Abstract
Sordaria macrospora, a self-fertile filamentous ascomycete, carries genes encoding three different α-subunits of heterotrimeric G proteins (gsa, G protein Sordaria alpha subunit). We generated knockout strains for all three gsa genes (Δgsa1, Δgsa2, and Δgsa3) as well as all combinations of double mutants. Phenotypic analysis of single and double mutants showed that the genes for Gα-subunits have distinct roles in the sexual life cycle. While single mutants show some reduction of fertility, double mutants Δgsa1Δgsa2 and Δgsa1Δgsa3 are completely sterile. To test whether the pheromone receptors PRE1 and PRE2 mediate signaling via distinct Gα-subunits, two recently generated Δpre strains were crossed with all Δgsa strains. Analyses of the corresponding double mutants revealed that compared to GSA2, GSA1 is a more predominant regulator of a signal transduction cascade downstream of the pheromone receptors and that GSA3 is involved in another signaling pathway that also contributes to fruiting body development and fertility. We further isolated the gene encoding adenylyl cyclase (AC) (sac1) for construction of a knockout strain. Analyses of the three ΔgsaΔsac1 double mutants and one Δgsa2Δgsa3Δsac1 triple mutant indicate that SAC1 acts downstream of GSA3, parallel to a GSA1–GSA2-mediated signaling pathway. In addition, the function of STE12 and PRO41, two presumptive signaling components, was investigated in diverse double mutants lacking those developmental genes in combination with the gsa genes. This analysis was further completed by expression studies of the ste12 and pro41 transcripts in wild-type and mutant strains. From the sum of all our data, we propose a model for how different Gα-subunits interact with pheromone receptors, adenylyl cyclase, and STE12 and thus cooperatively regulate sexual development in S. macrospora.
IN eukaryotes, heterotrimeric GTP-binding proteins consisting of α-, β-, and γ-subunits interact with activated heptahelical transmembrane receptors (G protein-coupled receptors, GPCRs) and transduce various environmental signals to stimulate morphogenesis and cellular response. Upon activation by an extracellular signal, the receptor promotes the exchange of GDP for GTP on the Gα-subunit of the heterotrimeric G protein. This in turn leads to the dissociation of Gα from the βγ-complex and each complex can bind and regulate effectors that then can propagate signals into the cell (Hamm 1998; Lengeler et al. 2000). During evolution, G protein subunit genes have expanded enormously in number and diversity. The most complex situation is found in the genome of humans where 27 different genes encoding for Gα-subunits are found (Albert and Robillard 2002). On the basis of sequence similarity, the mammalian Gα-subunits have been divided into four families: (1) Gs activates adenylyl cyclase (AC), (2) Gi inhibits adenylyl cyclase, (3) Gq activates phospholipase C (PLC), and (4) Gα-subunits currently having an unknown function (Hamm 1998). In the genome of the yeast Saccharomyces cerevisiae, only two genes for Gα-subunits (GPA1 and GPA2) have been detected and these are known to play significant roles in mating and filamentous growth (Kübler et al. 1997; Schrick et al. 1997). During sexual development of S. cerevisiae, two haploid mating types, a and α, communicate via pheromones. While a-cells express genes for a lipopeptide pheromone (a-factor) and the GPCR Ste2p sensing the extracellular α-pheromone, α-cells express genes for a peptide pheromone (α-factor) and the GPCR Ste3p sensing the a-factor. In both cell types, Ste2p and Ste3p are coupled to Gpa1p, one of the two Gα-subunits that forms a conventional heterotrimeric G protein with βγ-subunits Ste4p/18p (Dohlman and Thorner 2001). Recent studies by Slessareva et al. (2006) revealed a new function for Gpa1p, when they discovered that this Gα-subunit not only is located at the plasma membrane, but also is present at the endosomes where it stimulates phosphoinositide 3-kinase (PI3K) to produce PI 3-phosphate. Thus, this Gα-subunit contributes activity to the mating response pathway by signaling external signals to internal cellular compartments. The second Gα-subunit of S. cerevisiae, Gpa2p, senses nutrients and controls filamentous growth. This subunit acts upstream of the adenylyl cyclase that generates the second messenger cAMP in response to glucose (Versele et al. 2001).
To study the function of different Gα-subunits within a multicellular eukaryote, filamentous fungi are ideal model systems for such experimental investigations. Genomic sequencing has revealed that most filamentous ascomycetes have three Gα-subunits (Liu and Dean 1997; Chang et al. 2004; Kays and Borkovich 2004). Among them, Gα-subunits of the fungal group I share specific sequence similarities with the mammalian Gi subunits, while the subunits of group III have been assigned as Gs subunits on the basis of their functionality in stimulating adenylyl cyclases like their mammalian counterparts. The third fungal Gα-subunit placed in group II has no known mammalian counterpart (Bölker 1998). Currently, the best studied example among filamentous fungi is Neurospora crassa, where the three Gα-subunits are known to contribute significantly to sexual and vegetative development (Ivey et al. 1996; Baasiri et al. 1997; Kays et al. 2000; Kays and Borkovich 2004). In this heterothallic fungus, disruption of the genes encoding the Gα-subunits has an effect not only on fruiting body development, but also on the fertilization process. In N. crassa, fertilization is accomplished by growth of the trichogyne from a protoperithecium toward the male for which either a conidiospore or a somatic cell can act as a male cell. In both cases, the male cell must be derived from a strain of the opposite mating type. In N. crassa, this fertilization process is a prerequisite for fruiting body formation and ascospore development (Springer 1993; Davis 2000). Mutations of different components of the signaling pathway affect different steps of the complex fertilization process. For example, if the fusion of the male cell with the trichogyne is impaired as in the Gα-mutant Δgna-1 (Kim and Borkovich 2004), later steps of the sexual developmental pathway are blocked and therefore cannot be analyzed.
We previously established the homothallic ascomycete Sordaria macrospora as a model system to investigate sexual development and to determine key players controlling fruiting body differentiation. S. macrospora lacks any structures for asexual propagation like conidia, thus no overlapping developmental processes occur. Therefore, S. macrospora is an ideal model organism to study sexual differentiation. Moreover, developmental defects are immediately apparent in this self-fertile fungus without the necessity of fertilization (Pöggeler et al. 2006a). To date, several S. macrospora pro mutants that develop protoperithecia but no perithecia have been generated and characterized, thereby revealing essential components of fruiting body development (Masloff et al. 1999; Nowrousian et al. 1999, 2007a; Pöggeler and Kück 2004; Kück 2005; Engh et al. 2007). In addition, despite the fact that S. macrospora completes the sexual cycle without a mating partner, two pheromone-precursor (ppg1 and ppg2) and two pheromone-receptor genes (pre1 and pre2) were shown to be involved in sexual development (Pöggeler and Kück 2000; Mayrhofer and Pöggeler 2005; Mayrhofer et al. 2006; Pöggeler et al. 2006b). The two receptors show significant amino acid similarities to the pheromone receptors in S. cerevisiae (Pöggeler and Kück 2001).
Here, we present a genetic analysis of knockout strains Δgsa1, Δgsa2, and Δgsa3 that correspond to the three genes encoding Gα-subunits that act in different ways on sexual development and growth. We further address the question whether the Gα-subunits interact genetically with adenylyl cyclase, STE12 and PRO41. For this purpose, we generated 18 double mutants and a single triple mutant from the above-described mutant strains. With a total of 27 mutants, we genetically dissected the signaling pathway upstream and downstream of the Gα-subunits. To the best of our knowledge, double mutants carrying a deleted gsa gene together with a disrupted adenylyl cyclase, pheromone receptor, and ste12 transcription factor gene are described for the first time for a filamentous fungus. In addition, we analyzed the impact of the G protein signaling network on previously known developmentally regulated genes using expression analysis. The sum of our data led us to propose a model on how Gα-subunits interact differently with upstream or downstream signaling components and how they act on developmental processes.
MATERIALS AND METHODS
Strains, media, and growth conditions:
Cloning and propagation of recombinant plasmids were performed in Escherichia coli strain XL1Blue MRF′ (Stratagene, La Jolla, CA) under standard culture conditions (Sambrook and Russel 2001). All S. macrospora strains were cultivated on cornmeal or CM medium (Esser 1982; Nowrousian et al. 1999). For supplementation of the Δsac1 strain with cAMP, 3.4 mm of dibutyryl-cAMP (db-cAMP) (Biolog Life Science Institute, Bremen, Germany) was added to solid cornmeal medium. For RNA extraction, strains were grown for 5 days in synthetic crossing medium as described previously (Nowrousian et al. 2005). Growth rates were measured in race tubes as described by Nowrousian and Cebula (2005). Transformation of S. macrospora was performed according to Nowrousian et al. (1999) with 0.4 g Glucanex 200 G (Novozymes Switzerland AG, Neumatt, Dittingen, Switzerland) for cell wall degradation. Details for all S. macrospora strains are given in Table 1.
TABLE 1.
