Abstract
Background
Polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins, and poly-chlorinated dibenzofurans adversely affect the health of humans and various animals. Such effects might be partially exerted through the thyroid hormone (TH) system. We previously reported that one of the hydroxylated PCB congeners suppresses TH receptor (TR)-mediated transcription by dissociating TR from the TH response element (TRE). However, the binding site of PCB within TR has not yet been identified.
Objectives
We aimed to identify the functional TR domain responsible for the PCB-mediated suppression of TR action by comparing the magnitude of suppression using several representative PCB/dioxin congeners.
Materials and methods
We generated chimeric receptors by combining TR and glucocorticoid receptor (GR) and determined receptor-mediated transcription using transient transfection-based reporter gene assays, and TR-TRE binding using electrophoretic mobility shift assays.
Results
Although several PCB congeners, including the hydroxylated forms, suppressed TR-mediated transcription to various degrees, 2,3,7,8-tetrachlorodibenzo-p-dioxin did not alter TR action, but 2,3,4,7,8-pentachlorodibenzofuran weakly suppressed it. The magnitude of suppression correlated with that of TR–TRE dissociation. The suppression by PCB congeners was evident from experiments using chimeric receptors containing a TR DNA-binding domain (DBD) but not a GR-DBD.
Conclusions
Several nondioxin-like PCB congeners and hydroxylated PCB compounds suppress TR action by dissociating TR from TRE through interaction with TR-DBD.
Keywords: dioxin, DNA-binding domain, polychlorinated biphenyl, thyroid hormone receptor, transcription
Polychlorinated biphenyls (PCBs), poly-chlorinated dibenzo-p-dioxins (PCDDs), and polychlorinated dibenzofurans (PCDFs) are extremely persistent environmental compounds that adversely affect the health of humans and other animals. These compounds are toxic to the fetal and early post-natal developing brain, which is exposed via the placenta and breast milk as a result of maternal exposure (Gladen and Rogan 1991; Safe 1994; Tilson et al. 1990; Tilson and Kodavanti 1997), even if the exposure level is too low to induce maternal toxicity (Chen et al. 1992; Jacobson and Jacobson 1996a; Jacobson et al. 1985, 1990). Jacobson and Jacobson (1996b) suggested that exposure to PCBs in utero induced intellectual impairment in children born to mothers who consumed excessive amounts of sport fish obtained from the Great Lakes area in the United States. Disruption of cognitive development among children exposed to dioxins and PCBs has been documented in accidental human exposures, such as in the Yusho and Yu-cheng incidences (Aoki 2001), and confirmed in experimental animals (Giesy and Kannan 1998; Jacobson and Jacobson 1997). In addition, exposure to PCBs may alter dendrito-genesis in several brain regions during development (Kimura-Kuroda et al. 2005; Lein et al. 2007).
The effects of PCBs/dioxins on the brain have been interpreted in several ways. First, dioxin-like PCB congeners are able to bind to and activate aryl hydrocarbon receptors (AhRs), exerting various toxic effects. The degree of such effects is numerically expressed as the toxicity equivalency factor (TEF) and is standardized to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; TEF = 1) (Van den Berg et al. 2006). However, the TEF concept might not fully encompass the developmental neurotoxicity of PCBs, because AhR expression in the brain may be regional (Huang et al. 2000; Petersen et al. 2000) and because PCB congeners are considered to have neuro-toxicities via both AhR-dependent and AhR-independent mechanisms (Giesy and Kannan 1998; Tilson and Kodavanti 1997). Second, PCBs may disrupt intracellular signaling pathways that are essential not only for brain function but also for brain development (reviewed by Kodavanti 2005), which will disturb intra-cellular calcium homeostasis (Kodavanti et al. 1993). For example, ortho-substituted non-coplanar congeners might alter protein kinase C translocation, cellular dopamine uptake, and the formation of reactive oxygen species. Such toxicity is referred to as the neurotoxicity equivalent (reviewed by Kodavanti 2005; Simon et al. 2007).
In addition to these signaling pathways, we and several other groups have focused on the possible interactions of these chemicals with the thyroid hormone (TH) system (Bogazzi et al. 2003; Iwasaki et al. 2002; Kitamura et al. 2005; Miyazaki et al. 2004). TH is crucial for brain development, and TH deficiency during the critical perinatal period has been reported to cause cretinism, with severe cognitive and/or mental disorders in the offspring (Koibuchi and Chin 2000; Oppenheimer and Schwartz 1997; Porterfield and Hendrich 1993). PCB/dioxin congeners are considered to cause neurotoxicity by altering TH homeo-stasis in the developing brain. Some researchers have reported that exposure to PCB congeners results in thyroid enlargement and reduced serum total thyroxine (T4) levels with normal levels of triiodothyronine (T3), an active compound of TH (Brouwer et al. 1998; Hauser et al. 1998; Porterfield 2000), during a possible critical period of TH action. Certain PCB congeners were reported to induce the expression of a microsomal enzyme, uridine diphosphate glucuronosyl-transferase, which glucuronizes T4 to facilitate excretion (Hood et al. 2003; Liu et al. 1995). Exposure to TCDD also results in morphologic and functional alterations in the thyroid of adult rodents (Gorski and Rozman 1987; Henry and Gasiewicz 1987; Potter et al. 1986; Van Birgelen et al. 1995). Such exposure induces not only an increase in the volume of thyroid follicular cells, followed by hyperplasia, but also follicular thyroid tumors in rats (Huff et al. 1991; Sewall et al. 1995). Both PCDD and PCDF induce T3 and T4 excretion, thereby decreasing plasma T3 and T4 levels (Bastomsky 1977; Kohn et al. 1996; Nishimura et al. 2002). These results indicate that PCBs/dioxins disrupt the TH system by decreasing blood TH levels, which in turn induces hypothyroidism in various organs.
