: 10.1093/aje/kwi157. [DOI] [PubMed] [Google Scholar]
- Wong CM, Ma S, Hedley AJ, Lam TH. Does ozone have any effect on daily hospital admissions for circulatory diseases? J Epidemiol Community Health. 1999;53(9):580–581. doi: 10.1136/jech.53.9.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CM, Ma S, Hedley AJ, Lam TH. Effect of air pollution on daily mortality in Hong Kong. Environ Health Perspect. 2001;109:335–340. doi: 10.1289/ehp.01109335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. International Classification of Diseases, Ninth Revision. Geneva: World Health Organization; 1978. [PubMed] [Google Scholar]
- WHO. International Classification of Diseases, Tenth Revision. Geneva: World Health Organization; 1993. [Google Scholar]
- WHO. Air Quality Guideline for Europe. Copenhagen: World Health Organization; 2000. [Google Scholar]
- Xu Z. Effect evaluation on smoking control plan for one year in Shanghai-China/WHO smoking control capability construction cooperation items. Chin J Health Educ. 2005;21:412–416. [Google Scholar]
- Yunginger JW, Reed CE, O’Connell EJ, Melton LJ, III, O’Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis. 1992;146(4):888–894. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Race, gender, and social status as modifiers of the effects of PM10 on mortality. J Occup Environ Med. 2000;42(5):469–474. doi: 10.1097/00043764-200005000-00002. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeka A, Zanobetti A, Schwartz J. Individual-level modifiers of the effects of particulate matter on daily mortality. Am J Epidemiol. 2006;163(9):849–859. doi: 10.1093/aje/kwj116. [DOI] [PubMed] [Google Scholar]