Sordaria macrospora strains used in this study
| Strain | Relevant genotype and phenotype | Reference or source |
|---|---|---|
| S48977 | Wild type | Our culture collection |
| S23442 | fus1, spore color mutant | Our culture collection |
| S67813 | r2, spore color mutant | Our culture collection |
| S66001 | Δku70∷nat | Pöggeler and Kück (2006) |
| S2-2-1 | Δpre1∷hph | Mayrhofer et al. (2006) |
| S60441 | Δpre2∷hph | Mayrhofer et al. (2006) |
| J223 | Δgsa1∷hph | This study |
| 10-49-1 | Δgsa2∷hph | This study |
| S72902 | Δgsa3/r2∷hph | This study |
| K23 | Δsac1∷hph | This study |
| S68567 | Δste12/fus∷hph | Nolting and Pöggeler (2006) |
| S46357 | pro41 | Nowrousian et al. (2007a) |
| J283 | Δgsa1∷hph/Δgsa2∷hph | This study |
| J125 | Δgsa1∷hph/Δpre1∷hph | This study |
| S71427 | Δgsa1∷hph/Δpre2∷hph | This study |
| S73097 | Δgsa1∷hph/Δsac1∷hph | This study |
| S80762 | Δgsa1∷hph/Δste12/fus∷hph | This study |
| S81063 | Δgsa1∷hph/pro41 | This study |
| S75575 | Δgsa2∷hph/Δgsa3/r2∷hph | This study |
| S68487 | Δgsa2∷hph/Δpre1∷hph | This study |
| S68093 | Δgsa2∷hph/Δpre2∷hph | This study |
| S72594 | Δgsa2∷hph/Δsac1∷hph | This study |
| S83053 | Δgsa2∷hph/Δste12/fus∷hph | This study |
| S81167 | Δgsa2∷hph/pro41 | This study |
| S73402 | Δgsa3/r2∷hph/Δgsa1∷hph | This study |
| K101 | Δgsa3/r2∷hph/Δpre1∷hph | This study |
| S75162 | Δgsa3/r2∷hph/Δpre2∷hph | This study |
| J423 | Δgsa3/r2∷hph/Δsac1∷hph | This study |
| S83821 | Δgsa3/r2∷hph/Δste12/fus∷hph | This study |
| S81284 | Δgsa3/r2∷hph/pro41 | This study |
| S77487 | Δgsa2∷hph/Δgsa3/r2∷hph/Δsac1∷hph | This study |
Identification and DNA sequencing of three genes for G protein α-subunits and of a gene encoding adenylyl cyclase from S.macrospora:
To isolate the gsa genes encoding Gα-subunits, two different strategies were used. While gsa1 and gsa3 were isolated by direct amplification of S. macrospora genomic DNA with primers that were designed according to the sequence of the homologous N. crassa genes, gsa2 was identified by screening a S. macrospora cosmid library (Pöggeler et al. 1997). The heterologous oligonucleotides used for all three genes encoding Gα-subunits were based on the N. crassa sequence as previously described (accession nos. U56090.1, AF004846, and AF281862, Nowrousian et al. 2004; Pöggeler and Kück 2006). All DNA sequencing was performed by GATC Biotech AG (Konstanz, Germany). Primers were synthesized at MWG Biotech AG (Eversberg, Germany). PCR amplicons of the gsa1 and gsa3 open reading frames were obtained with primer pairs gna1-3 and gna1-4 and gna3-1 and gna3-6, respectively (Table 2). Sequences adjacent to the gsa1 and gsa3 genes were obtained by inverse PCR. Prior to amplification, genomic DNA from the S. macrospora wild type was digested with PvuI (5′ region) or NcoI (3′ region) (for gsa1) and AvaI (for gsa3 5′ region). Ligation and further amplification of the flanking regions were performed according to Nowrousian et al. (2007b) with the following primer pairs: gsa1-7 and gsa1-8 for gsa1 5′, gsa1-9 and gsa1-10 for gsa1 3′, and gsa3-7 and gsa3-8 for gsa3 5′ (Table 2). Using oligonucleotide primers gna2-1 and gna2-2, a S. macrospora indexed cosmid library was screened for gsa2 (Pöggeler et al. 1997). This led to the isolation of cosmid D10 containing the gsa2 gene of S. macrospora and its flanking sequences.
TABLE 2.
Oligonucleotides used in this study
| Oligonucleotide | Sequence (5′–3′) | Specificity |
|---|---|---|
| gna1-1 | GAAGCAGATGAAGCTTATCCA | gsa1 |
| gna1-2 | TCGAACATACGGTACGTAAGA | gsa1 |
| gna1-3 | ATGGGTTGCGGAATGAGTACAGAG | gna-1 N. crassa |
| gna1-4 | AAACCGCAGAGACGCAGGTTCTC | gna-1 N. crassa |
| gsa1-1 | CGATCGCATCGGTCTTCGTTTC | 5′ flank gsa1 |
| gsa1-2 | AGCGCTGCCATGCCCGACAAT | 5′ flank gsa1 |
| gsa1-3 | GAATTCTCAGTCCTGCTCCTTTTGGCGACTTGTTGTAACTCTTGG | 5′ flank gsa1 with hph overhang |
| gsa1-4 | TCCTCTAGAGTCGACCTGCAGCATTGAACCCAGTCTAATTTTTCAC | 3′ flank gsa1 with hph overhang |
| gsa1-5 | TTCCCCCACACTGCCCATGAAAG | 3′ flank gsa1 |
| gsa1-6 | CCATGGGCTTGAGTCCCACTAC | 3′ flank gsa1 |
| gsa1-7 | CTGTACTCATTCCGCAACCCAT | gsa1 (inverse PCR) |
| gsa1-8 | GTCGACCATCTTGAAGCAGATG | gsa1 (inverse PCR) |
| gsa1-9 | CCAGTGGCAACTCTAGGGACT | gsa1 (inverse PCR) |
| gsa1-10 | GAGAACCTGCGTCTCTGCGG | gsa1 (inverse PCR) |
| 5′gsa1 | TCAATGAGCGCTGCCATGCCCG | 5′ flank gsa1 |
| 3′gsa1 | ACTTTCCCCCACACTGCCCATGA | 3′ flank gsa1 |
| gna2-1 | AAGTTGATCTATGCACAAGG | gna-2 N. crassa |
| gna2-2 | AAATATTCTAGTGGTAGAGG | gna-2 N. crassa |
| gna2-3 | ATGTGTTTCGGGGGTCGTGG | gsa2 |
| gna2-4 | ACAGGATAAGTTGTTTGAGGTTC | gsa2 |
| gsa2-1 | GTGGGCCTAGCATGCAGAAT | 5′ flank gsa2 |
| gsa2-2 | TGATCGTCCCTGTCTCCATTG | 5′ flank gsa2 |
| gsa2-3 | GAATTCTCAGTCCTGCTCCTTATGTGTTGGATCCGTGTTGCAGAG | 5′ flank gsa2 with hph overhang |
| gsa2-4 | TCCTCTAGAGTCGACCTGCACACATCCGATACATCTGCTTCCGCA | 3′ flank gsa2 with hph overhang |
| gsa2-5 | CCAACGTGAGGGAGAGGTGA | 3′ flank gsa2 |
| gsa2-6 | TGCAAGCTAACTTGAAATCTCC | 3′ flank gsa2 |
| 5′gsa2 | GGCTCCCACCACACTCGCTGTCTGTC | 5′ flank gsa2 |
| 3′gsa2 | ATCATCGAGAATAGCTCCTCTGTAC | 3′ flank gsa2 |
| gna3-1 | ATGGGCGCATGCATGAGC | gsa3 S. macrospora; gna-3 N. crassa |
| gna3-2 | ACTTGGCGGCCTTGTTGAC | gsa3 |
| gna3-6 | TCATAGAATACCGGAGTCTTTAAG | gna-3 N. crassa |
| gsa3-1 | CCCATCTCCCTCCCGCAAGAT | 5′ flank gsa3 |
| gsa3-2 | TTGCCTGCCTGCCTTTCACCTTTACC | 5′ flank gsa3 |
| gsa3-3 | GAATTCTCAGTCCTGCTCCTATGAGGCAGTCGACGATGGTCCG | 5′ gsa3 with hph overhang |
| gsa3-4 | TCCTCTAGAGTCGACCTGCACGTAGCGAACGCAAGAAGTGGATT | 3′ gsa3 with hph overhang |
| gsa3-5 | CCGGAGTCTTTAAGAGCGTTGTTG | 3′ flank gsa3 |
| gsa3-6 | TCATAGAATACCGGAGTCTTTAAG | 3′ flank gsa3 |
| gsa3-7 | TTCTTCTGCTCCGTCTCCTC | gsa3 (inverse PCR) |
| gsa3-8 | AAGAAGTGGATTCACTGCTTCG | gsa3 (inverse PCR) |
| 5′gsa3 | GCATTGCCTGCCTGCCTTTCACCTTT | 5′ flank gsa3 |
| sac1 | ACTGCCGACGGGAAGCTCAATG | 5′ flank sac1 |
| sac2 | ATGGCAGATGGGAGTGGTGGTAC | 5′ flank sac1 |
| sac3 | GAATTCTCAGTCCTGCTCCTACTACTAGGAGACCACAGCCATTCCACG | 5′ sac1 with hph overhang |
| sac4 | TCCTCTAGAGTCGACCTGCATCAAGCTGCATCATCATCACCATCTGCTC | 3′ sac1 with hph overhang |
| sac5 | ATCCAGGACCCAAAGACTAACC | 3′ flank sac1 |
| sac6 | TATATGTAACTGTCGCAGGTGG | 3′ flank sac1 |
| sac-f | AGGCTGGTGCTTACCTACCG | sac1 |
| sac-r | ATTGAACAGATGAGAACGACC | 3′ flank sac1 |
| sac10s | ATGATAGAATTGAAGCGTGG | sac1 |
| 5′sac1 | ATAATGCTAGTTGTACCTCTAAGAGAGTCG | 5′ flank sac1 |
| 3′sac1 | ACATACACAGATTCGATCACCATCACCGATG | 3′ flank sac1 |
| 1091 | AAGACGGAAGGAGACGCATTTATG | cr-1 N. crassa |
| 1092 | CTCAAGGCCTTTGAGCTTCTTCTC | cr-1 N. crassa |
| u-cr-1 | TGCGGCTGATTATGGAGGA | cr-1 N. crassa |
| cr-1for | AGCGCAGCAATTCAAGGGACAGC | cr-1 N. crassa |
| cr-1rev | GTTGCAAGAGCGCCATAGGGC | cr-1 N. crassa |
| D5 | CACCACCACACAGAGGAAAC | 5′ flank ste12 |
| hph-f | AGGAGCAGGACTGAGAATTC | hph cassette |
| hph-r | TGCAGGTCGACTCTAGAGGA | hph cassette |
| hph-if | TCCAGTCAATGACCGCTGTTATG | hph cassette internal |
| hph-ir | TCCAACAATGTCCTGACGGACA | hph cassette internal |
| h3 | GGGCCCGAAACGAACTAGAGTTCTAG | hph cassette internal |
| SSU1 | ATCCAAGGAAGGCAGCAGGC | SSU rRNA (real-time PCR) |
| SSU2 | TGGAGCTGGAATTACCGCG | SSU rRNA (real-time PCR) |
| ste12for | CTTCGCAGCATGCCAATATG | ste12 (real-time PCR) |
| ste12rev | GCGCGGAAATGAGGAAATAC | ste12 (real-time PCR) |
| SMU2767for | GTGGCCGCTCGGTTTTATTG | pro41 (real-time PCR) |
| SMU2767rev | TCACCTGGTAAATCGCAGCGT | pro41 (real-time PCR) |
For the isolation of the sac1 gene encoding the adenylyl cyclase, the S. macrospora cosmid library was screened with a sac1-specific fragment that was generated by PCR amplification using S. macrospora genomic DNA as template and the N. crassa cr-1 specific oligonucleotides 1091 and 1092 (accession no. D00909.1, Table 2). This resulted in the isolation of cosmid H2, carrying the 3′ end of the coding region of sac1 that was further sequenced by primer walking and random insertion of pGPS2.1-hph plasmid (Dreyer et al. 2007) using the Tn7L and Tn7R sequences of the integrated transposable element. The 5′ end of the sac1 gene and its 5′ flanking region was amplified from genomic S. macrospora DNA with an oligonucleotide (u-cr-1) homologous to the corresponding N. crassa sequence and a S. macrospora-specific oligonucleotide (sac10s). The resulting 4.2 kb amplicon was cloned in pDrive (Qiagen GmbH, Hilden, Germany) and the recombinant plasmid pDAde was sequenced by means of primer walking. Details for the plasmids used in this study are given in Table 3.