Perinatal exposure to PCBs/dioxins in laboratory animals induces a decrease in plasma T4 levels without significantly altering the growth (Lein et al. 2007; Zoeller et al. 2000), and T3 levels remain within the normal range (Porterfield 2000), indicating that the toxicity of these chemicals does not manifest by altering blood TH levels. On the other hand, because the molecular structures of PCBs/dioxins are similar to those of TH, these chemicals are considered to act via TH receptors (TRs) (McKinney 1989). Furthermore, these compounds can be transferred across the blood–brain barrier and accumulate in the brain (Brouwer and van den Berg 1986; Cheek et al. 1999; Darnerud et al. 1996; McKinney 1989). These findings suggest that PCBs/dioxins induce abnormal brain development by directly acting on TR.
We therefore performed a series of experiments and found that a hydroxylated (OH) PCB compound [4-OH-2′,3,3′,4′,5′-penta-chlorobiphenyl (pentaCB); 4-OH-PCB-106] at a concentration of 10−10 M suppresses TR-mediated transcription induced by TH (Iwasaki et al. 2002). The magnitude of suppression induced by 4-OH-PCB-106 was cell-type dependent and most obvious in clonal TE671 cells derived from human cerebellar granule cells (Iwasaki et al. 2002), and this suppression was due to the partial dissociation of TR from the TH response element (TRE) (Miyazaki et al. 2004). These results suggest that PCBs directly act on TR, although the TR functional domain responsible for PCB action remains obscure.
Here, we report that the magnitude of the suppression of TR-mediated transcription differs among a variety of congeners of PCBs/dioxins. In addition, we identified the functional domain responsible for PCB action using chimeric receptors generated from TR and the glucocorticoid receptor (GR).
Materials and Methods
Chemicals
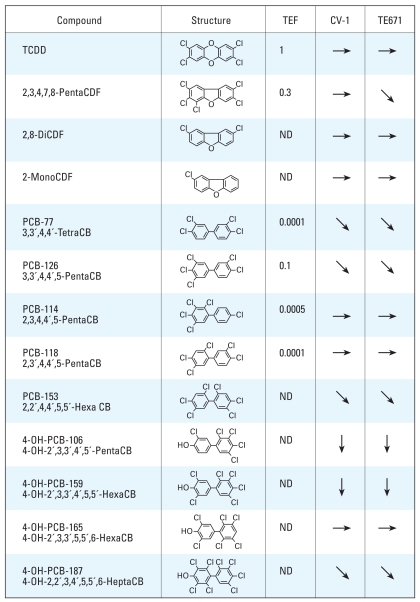
We purchased T3 from Sigma Chemical Co. (St. Louis, MO, USA), and TCDD and PCDFs [2,3,4,7,8-pentachloro-dibenzofuran (pentaCDF), 2,8-diCDF, and 2-monoCDF] from Cambridge Isotope Laboratory (Andover, MA, USA); all congeners were > 98% pure. We purchased all PCB congeners [3,3′,4,4′-tetraCB (PCB-77), 3,3′,4,4′,5-pentaCB (PCB-126), 2,3,4,4′,5-pentaCB (PCB-114); 2,3′,4,4′,5-pentaCB (PCB-118); and 2,2′,4,4′,5,5′-hexaCB (PCB-153)] and OH-PCBs [4-OH-2′,3,3′,4′,5′-pentaCB (4-OH-PCB-106); 4-OH-2,3,3′,4,5,5′-hexaCB (4-OH-PCB-159); 4-OH-2,3,3′,5,5′,6-hexaCB (4-OH-PCB-165); and 4-OH-2,2′,3,4′,5,5′,6-heptaCB (4-OH-PCB-187)] from AccuStandard Chemicals (New Haven, CT, USA). All PCB congeners were > 99% pure, and OH-PCBs were > 98% pure.
Plasmids
The expression vectors for human TRβ1, GR, and mouse retinoid X receptor β (RXRβ), as well as the 2× glucocorticoid response element-luciferase (LUC) reporter in pTAL-LUC (BD Biosciences Clontech, Palo Alto, CA, USA) are described elsewhere (Iwasaki et al. 2001). The LUC reporter constructs, the chick lysozyme (F2)–thymidine kinase (TK)-LUC, and the artificial direct repeat TRE, direct repeat 4 (DR4)-TK-LUC, in the PT109 vector are also described elsewhere (Koibuchi et al. 1999).
We subcloned restriction enzyme fragments of the cDNA inserts of human TRβ1 and GR into the KpnI and XbaI sites of pcDNA3. To create human TRβ1 with NotI and XhoI sites, we changed the oligo-nucleotides used to create the NotI site from Asp-97 to Arg, from Lys-98 to Pro, and from Asp-99 to Pro. The oligonucleotides we used to create the XhoI site were changed from Thr-171 to Leu and from Asp-172 to Gly (Thompson and Evans 1989). The creation of the NotI site on GR changed Pro-416 to Arg, whereas the creation of the XhoI site did not alter the GR amino acid sequence.
We constructed chimeric receptors by exchanging KpnI-NotI, NotI-XhoI, or XhoI-XbaI restriction fragments of human TRβ1 and GR with NotI and XhoI sites.
Cell culture
We maintained CV-1 and TE671 cells in Dulbecco’s modified Eagle’s medium supplemented with 5 μg/mL penicillin/streptomycin and 10% fetal bovine serum deprived of small lipophilic hormone at 37°C under a 5% CO2 atmosphere as previously described (Iwasaki et al. 2002).