TABLE 3.
Plasmids and cosmids used in this study
| Plasmid/cosmid | Feature | Reference |
|---|---|---|
| pDrive | UA-based PCR cloning | Qiagen |
| pGPS2.1-hph | pGPS2.1 carrying hph, Transprimer-2 element | Dreyer et al. (2007) |
| pD-NAT1 | pDrive with nat1 | Kück and Hoff (2006) |
| pD194.1 | pDrive with gsa3 and 5′ flanking region | This study |
| pD202 | pDrive with gsa1 and flanking regions | This study |
| pDAde | pDrive with sac1 5′ flanking region | This study |
| D10 | Cosmid from pool 2012-2116A containing gsa2 | Pöggeler et al. (1997) |
| H2 | Cosmid from pool 2213-2308VIB containing sac1 | Pöggeler et al. (1997) |
| H122 F6 | N. crassa cr-1 in pLorist6Xh | Fungal Genetics Stock Center (FGSC) |
Sequence analysis:
DNA and protein sequence data were obtained from the public databases at NCBI (http://www.ncbi.nlm.nih.gov/sites/entrez) or for N. crassa sequences, at the Broad Institute (http://www.broad.mit.edu/annotation/genome/neurospora/Home.html). BLAST analysis (Altschul et al. 1990) was performed at the NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi) or the Broad Institute (http://www.broad.mit.edu/annotation/genome/neurospora/Blast.html). Sequence alignments were carried out with ClustalW (http://align.genome.jp, Thompson et al. 1994), and the corresponding graphical editing was performed with GeneDoc (http://www.psc.edu/biomed/genedoc, Nicholas et al. 1997).
Preparation of nucleic acids, hybridization protocols, and PCR:
Isolation of S. macrospora genomic DNA was carried out as described by Pöggeler et al. (1997). Southern blotting and hybridization was done according to standard techniques (Sambrook and Russel 2001) using 32P-labeled DNA probes. For the construction of probes, PCR fragments carrying the gsa1, -2, -3, sac1, or hph gene were amplified from S. macrospora genomic DNA or pZHK2 (Kück and Pöggeler 2004) as template. PCR was performed with HotStarTaq DNA polymerase (Qiagen GmbH, Hilden, Germany), HotMaster Taq (Eppendorf AG, Hamburg, Germany), GoTaq (Promega, Madison, WI) or the Taq and Pwo DNA polymerase blend from the Expand Long Template PCR system (Roche AG, Basel, Switzerland) according to manufacturer's protocols. Extraction of total RNA and quantitative real-time PCR were performed as described previously (Nowrousian et al. 2005). Oligonucleotides that were used as primers for quantitative real-time PCR are given in Table 2.
Generation of Δgsa1, Δgsa2, Δgsa3, and Δsac1 knockout strains:
Knockout constructs for homologous recombination in S. macrospora were generated using PCR-based fusions (Shevchuk et al. 2004). The flanking regions of the three gsa and the sac1 genes were amplified with S. macrospora genomic DNA as template using primer pairs located upstream or downstream of the corresponding gene (Table 2; supplemental Figure S1A, oligonucleotides given in italics). The gene for the hph resistance marker was amplified with primer pair hph-f and hph-r (Table 2; supplemental Figure S1A, given in italics) from plasmid pZHK2 (Kück and Pöggeler 2004) and fused to the amplified flanking regions. The PCR products obtained served as template for the next step to generate the complete knockout constructs with nested primers (gsa1-2 and gsa1-5, gsa2-2 and gsa2-5, gsa3-2 and gsa3-5, and sac2 and sac5) as specified in Table 2 and supplemental Figure S1A (given in italics). To generate mutant strains Δgsa1, Δgsa2, Δgsa3, and Δsac1, the resulting PCR amplicons were used to transform the wild-type or Δku70 strain impaired in heterologous recombination (Pöggeler and Kück 2006). In each of the four strains, the corresponding gene was substituted by the hph gene through homologous recombination. Recombinant strains carrying the hph cassette instead of gsa1, -2, -3, or sac1 were identified by PCR with primer pairs that are specific for sequences external and internal of the knockout cassette (see Table 2 and supplemental Figure S1A, given in gray). The number of integrated hph copies in the genome of the mutant strains was determined by Southern analysis with an hph-specific probe amplified with the primer pair hph-f and hph-r (Table 2) from the plasmid pZHK2 (Kück and Pöggeler 2004). Fungal transformants are often heterokaryotic and thus mycelia carry transformed and nontransformed nuclei. Therefore, single spore isolates were generated by crossing the putative knockout mutants with strain r2 (S67813) or fus1 (S23442), having red-colored ascospores (Table 1). To rescue the phenotype of single mutants Δgsa1 (J223) and Δgsa3/r2 (S72902), plasmid pD202 and pD194.1, respectively, each carrying a corresponding wild-type copy of the disrupted genes, were cotransformed with plasmid pD-NAT1, carrying the nourseothricin resistance gene nat1 (Table 3, Kück and Hoff 2006). To complement the phenotype of the Δgsa1Δgsa2 (J283) and Δgsa1Δsac1 (S73097) double mutants, plasmid pD202 carrying the gsa1 gene, was cotransformed with plasmid pD-NAT1. Cosmid H122 F6 (FGSC, Table 3), containing the entire N. crassa cr-1 gene, was cotransformed with pD-NAT1 to rescue the Δsac1 mutant.
Double- and triple-knockout strains:
Double- and triple-knockout strains (Table 1) were generated by crossing the single knockout strains using conventional genetic methods as described by Esser and Straub (1958). Asci from recombinant perithecia were isolated and spore isolates of selected asci were analyzed by PCR and by backcrossing.
Microscopic investigations:
S. macrospora strains were grown on solid cornmeal medium on slides in glass petri dishes as described by Engh et al. (2007). Ascospore germination rates were determined after incubation for 5 hr on solid cornmeal medium containing 0.5% (w/v) sodium acetate. Light microscopy was carried out with an Axiophot microscope (Carl Zeiss AG, Oberkochen, Germany), and pictures were captured by an AxioCam using the Axiovision digital imaging system. Adobe Photoshop CS2 was used to edit images.
RESULTS
Isolation and characterization of three genes for G protein α-subunits and a gene for adenylyl cyclase from S. macrospora:
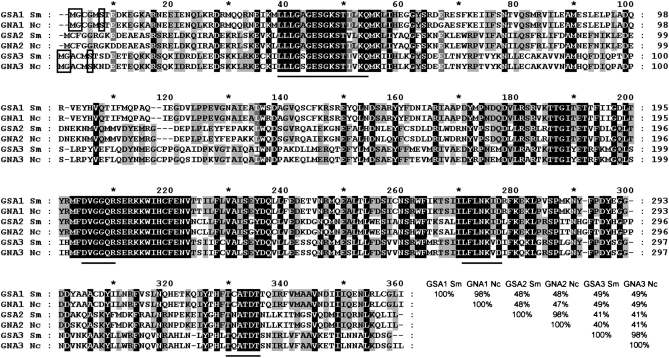
Previously, we showed that N. crassa and S. macrospora have a high degree of nucleic acid identity with an average of 89.5% within exonic sequences (Nowrousian et al. 2004). Therefore, primers based on the N. crassa sequence were designed and used for the PCR-mediated isolation of three genes encoding Gα-subunits from S. macrospora. The resulting amplicons were used for DNA sequencing and their identities were confirmed by comparison with the homologous sequences from N. crassa. Similar to the corresponding gene designations in N. crassa, the S. macrospora genes were named gsa1, gsa2, and gsa3 (G protein Sordaria alpha subunit). Using an inverse PCR-based strategy, the flanking sequences of the gsa1 and gsa3 genes were isolated. In the case of gsa2, a PCR-based strategy was used to isolate a cosmid clone encoding the GSA2 protein (Pöggeler et al. 1997). DNA sequencing of the three gsa genes revealed that the predicted amino acid sequences from all genes are closely related to the corresponding N. crassa homologs. The amino acid sequences of GSA1, -2, and -3 display 98% sequence identity with their counterparts from N. crassa (Figure 1). Further conservation is seen when the positions and numbers of introns are compared. All introns are located at similar positions when compared with the homologous genes from N. crassa (supplemental Figure S1A).