Transient transfection-based reporter gene assays
We plated cells in 24-well plates 2 days before transfection by calcium phosphate coprecipitation (Iwasaki et al. 2002). The internal control was a cytomegalovirus–β-galactosidase plasmid. Sixteen to 24 hr later, the cells were incubated for 24 hr in fresh medium containing T3 and/or PCBs/dioxins. We then harvested the cells to measure the LUC activities as previously described (Iwasaki et al. 2002). We balanced total amounts of DNA per well by adding pcDNA3 plasmids (Invitrogen, San Diego, CA, USA). LUC activities were normalized to that of β-galactosidase and then calculated as relative LUC activity. We repeated all transfection studies at least twice in triplicate. Data represent means ± SEs of triplicates. We analyzed the data by analysis of variance (ANOVA) and by post hoc comparisons using Bonferroni’s multiple range test.
Electrophoretic mobility shift assay
The methods for the electrophoretic mobility shift assay (EMSA) have been previously described (Satoh et al. 1996). Briefly, we labeled double-stranded oligonucleotides using the Klenow fragment with [α-32P]-dCTP. We incubated in vitro transcribed and translated human TRβ1, mouse RXRβ, and 1 × 104 counts per minute labeled nucleotides in binding buffer [25 mM HEPES (pH 7.6), 5 mM MgCl2, 4 mM EDTA, 110 mM NaCl, 5 μg/μL bovine serum albumin, 1 μg/μL of poly(deoxyinosinic-deoxycytidylic) acid sodium salt, 20% glycerol, and 2 mM dithiothreitol] with or without 10−6 M T3 and/or 10−8 M PCBs/dioxins for 30 min on ice. We added various amounts of control reticulocyte lysate to some samples to render a consistent total volume of lysate. After incubation, the samples were resolved by electrophoresis and visualized by autoradiography.
Results
Effects of PCBs/dioxins on TR-mediated transcription
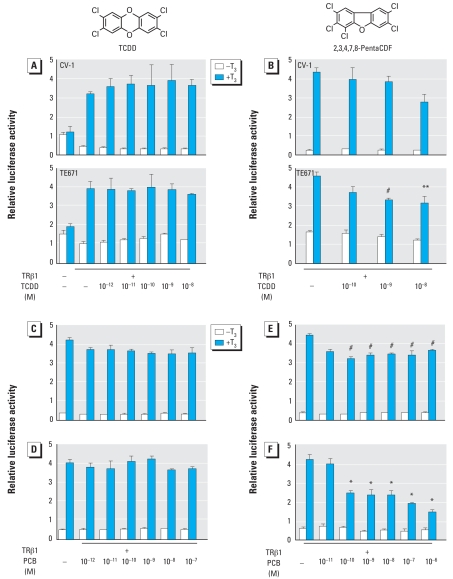
We previously reported that several PCB congeners, including OH metabolites such as 4-OH-PCB-106 and a PCB mixture (Aroclor 1254), suppress TR-mediated transcription (Iwasaki et al. 2002; Miyazaki et al. 2004). Here, we further investigated the effects of dioxins on TR-mediated transcription in monkey fibroblast-derived CV-1 and human medulloblastoma-derived TE671 cells using transient cotransfection experiments (Figure 1). The most toxic congener, TCDD (TEF = 1) (Van den Berg et al. 2006), in a range of concentrations from 10−16 to 10−6 M, did not suppress TR-mediated transcription activated by 10−7 M T3 (Figure 1A). TR-mediated transcription was weakly suppressed by 10−9 M pentaCDF (TEF = 0.3), which is relatively higher than the effective dose of 4-OH-PCB-106, in TE671 but not in CV-1 cells. Among the PCDF congeners, 2,8-diCDF and 2-monoCDF did not affect TR-mediated transcription (Figure 2).
Figure 1.
Effects of PCBs/dioxins on TR-mediated transcription (data represent mean ± SE of triplicates). (A and B) Expression plasmids encoding TRβ1 (10 ng) were cotransfected with F2-TK-LUC reporter plasmid (100 ng) into CV-1 and TE671 cells, and cells were incubated with or without 10−7 M T3 and indicated concentrations of TCDD (A) or pentaCDF (B). (C–F) Expression plasmids encoding TRβ1 (10 ng) were cotransfected with F2-TK-LUC reporter plasmid (100 ng) into CV-1 cells, and cells were incubated with or without T3 (10−7 M) and with indicated concentrations of PCB-114 (coplanar type; C), PCB-153 (D), 4-OH-PCB-165 (E), or 4-OH-PCB-106 (F).
*p < 0.01, **p < 0.02, and #p < 0.05 by ANOVA, compared with TRβ1 (+), T3 (+), and TCDD (−) in A, PCDF (−) in B, and PCB (−) in C–F.
Figure 2.
Effects of PCBs/dioxins on TR-mediated transcription in the presence of T3 (10−7 M) in CV-1 and TE671 cells. Down arrows indicate suppression > 50% at 10−8 M PCBs/dioxins; diagonal arrows indicate mild suppression (significant, but < 50% at 10−8 M); and right arrows represent no effect. TEF = 1 for the most toxic congener, TCDD. Congeners without TEF are indicated as ND (Van den Berg et al. 2006).
We also examined the effects of several representative PCB congeners, including coplanar PCBs (Figure 1C), noncoplanar PCBs (Figure 1D), and OH-PCBs (Figure 1E,F). We selected these congeners on the basis of their ortho-substitution profiles and 4-OH-PCB metabolites, which had significant effects on TR (Iwasaki et al. 2002; Miyazaki et al. 2004). Both PCB-77 (non-ortho; Figure 2), a coplanar PCB congener, and PCB-153 (di-ortho; Figure 1D), a noncoplanar PCB, slightly suppressed TR-mediated transcription at 10−10 M and 10−11 M, respectively, whereas PCB-114 (mono-ortho; Figure 1C) and 4-OH-PCB-165 (Figure 1E) had no effects. On the other hand, 4-OH-PCB-106 (Figure 1F) effectively suppressed TR-mediated transcription. Figure 2 summarizes the results of the effects of PCB/dioxin congeners on TR, including those from our previous studies under the same experimental conditions using CV-1 and TE671 cell lines (Iwasaki et al. 2002; Miyazaki et al. 2004), with a distinct difference among these compounds. Hydroxylation, degree of chlorination, and structural coplanarity do not correlate with the magnitude of suppression of TR-mediated transcription.