Figure 1.—
Comparison of amino acid sequences from S. macrospora (Sm) GSA1, GSA2, and GSA3 proteins (accession nos. CAP09209, CAP09210, and CAP09211) with their N. crassa (Nc) orthologs (accession nos. AAB37244.1, Q05424, and XP_962205.1). The amino acids with the solid background are identical in all three subunits of both species. Sequence identity between four or five sequences is indicated by shading. Conserved regions that are predicted to play a role in the interaction with GTP are underlined. Putative myristoylation sites are marked with boxes. Amino acid identity of Gα-subunits is given at the end in percentages.
The predicted polypeptides encoded by the three gsa genes exhibit conserved domains (Figure 1) that are considered to be directly involved in guanine-nucleotide interaction (Simon et al. 1991; Skiba et al. 1996; Bohm et al. 1997). Both GSA1 and GSA3 have a putative myristoylation site (MGXXXS) at the N terminus (Buss et al. 1987; Gordon et al. 1991), but this motif is absent from GSA2 (Figure 1). GSA1 is further characterized by the consensus sequence CXXX at the carboxy terminus that is susceptible to modification by the pertussis toxin (Simon et al. 1991; Bölker 1998). These two features indicate that GSA1, just like its N. crassa counterpart, is evolutionarily related to the Gαi-subfamily of mammals that inhibits adenylyl cyclase (Ivey et al. 1996). As already shown by others, the amino acid sequences for Gα-subunits are highly homologous within filamentous fungi (Bölker 1998; Kays et al. 2000; Parsley et al. 2003). The proteins encoded by gsa1, gsa2, and gsa3 from S. macrospora display significant identities to corresponding proteins from other fungi with the highest amino acid identity to the Gα-subunits from N. crassa (data not shown).
To isolate the gene encoding the adenylyl cyclase, a probe was amplified with S. macrospora genomic DNA as template and heterologous oligonucleotides specific for the N. crassa adenylyl cyclase gene (oligonucleotides 1091 and 1092, Table 2). The resulting fragment was used for screening of an indexed S. macrospora cosmid library (Pöggeler et al. 1997). This led to the identification of a cosmid clone that was subjected to DNA sequencing, resulting in the identification of an open reading frame for a predicted adenylyl cyclase. The corresponding gene was named sac1 (Sordaria adenylyl cyclase1) and the comparison of the S. macrospora sequence with the corresponding N. crassa cr-1 gene displayed a similar exon-intron structure with respect to position of the introns (supplemental Figure S1A). The deduced amino acid sequence (accession no. CAP09208) exhibits an identity of 92.5% to the N. crassa homolog CR-1 (accession no. BAA00755.1). SAC1 displays a domain distribution typical for fungal adenylyl cyclases. The conserved amino acid motif DXNLN is located close to the N terminus, representing a putative Gα-binding site (Ivey and Hoffman 2005), followed by a RA (Ras association) domain. The central core of the enzyme consists of a leucin-rich repeat domain. At the C terminus, a serine/threonine protein phosphatase-like catalytic domain (type 2C, PP2Cc) is located immediately upstream of the single catalytic domain of the adenylyl cyclase (Baker and Kelly 2004).
Construction and phenotypic description of three G protein α-subunit deletion strains:
To functionally and genetically analyze the Gα-subunit genes, we constructed three knockout strains. As shown in supplemental Figure S1A, linear fragments were generated that contain the hph gene, flanked by genomic sequences of the different gsa genes. For gsa1 and gsa2, the marker gene replaces the complete open reading frame for the corresponding Gα-subunit, while for gsa3, two-thirds of the N-terminal coding region are substituted by the hph resistance gene. S. macrospora transformants often contain wild-type as well as mutant nuclei and are thus heterokaryotic. To obtain homokaryotic strains, single ascospore isolates were generated from all three above-described knockout strains. Since we crossed these strains with the spore color mutants r2 or fus1 (Table 1), disruption strains often carry an additional spore color mutation. Apart from the red spore color, r2 and fus1 strains resemble the wild-type phenotype of S. macrospora. For Δgsa3, only single-spore isolates with an r2 background were isolated. In all knockout strains, the substitution of the gsa genes by the hygromycin B resistance gene (hph) was further verified by PCR and Southern analysis as outlined in the materials and methods and shown in supplemental Figure S1.
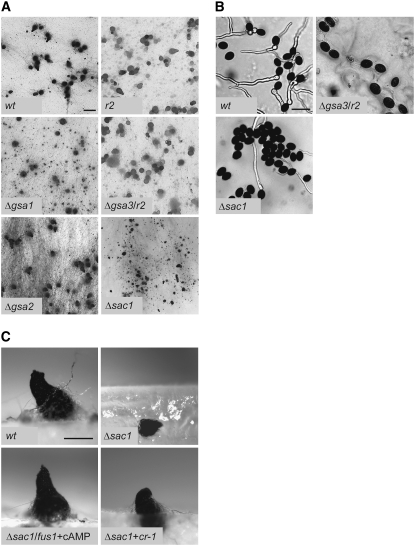
The characterization of the mutant strains revealed several phenotypic changes compared to the wild type. For example, we found a reduction of the growth rate ranging from 22% (Δgsa3) to 29% (Δgsa2) and 37% (Δgsa1). With respect to sexual development, we observed that Δgsa2 has wild-type-like fertility, while the two other mutant strains exhibit major differences. In Δgsa1, the number of fruiting bodies is reduced by ∼50% (Figure 2A). Moreover, in wild type grown on solid media, mature fruiting bodies appear after 7 days, whereas in Δgsa1, the first mature fruiting bodies did not appear until after 11 days of growth. The wild-type phenotype was restored when Δgsa1 was transformed with a full-length copy of the gsa1 gene (pD202, Table 3, data not shown). Δgsa3 has a wild-type-like phenotype as regards fruiting body development, and also similar to the wild type, fruiting bodies develop within 7 days (Figure 2A). However, Δgsa3 ascospores have a lower germination rate with a 95% reduction compared to wild type (Figure 2B). This phenotype can be rescued through complementation with a full-length gsa3 sequence (pD194.1, Table 3, data not shown).
Figure 2.—
Fruiting body development and ascospore germination of wild type, Δgsa, and Δsac1 mutant strains. r2 and fus1 are spore color mutants that show wild-type fertility. (A) Perithecia on solid cornmeal medium after 11 days of growth. The scale bar represents 1 mm. (B) Ascospore germination of wild-type and mutant strains Δsac1 and Δgsa3/r2. Spores were incubated for 5 hr on solid cornmeal medium with 0.5% (w/v) sodium acetate. The scale bar represents 50 μm. (C) Lateral view on perithecia of wild type, Δsac1, Δsac1/fus1 supplemented with cyclic AMP (+cAMP), and retransformant Δsac1 + cr-1 on solid cornmeal medium after 9 days of growth. The scale bar represents 200 μm.
Construction and phenotypic characterization of a Δsac1 disruption strain:
Similar to the disruption strategy described above for the gsa genes, a sac1 deletion strain was generated for further functional analysis. A set of primers was used to generate a DNA fragment containing the hph gene flanked by genomic sequences from the sac1 gene (see supplemental Figure S1A). This linear DNA fragment was used to transform a Δku70 recipient strain and the resulting transformants were analyzed with different sets of primers (Table 2, supplemental Figure S1A, data not shown). Six fungal transformants were generated in this way and all of them contained a disrupted sac1 gene, but only three of them were homokaryotic. By crossing of a primary, homokaryotic Δsac1 mutant with a fus1 strain, we obtained a Δsac1 strain lacking the ku70 deletion but carrying a single copy of the hph gene (see corresponding Southern analysis in supplemental Figure S1B). This strain served for further functional analysis. Although this strain is fertile, the fruiting bodies have a size that is reduced by 30% compared with wild-type perithecia (Figure 2A). In addition, a significant number of fruiting bodies are embedded in the agar and the germination rate of the ascospores is only ∼30% (Figure 2B). When the Δsac1 strain was transformed with cosmid H122 F6 (Table 3) carrying the entire N. crassa cr-1 gene the ascospore germination rate was restored to the wild-type level, thus demonstrating that deletion of sac1 is causally related to the observed phenotype (data not shown). Additionally, perithecia of the rescued strain developed at the air-to-surface interface of solid medium, as shown in Figure 2C (Δsac1 + cr-1). Perithecial formation at the air-to-surface interface was also restored by adding cAMP-derivative dibutyryl-cAMP to the culture medium, indicating that the embedded perithecia of the Δsac1 mutant are due to a lack of cAMP (Figure 2C, Δsac1/fus1 + cAMP). Besides, the growth rate of the mutant was increased from 59 to 74% compared to the wild type when supplemented with cAMP.
gsa double mutants carrying the Δgsa1 mutation are unable to develop fruiting bodies:
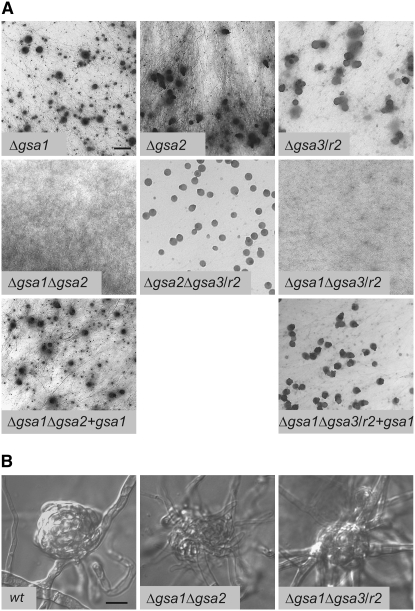
To study the genetic interactions between S. macrospora gsa genes, all three possible double mutants Δgsa1Δgsa2, Δgsa1Δgsa3, and Δgsa2Δgsa3 were generated by conventional crossing of single mutants. The genotype of the double mutants was confirmed by PCR analysis (data not shown). The two Δgsa1Δgsa2 and Δgsa1Δgsa3 double mutants are completely blocked in their sexual development. As can been seen in Figure 3A, development to mature fruiting bodies is prevented in these two double mutants even after prolonged growth. Moreover, both double mutants only produce protoperithecia with a frequency that is below that seen for each single mutant or the wild type (Figure 3B). To exclude the possibility that a further mutation is responsible for the sterile phenotype, fertility was restored by introducing a full-length copy of gsa1 (pD202, Table 3) into the Δgsa1Δgsa2 and Δgsa1Δgsa3 double mutants (Figure 3A, Δgsa1Δgsa2 + gsa1; Δgsa1Δgsa3/r2 + gsa1). Characterization of the strains was done by PCR amplification with primer pairs specific for the two gsa deletions or the wild-type gsa genes (data not shown). The phenotype of the rescued strains resembled the Δgsa2 phenotype (Δgsa1Δgsa2 + gsa1) and the Δgsa3 phenotype with reduced ascospore germination (Δgsa1Δgsa3/r2 + gsa1). In contrast to the double mutants carrying the Δgsa1 disruption, Δgsa2Δgsa3 displays a phenotype that resembles that of the Δgsa3 single mutant (Figure 3A) including reduced ascospore germination and growth rates.