Correlation of suppression of TR action and TR-TRE dissociation
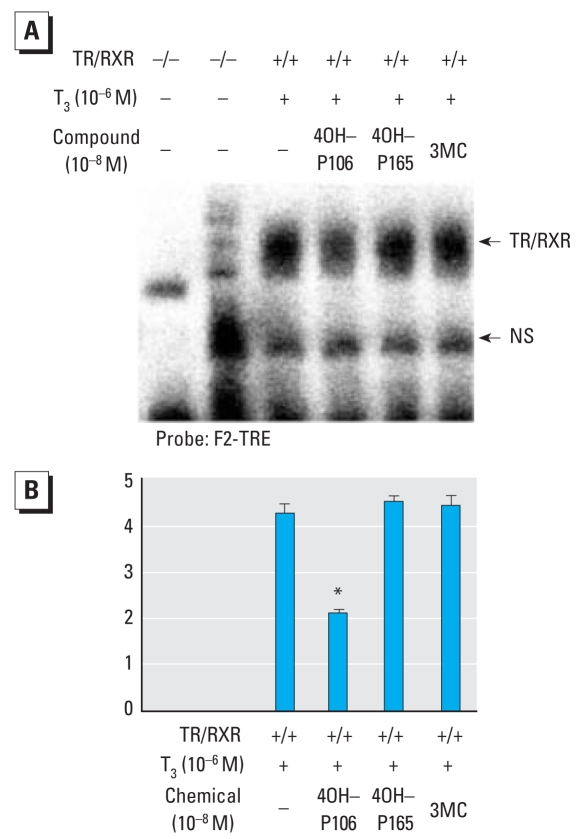
We previously reported that suppression of TR-mediated transcription by 4-OH-PCB-106 is induced by partial dissociation of TR from TRE (Miyazaki et al. 2004). We examined the effects of PCBs/dioxins on TR-TRE binding using EMSA. Some of the PCB/dioxin congeners neither altered TR-mediated transcription nor affected TR-TRE binding. On the other hand, the PCB congeners that suppressed TR-mediated transcription effectively dissociated TR-TRE binding, as shown as a representative data in Figure 3. These results suggest that the magnitude of suppression of TR-mediated transcription by PCBs/dioxins correlates with those of the dissociation of TR-TRE binding, and that the site of action of PCBs in TR may be located within the DNA-binding domain (DBD).
Figure 3.
Magnitude of TR dissociation from TRE correlated with that of suppression by PCBs/dioxins. NS, nonspecific. (A) In vitro translated TRβ1 (1.5 μL) and/or RXRβ (3 μL) incubated with [32P]-labeled F2-TRE with or without 10−6 M T3 and 10−8 M 4-OH-PCB-106 (4OH-P106), 4-OH-PCB-165 (4OH-P165), or 3-methyl coranthrene (3MC). The results were similar in three independent repeats of the same experiment, and in experiments using DR4-TRE. (B) Histogram of relative intensity of dissociated TRβ1 from TRE by adding PCBs and 3MC. The intensity values of bands are ratios of intensity values with T3 without PCB/3MC. Results are mean ± SE of three independent experiments.
*p < 0.01 by ANOVA and post hoc comparison using Bonferroni’s multiple range test compared with TR/RXR (+), T3 (+), and PCB (−).
PCBs alter TR-mediated transcription through the DBD
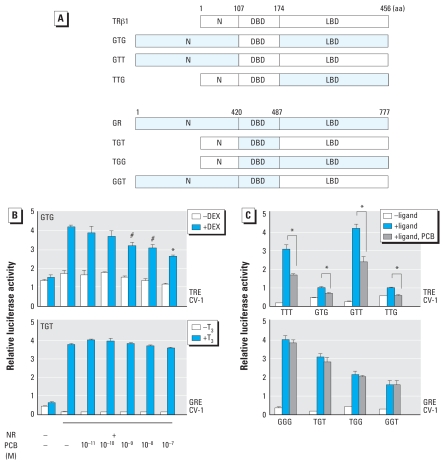
Because several PCB congeners and their OH metabolites affect TR-mediated transcription by partially dissociating TR from TRE, we determined which functional domain of TR is affected by using 4-OH-PCB-106, which had the most suppressive effect among the PCB congeners and their OH metabolites. We previously showed that 4-OH-PCB-106 did not affect GR-mediated transcription (Iwasaki et al. 2002). We therefore constructed a series of chimeric receptors containing TR and GR functional domains (Figure 4A). Transcription of chimeric receptors containing TR-DBD was suppressed by 4-OH-PCB-106 (Figure 4B,C), whereas 4-OH-PCB-106 was not significantly suppressive when the chimeric receptors contained GR-DBD (Figure 4B,C). These results indicate that PCB congeners act on the TR through DBD rather than on the TR N-terminus or TR-ligand binding domain (LBD).
Figure 4.
TR-mediated transcription altered by 4-OH-PCB-106 through TR-DBD. (A) Schematic structures and chimeric proteins used in the present study. Abbreviations: G, glucocorticoid receptor; N, N-terminal domain; NR, nuclear hormone receptor; T, thyroid hormone receptor. (B) Representative examples of PCB actions on chimeric receptor-induced transcription. Chimeric receptors (10 ng) were cotransfected with F2-TK-LUC or GRE-LUC reporter plasmid (100 ng) into CV-1 cells and incubated with or without T3 (10−7 M) or dexamethasone (DEX; 10−7 M) and 10−11–10−7 M 4-OH-PCB-106. (C) Effect of PCB on transcription through chimeric receptors containing TR-DBD or GR-DBD. Chimeric receptors (10 ng) were cotransfected with F2-TRE-LUC or GRE-LUC reporter plasmid (100 ng) into CV-1 cells and incubated with or without T3 (10−7 M) or DEX (10−7 M) and 10−11–10−7 M 4-OH-PCB-106. For B and C, data represent mean ± SE of triplicates.