Figure 3.—
Phenotypic characterization of fruiting body development of Gα-single and -double mutants. (A) Perithecial development on solid cornmeal medium after 11 days. Bar, 1 mm. (B) Microscopic images of protoperithecia from wild-type and double mutants Δgsa1Δgsa2 and Δgsa1Δgsa3/r2. Bar, 10 μm.
ΔgsaΔpre double mutants:
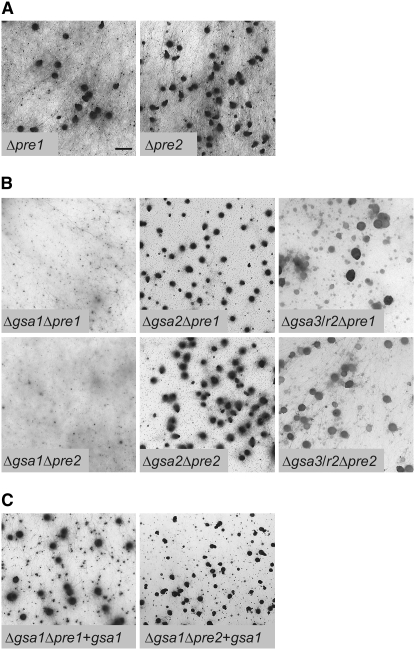
Previously, we identified two transcriptionally expressed pheromone receptor genes, pre1 and pre2, in the genome of S. macrospora (Pöggeler and Kück 2001). Functional characterization of the pre genes has shown that single knockout strains have a wild-type-like fruiting body development. However, deletion of both receptor genes completely eliminates fruiting body formation (Mayrhofer et al. 2006).
To determine which of the Gα-subunits transmits the pheromone signal from the G protein-coupled receptors, six double mutants were generated by crossing all Δgsa strains with either Δpre1 or Δpre2 (Figure 4A). Similar to the procedures described above, the genotypes of double mutants were confirmed by PCR analysis (data not shown). Phenotypic characterization of the six double mutants showed clear differences in their capacity to complete the sexual cycle. While Δgsa2Δpre1 and Δgsa2Δpre2 have a wild-type-like phenotype, both Δgsa1Δpre1 and Δgsa1Δpre2 display a severe impairment of fruiting body development (Figure 4B). For example, the number of perithecia is drastically reduced to a level of 0.5% when compared to the Δpre1 and Δpre2 single mutants. To confirm that this reduction is not due to the acquisition of another mutation, both double mutants (Δgsa1Δpre1; Δgsa1Δpre2) were complemented with a full-length copy of the gsa1 gene (pD202, Table 3). As shown in Figure 4C, Δgsa1Δpre1 + gsa1 and Δgsa1Δpre2 + gsa1 strains are phenotypically identical to the Δpre1 and Δpre2 single mutants.
Figure 4.—
Phenotypic characterization of fruiting body development in Gα-subunits/pheromone receptor mutants. Δpre receptor mutants (A) and ΔgsaΔpre double mutants (B) are shown after growth for 11 days on solid cornmeal medium. (C) Transformation with gsa1 (+gsa1) restores fertility in the Δgsa1Δpre mutants. Bar, 1 mm.
In addition to the above-described double mutants, Δgsa3Δpre1 and Δgsa3Δpre2 mutants were generated and both double mutants exhibit a Δgsa3-like phenotype (reduced germination rate of ascospores).
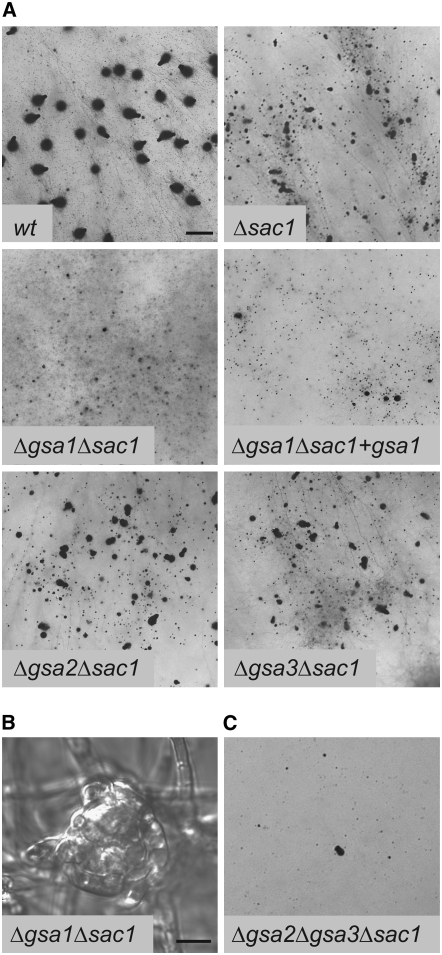
ΔgsaΔsac1 double and triple mutants:
To study the genetic interactions between the adenylyl cyclase and the Gα-subunits, the three possible double mutants (Δgsa1Δsac1, Δgsa2Δsac1, and Δgsa3Δsac1) and a Δgsa2Δgsa3Δsac1 triple mutant were generated by conventional crossings. The genotype of the mutants was confirmed by PCR analysis (data not shown). The morphological characterization of the mutants showed the following phenotypes: Δgsa2Δsac1 and Δgsa3Δsac1 resemble Δsac1 with small perithecia embedded in the agar and a highly reduced germination rate of ascospores (Figure 5A). A dramatic change in phenotype was observed in the Δgsa1Δsac1 double mutant. As shown in Figure 5A, this strain is completely sterile and unable to form any perithecia, and only protoperithecia are detectable (Figure 5B). When Δgsa1Δsac1 was transformed with a full-length copy of gsa1 (pD202, Table 3), development of a few perithecia compared to the Δsac1 single mutant was restored (Figure 5A, Δgsa1Δsac1 + gsa1).
Figure 5.—
Phenotypic characterization of Δgsa/Δsac1 double mutants and Δgsa2Δgsa3Δsac1 triple mutant. (A) Perithecial development of wild type, Δsac1, and Δgsa/Δsac1 double mutants after 11 days of growth on solid cornmeal medium. Bar, 1 mm. (B) Protoperithecial development in the sterile double mutant Δgsa1/Δsac1. Bar, 10 μm. (C) Δgsa2Δgsa3Δsac1 triple mutant after 11 days of growth on solid cornmeal medium.
Finally, the triple-mutant strain Δgsa2Δgsa3Δsac1 exhibits a phenotype similar to the above-described Δgsa1Δpre strains showing a drastically reduced number of perithecia compared to the wild type (Figure 5C). To the best of our knowledge, this is the first description in filamentous fungi of all possible double mutants carrying a disrupted adenylyl cyclase gene together with one of the deleted gsa genes.
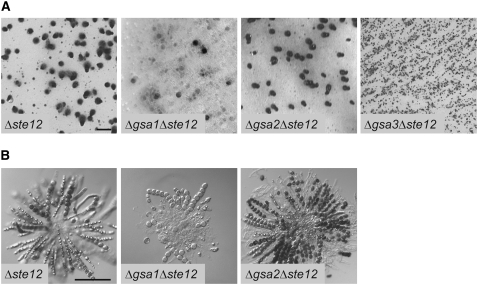
ΔgsaΔste12 double mutants:
In S. cerevisiae, the Ste12p transcription factor acts downstream of a signaling cascade that links pheromone receptors to a MAP kinase cascade via heterotrimeric G protein signaling (Dohlman and Thorner 2001). Loss of the Ste12p function results in the inability of haploid S. cerevisiae cells to mate (Hartwell 1980). However, the orthologous transcription factor STE12 of S. macrospora is required only for the correct morphogenesis of asci and ascospores. The cell walls of asci and ascospores of the Δste12 mutant strain are fragile and few of the spores are able to germinate (Nolting and Pöggeler 2006). To study the functional connections between the GSA subunits and the STE12 transcription factor in S. macrospora, three ΔgsaΔste12 double mutants were generated by conventional crossings. The genotype of the mutants was verified by PCR analysis (data not shown). While Δste12 single and the Δgsa2Δste12 double mutants show a wild-type-like formation of perithecia containing fragile asci and ascospores, the Δgsa1Δste12 mutant develops few fruiting bodies (Figure 6, A and B). Furthermore, perithecia of the Δgsa1Δste12 mutant contain only few asci compared with the Δste12 single or the Δgsa2Δste12 double mutants (Figure 6B). The Δgsa3Δste12 mutant exhibits a more severe phenotype lacking any perithecia. Instead only protoperithecia without asci initials are produced (Figure 6A).