*p < 0.01, and #p < 0.05 by ANOVA; for B, compared with GTG (+), DEX (+), and PCB (−).
Discussion
In the present study, we examined how PCBs/dioxins affect TR-mediated transcription and found distinct effects of several PCB congeners on TR. For example, several PCB congeners exerted significant suppression, whereas TCDD, the most toxic congener, did not. The magnitude of suppression was correlated with that of TR dissociation from TRE. Furthermore, we showed that these chemicals might act on TR-DBD.
We previously reported that the transcription mediated by TR is suppressed by 4-OH-PCB-106 (Iwasaki et al. 2002), and others have shown that dioxins and coplanar PCBs may disrupt the TH system (Bastomsky 1977; Gorski and Rozman 1987; Henry and Gasiewicz 1987; Kohn et al. 1996; Nishimura et al. 2002; Potter et al. 1986; Van Birgelen et al. 1995). Thus, we initially postulated that dioxins, coplanar PCBs, and OH-PCB compound suppress TR-mediated transcription. Although TCDD did not exert any effects, one PCDF congener and several PCB congeners, including mono-ortho–substituted congeners, suppressed TR action. On the other hand, 4-OH-PCB-165 had no suppressive effects. These results indicate that 4-hydroxylation and coplanarity are not essential for inducing the suppression.
We investigated which functional domain of TR is responsible for PCB action in a system using a series of chimeric receptors (Figure 4) and found that PCBs act on TR-DBD, but not on LBD. Although PCBs do not have high affinity for TR-LBD (Cheek et al. 1999), there are several possibilities for the interaction of PCBs and TR-DBD. It is conceivable that PCBs bind to and change the conformation of TR-DBD because we found that PCBs dissociate TR-coactivator complexes from TRE but not from TR-corepressor complexes (Miyazaki et al. 2004) and alter the binding between coactivators or corepressors and TR (Miyazaki et al. 2004). Coactivators bind to the activation function-2 domain of TR, which is located at the C-terminus of the LBD. In contrast, corepressors bind to a broad region of TRs, including the hinge region that is located immediately adjacent to the DBD. These observations are consistent with the notion that PCBs bind to the DBD and subsequently change the conformation of the domain and its surrounding region to induce the dissociation from TRE. Other possible interactions of PCBs and TR-DBD would be masking of the PCB-binding region of TR by corepressors and/or alteration of the TR-DBD conformation by PCBs binding or recruitment of a “PCB-responsive TR-binding protein.”
Although the present study revealed that PCBs suppress TR action, Gauger et al. (2007) found that some PCB congeners, such as PCB-105 and/or PCB-118 (mono-ortho PCB), may exert agonistic action toward TR-mediated transcription in rat somatomam-motroph-derived GH3 cells. Their study suggested that OH-PCB-105 or OH-PCB-118 may be responsible for this agonistic action toward TR because this action occurred when cytochrome P450 (CYP) expression was induced by PCB-126. Thus, a mixture of coplanar and noncoplanar PCBs might result in an agonistic effect on TR-mediated transcription. On the other hand, we confirmed by semiquantitative reverse transcriptase-polymerase chain reaction that CYP1A1 is not expressed in CV-1 cells and that various PCB congeners do not induce CYP1A1 expression [Supplemental Material, Figure 1S (available online at http://www.ehponline.org/members/2008/11176/suppl.pdf)]. Thus, we could refute the possible involvement of CYP1A1 and AhR in our experimental system, and the PCB congeners each might directly act on TR.
Dioxins/PCBs cause learning and memory impairment in children (Koopman-Esseboom et al. 1994) and in laboratory animals (Hojo et al. 2008; Schantz et al. 1996; Seo et al. 1999, 2000). However, the molecular mechanisms of PCB action in the brain have not been clarified. Because the amounts of AhR expressed in the brain are limited, AhR’s involvement in PCB/dioxin actions in the brain might be less than that in other organs where it is abundant, such as the liver and reproductive organs. Thus, other signaling pathways may be involved in the neurotoxic manifestations. A possible mechanism is the disruption of intracellular signaling pathways that depend on Ca2+ homeostasis (Kodavanti 2005; Simon et al. 2007). We have also shown that PCB congeners alter the intracellular Ca2+ levels in cultured neurons (Okada et al. 2005), which may be relevant to the altered expression of Ca2+ sensitive genes, such as c-Jun (Shimokawa et al. 2006). Another possible mechanism of neurotoxicity may be relevant to a decreasing trend in total T4 levels in people living in the general environment (Koopman-Esseboom et al. 1994; Nagayama et al. 1998). In utero and lactational exposures to TCDD have been reported to induce thyroid gland hyperplasia (Nishimura et al. 2003) or to induce the liver uridine diphosphate glucuronosyltransferase 1 family that catalyzes TH (Nishimura et al. 2005), which could induce hypothyroidism. However, the magnitude of decrease in brain TH levels might not be sufficient to induce hypothyroidism in the brain (Meerts et al. 2002). Instead, PCBs might suppress TR action in the brain by dissociating TR from TRE as shown in the present study, or act as agonists of TR in cells that express AhR and CYP1A1, as noted above (Gauger et al. 2007). Thus, we consider multiple pathways of PCB action to be involved in the induction of learning and memory disorders. Further analysis is required to clarify the distinct role of each of these possible signaling pathways.
In summary, we examined the effects of several representative PCB/dioxin congeners on TR-mediated transcription. We also generated chimeric receptors from TR and GR to identify the functional domain responsible for PCB action. Under our experimental conditions, PCBs apparently acted on the DBD of TR. We propose that several pathways should be considered to determine how PCB and its related compounds exert their toxic effects.
Footnotes
Supplemental Material is available online at http://www.ehponline.org/members/2008/11176/suppl.pdf
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (17510039 to T.I.; 17390060 to N.K.; 187859 to W.M.) and a grant for Long-Range Research Initiation from the Japan Chemical Industry Association (T.I. and N.K.).
References
- Aoki Y. Polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans as endocrine disrupters—what we have learned from Yusho disease. Environ Res. 2001;86:2–11. doi: 10.1006/enrs.2001.4244. [DOI] [PubMed] [Google Scholar]
- Bastomsky CH. Enhanced thyroxine metabolism and high uptake goiters in rats after a single dose of 2,3,7,8-tetra-chlorodibenzo-p-dioxin. Endocrinology. 1977;101:292–296. doi: 10.1210/endo-101-1-292. [DOI] [PubMed] [Google Scholar]
- Bogazzi F, Raggi F, Ultimieri F, Russo D, Campomori A, McKinney JD, et al. Effects of a mixture of poly-chlorinated biphenyls (Aroclor 1254) on the transcriptional activity of thyroid hormone receptor. J Endocrinol Invest. 2003;26:972–978. doi: 10.1007/BF03348194. [DOI] [PubMed] [Google Scholar]
- Brouwer A, Morse DC, Lans MC, Schuur AG, Murk AJ, Klasson-Wehler E, et al. Interactions of persistent environmental organohalogens with the thyroid hormone system: mechanisms and possible consequences for animal and human health. Toxicol Ind Health. 1998;14:59–84. doi: 10.1177/074823379801400107. [DOI] [PubMed] [Google Scholar]
- Brouwer A, van den Berg KJ. Binding of a metabolite of 3,4,3’,4’-tetrachlorobiphenyl to transthyretin reduces serum vitamin A transport by inhibiting the formation of the protein complex carrying both retinol and thyroxin. Toxicol Appl Pharmacol. 1986;85:301–312. doi: 10.1016/0041-008x(86)90337-6. [DOI] [PubMed] [Google Scholar]
- Cheek AO, Kow K, Chen J, McLachlan JA. Potential mechanisms of thyroid disruption in humans: interaction of organochlorine compounds with thyroid receptor, transthyretin, and thyroid-binding globulin. Environ Health Perspect. 1999;107:273–278. doi: 10.1289/ehp.99107273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Guo YL, Hsu CC. Cognitive development of children prenatally exposed to polychlorinated biphenyls (Yu-Cheng children) and their siblings. J Formos Med Assoc. 1992;91:704–707. [PubMed] [Google Scholar]
- Darnerud PO, Morse D, Klasson-Wehler E, Brouwer A. Binding of a 3,3’,4,4’-tetrachlorobiphenyl (CB-77) metabolite to fetal transthyretin and effects on fetal thyroid hormone levels in mice. Toxicology. 1996;106(1–3):105–114. doi: 10.1016/0300-483x(95)03169-g. [DOI] [PubMed] [Google Scholar]
- Gauger KJ, Giera S, Sharlin DS, Bansal R, Iannacone E, Zoeller RT. Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells. Environ Health Perspect. 2007;115:1623–1630. doi: 10.1289/ehp.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesy JP, Kannan K. Dioxin-like and non-dioxin-like toxic effects of polychlorinated biphenyls (PCBs): implications for risk assessment. Crit Rev Toxicol. 1998;28:511–569. doi: 10.1080/10408449891344263. [DOI] [PubMed] [Google Scholar]
- Gladen BC, Rogan WJ. Effects of perinatal polychlorinated biphenyls and dichlorodiphenyl dichloroethene on later development. J Pediatr. 1991;119:58–63. doi: 10.1016/s0022-3476(05)81039-x. [DOI] [PubMed] [Google Scholar]
- Gorski JR, Rozman K. Dose-response and time course of hypothyroxinemia and hypoinsulinemia and characterization of insulin hypersensitivity in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-treated rats. Toxicology. 1987;44:297–307. doi: 10.1016/0300-483x(87)90031-x. [DOI] [PubMed] [Google Scholar]
- Hauser P, McMillin JM, Bhatara VS. Resistance to thyroid hormone: implications for neurodevelopmental research on the effects of thyroid hormone disruptors. Toxicol Ind Health. 1998;14:85–101. doi: 10.1177/074823379801400108. [DOI] [PubMed] [Google Scholar]
- Henry EC, Gasiewicz TA. Changes in thyroid hormones and thyroxine glucuronidation in hamsters compared with rats following treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1987;89:165–174. doi: 10.1016/0041-008x(87)90037-8. [DOI] [PubMed] [Google Scholar]
- Hojo R, Kakeyama M, Kurokawa Y, Aoki Y, Yonemoto J, Tohyama C. Learning behavior in rat offspring after in utero and lactational exposure to either TCDD or PCB126. Environ Health Prevent Med. 2008;13:169–180. doi: 10.1007/s12199-008-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood A, Allen ML, Liu Y, Liu J, Klaassen CD. Induction of T(4) UDP-GT activity, serum thyroid stimulating hormone, and thyroid follicular cell proliferation in mice treated with microsomal enzyme inducers. Toxicol Appl Pharmacol. 2003;188:6–13. doi: 10.1016/s0041-008x(02)00071-6. [DOI] [PubMed] [Google Scholar]
- Huang P, Rannug A, Ahlbom E, Håkansson H, Ceccatelli S. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the expression of cytochrome P450 1A1, the aryl hydrocarbon receptor, and the aryl hydrocarbon receptor nuclear translocator in rat brain and pituitary. Toxicol Appl Pharmacol. 2000;169:159–167. doi: 10.1006/taap.2000.9064. [DOI] [PubMed] [Google Scholar]