Figure 6.—
Phenotypic characterization of Δste12 single and ΔgsaΔste12 double mutants. (A) Perithecial development of Δste12 single and Δgsa/Δste12 double mutants after 11 days of growth on solid cornmeal medium. Bar, 1 mm. (B) Microscopic images of asci from Δste12 single and Δgsa1Δste12 and Δgsa2Δste12 double mutants after 11 days of growth on solid cornmeal medium. The Δgsa3Δste12 mutant does not produce perithecia and therefore lacks any asci. Bar, 100 μm.
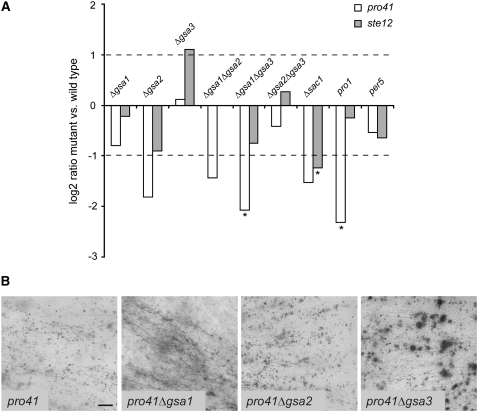
Expression analysis of developmentally regulated genes in Δgsa and Δsac1 mutants:
Microarray studies in the chestnut blight fungus Cryphonectria parasitica have revealed a downregulation of the ste12 homolog mst12 in a Δcpg-1 mutant strain that carries a deletion of the Gα-gene that is orthologous to gsa1 (Dawe et al. 2004). We therefore studied the expression of the ste12 transcript of S. macrospora in all Gα-single and -double mutants, in the Δsac1 mutant, and in the two previously characterized mutants pro1 and per5 (Masloff et al. 1999; Nowrousian et al. 1999) by quantitative real-time PCR. To connect the Gα-genes to other factors involved in fruiting body formation, we included the gene pro41 in the analysis, which is developmentally regulated during fruiting body development (Nowrousian et al. 2007a). The pro41 gene encodes a membrane protein of the endoplasmic reticulum and is essential for fruiting body development; the corresponding mutant forms protoperithecia but no mature perithecia (Nowrousian et al. 2007a). As shown in Figure 7A, ste12 transcript levels are significantly downregulated in the Δsac1 mutant in S. macrospora, but not in any of the other strains that were analyzed including Δgsa1. This indicates a high degree of diversification in the functions of G protein subunits in different fungal species. As was shown previously, pro41 is significantly downregulated in mutant pro1 (Nowrousian et al. 2007a). The only other mutant that displays a significant transcriptional downregulation of pro41 is the Δgsa1Δgsa3 double mutant. This might indicate that G protein signaling has some involvement in the regulation of pro41 expression; however, only deletion of major parts of the pathway leads to a significant reduction in pro41 transcript amounts. Taken together with the phenotypes of Gα-protein single and double mutants, this is another indication that the Gα-proteins are part of a signaling network that is at least partly buffered against loss of one subunit. We also generated double mutants of pro41 with all three Gα-genes, and as expected the mutants display the same phenotype as pro41 in that they are sterile and form only protoperithecia (Figure 7B).
Figure 7.—
Comparison of transcript levels of pro41 and ste12 between different mutants and phenotypes of pro41Δgsa double mutants. (A) Quantitative real-time PCR data are given as logarithmic values of the mutant/wild-type ratios (logarithm to the base 2 for the mean of at least two independent experiments). Real-time PCR results were tested for the significance of differential expression at P = 0.001 using REST (Pfaffl et al. 2002); genes that are expressed significantly differently in the mutant compared to the wild type are indicated by an asterisk. (B) Perithecial development of pro41 single and pro41Δgsa double mutants after 11 days of growth on solid cornmeal medium. Bar, 1 mm.
DISCUSSION
Three Gα-subunits have different functions during fungal development:
All three predicted GSA polypeptides are structurally similar to those described for other ascomycetes and can be classified into fungal groups I–III as previously described (Bölker 1998). While GSA1 corresponds to Gpa1p from S. cerevisiae (group I), GSA2 is more similar to Gpa1 from Schizosaccharomyces pombe (group II). The GSA3 subunit shows the highest homology to Gpa2 from both S. cerevisiae and S. pombe (group III, Bölker 1998). Similar to the Δgna mutants from N. crassa, the S. macrospora Δgsa deletion strains exhibit a reduced vegetative growth rate. Like its N. crassa counterpart, Δgsa1 shows defects in sexual development and reduced perithecium number. However, unlike the N. crassa mutant that produces aberrant perithecia without any ascospores (Ivey et al. 1996), Δgsa1 forms wild-type-like fruiting bodies with fertile ascospores. In Aspergillus nidulans, the corresponding ΔfadA mutant strain displays a different phenotype that has reduced vegetative growth and a complete block in cleistothecia formation (Rosén et al. 1999); therefore, these mutant strains illustrate the high degree of diversity in the function of this Gα-subunit.
The phenotype of the Δgsa2 strain is very close to the wild type, indicating a minor role of this Gα-subunit in sexual development. Single and double mutants suggest that the function of GSA2 can be substituted by GSA1 in the Δgsa2 mutant. However, as Δgsa1 has reduced fertility, GSA2 cannot fully substitute the GSA1 function in a Δgsa1 mutant, indicating that the activity of GSA1 dominates over that of GSA2 in S. macrospora with respect to sexual development. These findings correlate well with those obtained with the corresponding N. crassa Δgna mutants (Kays and Borkovich 2004).
As mentioned before, GSA3 from S. macrospora belongs to the same group (III) of fungal Gα-subunits as Gpa2 from the yeasts S. cerevisiae and S. pombe. The Gpa2 subunits from these yeasts are responsible for nutrient sensing and control of filamentous growth (Lengeler et al. 2000; Versele et al. 2001; Ivey and Hoffman 2005). The group III fungal Gα-subunits have also been proven to stimulate the adenylyl cyclase pathway in many different fungal species (Bölker 1998; Lengeler et al. 2000; D'Souza and Heitman 2001; Versele et al. 2001). The S. macrospora Δgsa3 mutant shows a late developmental block, resulting in a drastically reduced germination rate of the ascospores. This finding is similar to the ΔGPA2 mutant of S. pombe and to the defect observed in the corresponding Δgna-3 mutant from N. crassa, indicating that activation of this pathway might be an essential step in the germination process (Kays et al. 2000; Hatanaka and Shimoda 2001). Interestingly, the corresponding Δgna-3 mutant from N. crassa develops smaller perithecia that are submersed in the agar (Kays et al. 2000), a phenotype that was not observed in the corresponding S. macrospora mutant, but in the Δsac1 disruption strain. The differences between Δgsa1, Δgsa2, and Δgsa3 mutants suggest that they are involved in different steps of sexual development, such as fruiting body development and germination of the ascospores. Expression analysis of several developmentally regulated genes in the Gα-mutants also indicates distinct roles for each of the subunits.
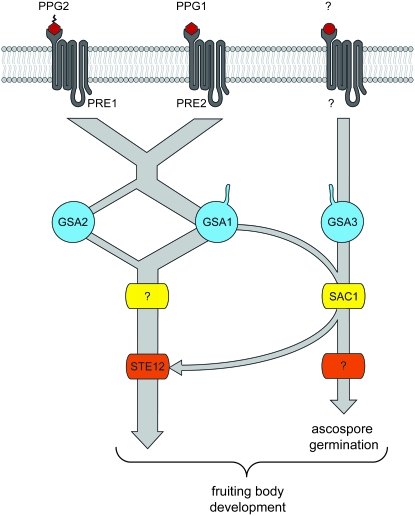
In Figure 8, a summary of our data is displayed in a model. According to this model different Gα-subunits interact with pheromone receptors or adenylyl cyclase and thus cooperatively regulate sexual development in S. macrospora. The S. macrospora double mutants Δgsa1Δgsa2 and Δgsa1Δgsa3 show a developmental block at the stage of protoperithecium formation. As depicted in Figure 8, in the case of the Δgsa1Δgsa2 mutant this would be explained by inactivation of the pathway downstream of the pheromone receptors. In the case of the Δgsa1Δgsa3 mutant, GSA2 is not able to compensate for the lack of GSA1. The corresponding N. crassa Δgna-1Δgna-2 and Δgna-1Δgna-3 mutants are also sterile, but progress somewhat further in development in that they develop aberrant perithecia lacking any ascospores (Kays and Borkovich 2004). Moreover, comparison of the Δgsa2Δgsa3 double mutant with its N. crassa counterpart revealed that both mutants produce perithecia, but in N. crassa perithecia are submersed in the agar whereas they are present in their normal position in S. macrospora. In summary, our genetic data lead us to conclude that gsa2 partially substitutes for gsa1, while gsa3 is part of a parallel pathway also necessary for wild-type-like fertility. The sterile phenotype of both Δgsa1 double mutants and the fertile phenotype of the Δgsa2Δgsa3 strain indicate that GSA1 is the major component for fruiting body development, while GSA2 and GSA3 play a supplementary role. In addition, GSA3 is involved in ascospore germination (Figure 8).
Figure 8.—
A model for the predicted G protein α-subunit signaling in S. macrospora. GSA1 and GSA2 propagate signals within the pheromone signaling pathway, in which GSA1 is the predominant regulator of fruiting body development upstream of the STE12 transcription factor. GSA3 and SAC1 act on sexual development in a less characterized, parallel signaling pathway. Putative myristoylation of Gα-subunits GSA1 and GSA3 is indicated by tails. Putative farnesylation of PPG2 is shown by a serrated tail.