- Huff JE, Salmon AG, Hooper NK, Zeise L. Long-term carcinogenesis studies on 2,3,7,8-tetrachlorodibenzo-p-dioxin and hexachlorodibenzo-p-dioxins. Cell Biol Toxicol. 1991;7:67–94. doi: 10.1007/BF00121331. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Chin WW, Ko L. Identification and characterization of RRM-containing coactivator activator (CoAA) as TRBP-interacting protein, and its splice variant as a coactivator modulator (CoAM) J Biol Chem. 2001;276:33375–33383. doi: 10.1074/jbc.M101517200. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Miyazaki W, Takeshita A, Kuroda Y, Koibuchi N. Polychlorinated biphenyls suppress thyroid hormone-induced transactivation. Biochem Biophys Res Commun. 2002;299:384–388. doi: 10.1016/s0006-291x(02)02659-1. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Dose-response in perinatal exposure to polychlorinated biphenyls (PCBs): the Michigan and North Carolina cohort studies. Toxicol Ind Health. 1996a;12:435–445. doi: 10.1177/074823379601200315. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996b;335:783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Evidence for PCBs as neuro-developmental toxicants in humans. Neurotoxicology. 1997;18:415–424. [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW, Humphrey HE. Effects of exposure to PCBs and related compounds on growth and activity in children. Neurotoxicol Teratol. 1990;12:319–326. doi: 10.1016/0892-0362(90)90050-m. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK. The effect of intrauterine PCB exposure on visual recognition memory. Child Dev. 1985;56:853–860. [PubMed] [Google Scholar]
- Kimura-Kuroda J, Nagata I, Kuroda Y. Hydroxylated metabolites of polychlorinated biphenyls inhibit thyroid-hormone-dependent extension of cerebellar Purkinje cell dendrites. Brain Res Dev Brain Res. 2005;154:259–263. doi: 10.1016/j.devbrainres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Jinno N, Suzuki T, Sugihara K, Ohta S, Kuroki H, et al. Thyroid hormone-like and estrogenic activity of hydroxylated PCBs in cell culture. Toxicology. 2005;208:377–387. doi: 10.1016/j.tox.2004.11.037. [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS. Neurotoxicity of persistent organic pollutants: possible mode(s) of action and further considerations. Dose Response. 2005;3:273–305. doi: 10.2203/dose-response.003.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti PRS, Shin D, Tilson HA, Harry GJ. Comparative effects of two polychlorinated biphenyl congers on calcium homeostasis in rat cerebellar granule cells. Toxicol Appl Pharmacol. 1993;123:97–106. doi: 10.1006/taap.1993.1226. [DOI] [PubMed] [Google Scholar]
- Kohn MC, Sewall CH, Lucier GW, Portier CJ. A mechanistic model of effects of dioxin on thyroid hormones in the rat. Toxicol Appl Pharmacol. 1996;136:29–48. doi: 10.1006/taap.1996.0004. [DOI] [PubMed] [Google Scholar]
- Koibuchi N, Chin WW. Thyroid hormone action and brain development. Trends Endocrinol Metab. 2000;11:123–128. doi: 10.1016/s1043-2760(00)00238-1. [DOI] [PubMed] [Google Scholar]
- Koibuchi N, Liu Y, Fukuda H, Takeshita A, Yen PM, Chin WW. ROR alpha augments thyroid hormone receptor-mediated transcriptional activation. Endocrinology. 1999;140:1356–1364. doi: 10.1210/endo.140.3.6562. [DOI] [PubMed] [Google Scholar]
- Koopman-Esseboom C, Morse DC, Weisglas-Kuperus N, Lutkeschipholt IJ, Van der Paauw CG, Tuinstra LG, et al. Effects of dioxins and polychlorinated biphenyls on thyroid hormone status of pregnant women and their infants. Pediatr Res. 1994;36:468–473. doi: 10.1203/00006450-199410000-00009. [DOI] [PubMed] [Google Scholar]
- Lein PJ, Yang D, Bachstetter AD, Tilson HA, Harry GJ, Mervis RF, et al. Ontogenetic alterations in molecular and structural correlates of dendritic growth after developmental exposure to polychlorinated biphenyls. Environ Health Perspect. 2007;115:556–563. doi: 10.1289/ehp.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu Y, Barter RA, Klaassen CD. Alteration of thyroid homeostasis by UDP-glucuronosyltransferase inducers in rats: a dose-response study. J Pharmacol Exp Ther. 1995;273:977–985. [PubMed] [Google Scholar]
- McKinney JD. Multifunctional receptor model for dioxin and related compound toxic action: possible thyroid hormone-responsive effector-linked site. Environ Health Perspect. 1989;82:323–336. doi: 10.1289/ehp.8982323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IATM, Assink Y, Cenijn PH, van den Berg JHJ, Weijers BM, Bergman Å, et al. Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat. Toxicol Sci. 2002;68:361–371. doi: 10.1093/toxsci/68.2.361. [DOI] [PubMed] [Google Scholar]
- Miyazaki W, Iwasaki T, Takeshita A, Kuroda Y, Koibuchi N. Polychlorinated biphenyls suppress thyroid hormone receptor-mediated transcription through a novel mechanism. J Biol Chem. 2004;279:18195–18202. doi: 10.1074/jbc.M310531200. [DOI] [PubMed] [Google Scholar]
- Nagayama J, Okamura K, Iida T, Hirakawa H, Matsueda T, Tsuji H, et al. Postnatal exposure to chlorinated dioxins and related chemicals on thyroid hormone status in Japanese breast-fed infants. Chemosphere. 1998;37:1789–1793. doi: 10.1016/s0045-6535(98)00244-6. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Miyabara Y, Sato M, Yonemoto J, Tohyama C. Immunohistochemical localization of thyroid stimulating hormone induced by a low oral dose of 2,3,7,8-tetra-chlorodibenzo-p-dioxin in female Sprague-Dawley rats. Toxicology. 2002;171:73–82. doi: 10.1016/s0300-483x(01)00559-5. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Yonemoto J, Miyabara Y, Sato M, Tohyama C. Rat thyroid hyperplasia induced by gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Endocrinology. 2003;144:2075–2083. doi: 10.1210/en.2002-220737. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Yonemoto J, Nishimura H, Ikushiro S, Tohyama C. Disruption of thyroid hormone homeostasis at weaning of Holtzman rats by lactational but not in utero exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2005;85:607–614. doi: 10.1093/toxsci/kfi122. [DOI] [PubMed] [Google Scholar]
- Okada J, Shimokawa N, Koibuchi N. Polychlorinated biphenyl (PCB) alters acid-sensitivity of cultured neurons derived from the medulla oblongata. Int J Biochem Cell Biol. 2005;37:1368–1374. doi: 10.1016/j.biocel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, Schwartz HL. Molecular basis of thyroid hormone-dependent brain development. Endocr Rev. 1997;18:462–475. doi: 10.1210/edrv.18.4.0309. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Curran MA, Marconi SA, Carpenter CD, Lubbers LS, McAbee MD. Distribution of mRNAs encoding the arylhydrocarbon receptor, arylhydrocarbon receptor nuclear translocator, and arylhydrocarbon receptor nuclear translocator-2 in the rat brain and brainstem. J Comp Neurol. 2000;427:428–439. doi: 10.1002/1096-9861(20001120)427:3<428::aid-cne9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Porterfield SP. Thyroidal dysfunction and environmental chemicals—potential impact on brain development. Environ Health Perspect. 2000;108(suppl 3):433–438. doi: 10.1289/ehp.00108s3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development—current perspectives. Endocr Rev. 1993;14:94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- Potter CL, Moore RW, Inhorn SL, Hagen TC, Peterson RE. Thyroid status and thermogenesis in rats treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1986;84:45–55. doi: 10.1016/0041-008x(86)90415-1. [DOI] [PubMed] [Google Scholar]
- Safe SH. Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol. 1994;24:87–149. doi: 10.3109/10408449409049308. [DOI] [PubMed] [Google Scholar]
- Satoh T, Yamada M, Iwasaki T, Mori M. Negative regulation of the gene for the preprothyrotropin-releasing hormone from the mouse by thyroid hormone requires additional factors in conjunction with thyroid hormone receptors. J Biol Chem. 1996;271:27919–27926. doi: 10.1074/jbc.271.44.27919. [DOI] [PubMed] [Google Scholar]
- Schantz SL, Seo BW, Moshtaghian J, Peterson RE, Moore RW. Effects of gestational and lactational exposure to TCDD or coplanar PCBs on spatial learning. Neurotoxicol Teratol. 1996;18:305–313. doi: 10.1016/s0892-0362(96)90033-1. [DOI] [PubMed] [Google Scholar]
- Seo BW, Powers BE, Widholm JJ, Schantz SL. Radial arm maze performance in rats following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Neurotoxicol Teratol. 2000;22:511–519. doi: 10.1016/s0892-0362(00)00070-2. [DOI] [PubMed] [Google Scholar]
- Seo BW, Sparks AJ, Medora K, Amin S, Schantz SL. Learning and memory in rats gestationally and lactationally exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Neurotoxicol Teratol. 1999;21:231–239. doi: 10.1016/s0892-0362(98)00049-x. [DOI] [PubMed] [Google Scholar]
- Sewall CH, Flagler N, Vanden Heuvel JP, Clark GC, Tritscher AM, Maronpot RM, et al. Alterations in thyroid function in female Sprague-Dawley rats following chronic treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1995;132:237–244. doi: 10.1006/taap.1995.1104. [DOI] [PubMed] [Google Scholar]
- Shimokawa N, Miyazaki W, Iwasaki T, Koibuchi N. Low dose hydroxylated PCB induces c-Jun expression in PC12 cells. Neurotoxicology. 2006;27:176–183. doi: 10.1016/j.neuro.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Simon T, Britt JK, James RC. Development of a neurotoxic equivalence scheme of relative potency for assessing the risk of PCB mixtures. Regul Toxicol Pharmacol. 2007;48:148–170. doi: 10.1016/j.yrtph.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Tilson HA, Jacobson JL, Rogan WJ. Polychlorinated biphenyls and the developing nervous system: cross-species comparisons. Neurotoxicol Teratol. 1990;12:239–248. doi: 10.1016/0892-0362(90)90095-t. [DOI] [PubMed] [Google Scholar]
- Tilson HA, Kodavanti PR. Neurochemical effects of poly-chlorinated biphenyls: an overview and identification of research needs. Neurotoxicology. 1997;18:727–743. [PubMed] [Google Scholar]
- Thompson CC, Evans RM. Trans-activation by thyroid hormone receptors: functional parallels with steroid hormone receptors. Proc Natl Acad Sci USA. 1989;86:3494–3498. doi: 10.1073/pnas.86.10.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Birgelen AP, Smit EA, Kampen IM, Groeneveld CN, Fase KM, Van der Kolk J, et al. Subchronic effects of 2,3,7,8-TCDD or PCBs on thyroid hormone metabolism: use in risk assessment. Eur J Pharmacol. 1995;293:77–85. doi: 10.1016/0926-6917(95)90021-7. [DOI] [PubMed] [Google Scholar]
- Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Dowling AL, Vas AA. Developmental exposure to polychlorinated biphenyls exerts thyroid hormone-like effects on the expression of RC3/neurogranin and myelin basic protein messenger ribonucleic acids in the developing rat brain. Endocrinology. 2000;141:181–189. doi: 10.1210/endo.141.1.7273. [DOI] [PubMed] [Google Scholar]