Double mutants of Gα-subunits and pheromone receptors indicate a predominant role of gsa1 in fruiting body development:
Here we report for the first time phenotypes resulting from deletion of all Gα-subunit genes in combination with pheromone receptor genes in filamentous fungi. All receptor mutants in a Δgsa1 genetic background can be clearly distinguished from those in a Δgsa2 background. Whereas the Δgsa2Δpre1 and Δgsa2Δpre2 double mutants are fertile, the Δgsa1Δpre1 and Δgsa1Δpre2 mutants are almost totally blocked in sexual development, producing a strongly reduced number of perithecia compared with the single mutants and the wild-type strain. This phenotype resembles that of the recently described Δpre1Δppg1 and Δpre2Δppg2 double mutants (Mayrhofer et al. 2006). The drastically reduced fertility of the Δgsa1Δpre mutants and the wild-type-like fruiting body formation of Δgsa2Δpre mutants point to the crucial role of the GSA1 subunit in transducing the signals from the PRE pheromone receptors in S. macrospora. The data are consistent with our model in which GSA1 is the main player in transducing the signal from the pheromone receptors whereas GSA2 plays a minor role (Figure 8). As indicated by the severe phenotype of the Δgsa1Δpre double mutants compared to the Δgsa1 single mutant, GSA2 alone can not properly transmit the signal, especially with only half of the receptors present. Thus, the data obtained with double mutants carrying a disrupted gsa gene together with a disrupted receptor gene indicate that the pheromone receptors interact differently with GSA1 or GSA2. S. macrospora is homothallic and both receptor genes are coexpressed within a single cell (Pöggeler and Kück 2001). The knockout of a single receptor in S. macrospora has no obvious effect on fruiting body development and indicates that one receptor can compensate for the loss of the other receptor (Mayrhofer et al. 2006). This is supported by a growing body of evidence suggesting that G protein-coupled receptors exist as homo- or heterooligomers (Bulenger et al. 2005; Overton et al. 2005). Overton et al. (2005) propose in a model that oligomerized receptors can activate a single G protein heterotrimer or alternatively each receptor monomer activates individual G proteins.
Deletion of adenylyl cyclase affects vegetative growth and sexual development in S.macrospora:
In S. macrospora, the deletion of the adenylyl cyclase-encoding gene sac1 leads to reduced mycelial growth. Similar phenotypes were described also for other adenylyl cyclase mutant strains from filamentous fungi including Magnaporthe grisea, Sclerotinia sclerotiorum, Aspergillus fumigatus, Trichoderma virens and N. crassa (Terenzi et al. 1974; Choi and Dean 1997; Liebmann et al. 2003; Jurick and Rollins 2007; Mukherjee et al. 2007). The addition of dibutyryl-cAMP to the Δsac1 strain of S. macrospora partially restores the growth defect as was previously shown for the close relative N. crassa (Terenzi et al. 1974; Rosenberg and Pall 1979). Besides the vegetative phenotype, the deletion of the adenylyl cyclase gene leads to an impairment in fruiting body development. In M. grisea, disruption of the adenylyl cyclase gene causes female sterility as no perithecia are produced (Choi and Dean 1997) and similarly, apothecium formation is eliminated in S. sclerotiorum (Jurick and Rollins 2007). In N. crassa, the cr-1 mutant exhibits a delay in fruiting body and ascospore formation (Ivey et al. 2002). However, the S. macrospora Δsac1 mutant has reduced fertility with a significant number of the fruiting bodies being embedded in the solid media, thereby leading to fewer ascospores being discharged by the perithecia. Similar findings were also observed in the N. crassa Δgna-3 mutant (Kays et al. 2000). The Δsac1 ascospores have a highly reduced germination rate and this phenotype also resembles that of Gα-subunit 3 disruption mutants from N. crassa (Kays et al. 2000) and S. macrospora. A severe block in the initial step of ascospore germination has also been reported for an adenylyl cylase disruption strain of S. pombe (Hatanaka and Shimoda 2001). While we were able to restore perithecial formation of the Δsac1 mutant at the air-to-surface interface by adding db-cAMP to the culture medium, the ascospore germination rate was not elevated by this cAMP analog (data not shown). This might be due to the highly impermeable ascospore cell wall that may prevent the uptake of cAMP in the cell. While a vegetative fungal cell is surrounded by a two-layer cell wall, an ascospore cell wall consists of four layers, of which the two outer layers have an ascospore-specific composition (Neiman 2005). The most outward layer, composed predominantly of dityrosine molecules, is highly impermeable (Briza et al. 1990).
Genetic interaction of Gα-subunits and the adenylyl cyclase of S.macrospora:
G protein-mediated signaling in fungi is transmitted via three major signal transduction pathways. Besides the MAP kinase and the phospholipase C (PLC)/PKC pathway, the activity of the AC can be regulated by heterotrimeric G proteins (McCudden et al. 2005). The latter pathway leads to the generation of the second messenger cAMP which in turn modulates the activity of protein kinase A (PKA) and thereby the activity of downstream effectors. In some fungi, the activity of the adenylyl cyclase is additionally regulated by Ras-GTPases. However, Gα-subunits of heterotrimeric G proteins are well known to regulate the activity of fungal adenylyl cyclases in different ascomycetes and basidiomycetes (Bölker 1998; Lengeler et al. 2000; D'Souza and Heitman 2001; Versele et al. 2001).
To analyze the genetic interactions between the Gα-subunits and adenylyl cyclase in S. macrospora, we generated double mutants lacking each of the Gα-subunits in combination with the adenylyl cyclase. As one would expect from crossing the wild-type-like Δgsa2 mutant, the Δgsa2Δsac1 has the same phenotype as the Δsac1 mutant, similar to the Δgsa2Δgsa3 mutant that displays the Δgsa3 phenotype. The Δgsa3Δsac1 double mutant resembles the Δsac1 phenotype as it develops small perithecia that are embedded in the agar, indicating that sac1 is epistatic to gsa3 (Figure 8). This is further verified by the ascospore germination deficiency found in both mutants. The genetic interaction between gsa3 and sac1 is in agreement with the observations made for N. crassa, where GNA-3, the corresponding Gα-subunit, regulates the protein level of adenylyl cyclase (Kays et al. 2000). In S. pombe, a direct interaction of the GSA3 ortholog Gpa2 with the adenylyl cyclase Git2 was demonstrated (Ivey and Hoffman 2005). Disruption of gpa2 or git2 results in retarded spore germination (Hatanaka and Shimoda 2001), and thus resembles the reduced rate of ascospore germination in Δgsa3 or Δsac1 from S. macrospora and Δgna-3 from N. crassa (Kays et al. 2000). We therefore propose that the S. macrospora GSA3-SAC1 pathway is a prerequisite for efficient spore germination supposedly by sensing nutrients through a yet unidentified receptor (Figure 8). This signal transduction resembles glucose sensing in S. cerevisiae through the Gpr1p-Gpa2p-Cyr1p pathway (Colombo et al. 1998; Kraakman et al. 1999).
The most significant phenotype concerning fruiting body development was observed in the Δgsa1Δsac1 double mutant that produces protoperithecia, but is unable to develop any perithecia. This implies that cAMP is required to develop mature perithecia in a Δgsa1 background. To further analyze the function of SAC1 in fruiting body development, we generated a Δgsa2Δgsa3Δsac1 triple mutant. While the Δgsa2Δgsa3 mutant is fully fertile concerning fruiting body development, the triple mutant is almost sterile showing a drastically reduced number of perithecia, thus confirming a functional interaction of the Gα-subunit GSA1 and the adenylyl cyclase SAC1 in fruiting body development. Interestingly, a direct regulation of adenylyl cyclase activity through the corresponding Gα-subunit GNA-1 from N. crassa has already been suggested (Ivey et al. 1999; Kays and Borkovich 2004). The involvement of both parallel pathways in fruiting body formation is verified by the sterile phenotype of the Δgsa3Δste12 double mutant, which only develops protoperithecia.
Taken together, we propose that both gene products, GSA3 and SAC1, act in a common signaling pathway. Additionally, the sterility of the Δgsa1Δsac1 double mutant indicates that the GSA3/SAC1 pathway functions in parallel to the GSA1/GSA2 pathway in fruiting body development. Both pathways are linked by the functional interaction of GSA1 with SAC1, as indicated by the Δgsa2Δgsa3Δsac1 triple mutant. The sterility of the Δgsa3Δste12 mutant is strong evidence for STE12 being one of several key regulators downstream of the pheromone receptors. This is reminiscent of signaling networks in the basidiomycete Ustilago maydis, where the pheromone-response factor Prf1, a transcription factor which recognizes pheromone response elements, is a key signaling node that mediates crosstalk between cAMP and MAP kinase pathways (Krüger et al. 1998; Feldbrügge et al. 2006).
In summary, the data presented here represent a comprehensive analysis of the contribution of all Gα-subunits combined with both pheromone receptor genes as well as downstream effector adenylyl cyclase and the transcription factor STE12 in fungal fruiting body development. Use of this powerful genetic approach will allow further dissection and better understanding of the key components of signaling pathways in filamentous fungi.
Acknowledgments
The authors wish to thank Susanne Schlewinski, Ingeborg Godehardt, and Swenja Ellßel for excellent technical assistance, and Eva Szczypka for the artwork. This work was funded by the Collaborative Research Center SFB480 (Project A1) and PO 532/3-2 of the Deutsche Forschungsgemeinschaft (Bonn, Germany).
References
- Albert, P. R., and L. Robillard, 2002. G protein specificity: traffic direction required. Cell Signal 14 407–418. [DOI] [PubMed] [Google Scholar]
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers and D. J. Lipman, 1990. Basic local alignment search tool. J. Mol. Biol. 215 403–410. [DOI] [PubMed] [Google Scholar]
- Baasiri, R. A., X. Lu, P. S. Rowley, G. E. Turner and K. A. Borkovich, 1997. Overlapping functions for two G protein α-subunits in Neurospora crassa. Genetics 147 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, D. A., and J. M. Kelly, 2004. Structure, function and evolution of microbial adenylyl and guanylyl cyclases. Mol. Microbiol. 52 1229–1242. [DOI] [PubMed] [Google Scholar]
- Bohm, A., R. Gaudet and P. B. Sigler, 1997. Structural aspects of heterotrimeric G-protein signaling. Curr. Opin. Biotechnol. 8 480–487. [DOI] [PubMed] [Google Scholar]
- Bölker, M., 1998. Sex and crime: heterotrimeric G proteins in fungal mating and pathogenesis. Fungal Genet. Biol. 25 143–156. [DOI] [PubMed] [Google Scholar]
- Briza, P., M. Breitenbach, A. Ellinger and J. Segall, 1990. Isolation of two developmentally regulated genes involved in spore wall maturation in Saccharomyces cerevisiae. Genes Dev. 4 1775–1789. [DOI] [PubMed] [Google Scholar]
- Bulenger, S., S. Marullo and M. Bouvier, 2005. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol. Sci. 26 131–137. [DOI] [PubMed] [Google Scholar]
- Buss, J. E., S. M. Mumby, P. J. Casey, A. G. Gilman and B. M. Sefton, 1987. Myristoylated α subunits of guanine nucleotide-binding regulatory proteins. Proc. Natl. Acad. Sci. USA 84 7493–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, M. H., K. S. Chae, D. M. Han and K. Y. Jahng, 2004. The GanB Gα-protein negatively regulates asexual sporulation and plays a positive role in conidial germination in Aspergillus nidulans. Genetics 167 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, W., and R. A. Dean, 1997. The adenylate cyclase gene MAC1 of Magnaporthe grisea controls appressorium formation and other aspects of growth and development. Plant Cell 9 1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo, S., P. Ma, L. Cauwenberg, J. Winderickx, M. Crauwels et al., 1998. Involvement of distinct G-proteins, Gpa2 and Ras, in glucose- and intracellular acidification-induced cAMP signalling in the yeast Saccharomyces cerevisiae. EMBO J. 17 3326–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza, C. A., and J. Heitman, 2001. Conserved cAMP signaling cascades regulate fungal development and virulence. FEMS Microbiol. Rev. 25 349–364. [DOI] [PubMed] [Google Scholar]
- Davis, R. H., 2000. Neurospora: Contributions of a Model Organism. Oxford University Press, New York.
- Dawe, A. L., G. C. Segers, T. D. Allen, V. C. McMains and D. L. Nuss, 2004. Microarray analysis of Cryphonectria parasitica Gα- and Gβγ-signalling pathways reveals extensive modulation by hypovirus infection. Microbiology 150 4033–4043. [DOI] [PubMed] [Google Scholar]
- Dohlman, H. G., and J. W. Thorner, 2001. Regulation of G protein-initiated signal transduction in yeast: paradigms and principles. Annu. Rev. Biochem. 70 703–754. [DOI] [PubMed] [Google Scholar]
- Dreyer, J., H. Eichhorn, E. Friedlin, H. Kürnsteiner and U. Kück, 2007. A homologue of the Aspergillus velvet gene regulates both cephalosporin C biosynthesis and hyphal fragmentation in Acremonium chrysogenum. Appl. Environ. Microbiol. 73 3412–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engh, I., C. Würtz, K. Witzel-Schlömp, H. Y. Zhang, B. Hoff et al., 2007. The WW domain protein PRO40 is required for fungal fertility and associates with Woronin bodies. Eukaryot. Cell 6 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser, K., 1982. Cryptogams-Cyanobacteria, Algae, Fungi, Lichens. Cambridge University Press, London.
- Esser, K., and J. Straub, 1958. Genetische Untersuchungen an Sordaria macrospora Auersw.: Kompensation und Induktion bei genbedingten Entwicklungsdefekten. Z. Vererbungsl. 89 729–746. [PubMed] [Google Scholar]
- Feldbrügge, M., M. Bölker, G. Steinberg, J. Kämper and R. Kahmann, 2006. Regulatory and structural networks orchestrating mating, dimorphism, cell shape, and pathogenesis in Ustilago maydis, pp. 375–387 in The Mycota I, edited by U. Kües and R. Fischer. Springer-Verlag, Berlin, Heidelberg, Germany.
- Gordon, J. I., R. J. Duronio, D. A. Rudnick, S. P. Adams and G. W. Gokel, 1991. Protein N-myristoylation. J. Biol. Chem. 266 8647–8650. [PubMed] [Google Scholar]
- Hamm, H. E., 1998. The many faces of G protein signaling. J. Biol. Chem. 273 669–672. [DOI] [PubMed] [Google Scholar]
- Hartwell, L. H., 1980. Mutants of Saccharomyces cerevisiae unresponsive to cell division control by polypeptide mating hormone. J. Cell Biol. 85 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka, M., and C. Shimoda, 2001. The cyclic AMP/PKA signal pathway is required for initiation of spore germination in Schizosaccharomyces pombe. Yeast 18 207–217. [DOI] [PubMed] [Google Scholar]
- Ivey, F. D., and C. S. Hoffman, 2005. Direct activation of fission yeast adenylate cyclase by the Gpa2 Gα of the glucose signaling pathway. Proc. Natl. Acad. Sci. USA 102 6108–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey, F. D., P. N. Hodge, G. E. Turner and K. A. Borkovich, 1996. The Gαi homologue gna-1 controls multiple differentiation pathways in Neurospora crassa. Mol. Biol. Cell 7 1283–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey, F. D., Q. Yang and K. A. Borkovich, 1999. Positive regulation of adenylyl cyclase activity by a Gαi homolog in Neurospora crassa. Fungal Genet. Biol. 26 48–61. [DOI] [PubMed] [Google Scholar]
- Ivey, F. D., A. M. Kays and K. A. Borkovich, 2002. Shared and independent roles for a Gαi protein and adenylyl cyclase in regulating development and stress responses in Neurospora crassa. Eukaryot. Cell 1 634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurick, 2nd, W. M., and J. A. Rollins, 2007. Deletion of the adenylate cyclase (sac1) gene affects multiple developmental pathways and pathogenicity in Sclerotinia sclerotiorum. Fungal Genet. Biol. 44 521–530. [DOI] [PubMed] [Google Scholar]
- Kays, A. M., and K. A. Borkovich, 2004. Severe impairment of growth and differentiation in a Neurospora crassa mutant lacking all heterotrimeric Gα-proteins. Genetics 166 1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kays, A. M., P. S. Rowley, R. A. Baasiri and K. A. Borkovich, 2000. Regulation of conidiation and adenylyl cyclase levels by the Gα protein GNA-3 in Neurospora crassa. Mol. Cell. Biol. 20 7693–7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H., and K. A. Borkovich, 2004. A pheromone receptor gene, pre-1, is essential for mating type-specific directional growth and fusion of trichogynes and female fertility in Neurospora crassa. Mol. Microbiol. 52 1781–1798. [DOI] [PubMed] [Google Scholar]
- Kraakman, L., K. Lemaire, P. Ma, A. W. Teunissen, M. C. Donaton et al., 1999. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol. Microbiol. 32 1002–1012. [DOI] [PubMed] [Google Scholar]
- Krüger, J., G. Loubradou, E. Regenfelder, A. Hartmann and R. Kahmann, 1998. Crosstalk between cAMP and pheromone signalling pathways in Ustilago maydis. Mol. Gen. Genet. 260 193–198. [DOI] [PubMed] [Google Scholar]
- Kübler, E., H. U. Mosch, S. Rupp and M. P. Lisanti, 1997. Gpa2p, a G-protein α-subunit, regulates growth and pseudohyphal development in Saccharomyces cerevisiae via a cAMP-dependent mechanism. J. Biol. Chem. 272 20321–20323. [DOI] [PubMed] [Google Scholar]
- Kück, U., 2005. A Sordaria macrospora mutant lacking the leu1 gene shows a developmental arrest during fruiting body formation. Mol. Genet. Genomics 274 307–315. [DOI] [PubMed] [Google Scholar]
- Kück, U., and B. Hoff, 2006. Application of the nourseothricin acetyltransferase gene (nat1) as dominant marker for the transformation of filamentous fungi. Fungal Genet. Newsl. 53 9–11. [Google Scholar]
- Kück, U., and S. Pöggeler, 2004. pZHK2, a bi-functional transformation vector, suitable for two step gene targeting. Fungal Genet. Newsl. 51 4–6. [Google Scholar]
- Lengeler, K. B., R. C. Davidson, C. D'Souza, T. Harashima, W. C. Shen et al., 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol. Mol. Biol. Rev. 64 746–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebmann, B., S. Gattung, B. Jahn and A. A. Brakhage, 2003. cAMP signaling in Aspergillus fumigatus is involved in the regulation of the virulence gene pksP and in defense against killing by macrophages. Mol. Genet. Genomics 269 420–435. [DOI] [PubMed] [Google Scholar]
- Liu, S., and R. A. Dean, 1997. G protein α subunit genes control growth, development, and pathogenicity of Magnaporthe grisea. Mol. Plant-Microbe Interact. 10 1075–1086. [DOI] [PubMed] [Google Scholar]
- Masloff, S., S. Pöggeler and U. Kück, 1999. The pro1(+) gene from Sordaria macrospora encodes a C6 zinc finger transcription factor required for fruiting body development. Genetics 152 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer, S., and S. Pöggeler, 2005. Functional characterization of an α-factor-like Sordaria macrospora peptide pheromone and analysis of its interaction with its cognate receptor in Saccharomyces cerevisiae. Eukaryot. Cell 4 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrhofer, S., J. M. Weber and S. Pöggeler, 2006. Pheromones and pheromone receptors are required for proper sexual development in the homothallic ascomycete Sordaria macrospora. Genetics 172 1521–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCudden, C. R., M. D. Hains, R. J. Kimple, D. P. Siderovski and F. S. Willard, 2005. G-protein signaling: back to the future. Cell. Mol. Life Sci. 62 551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee, M., P. K. Mukherjee and S. P. Kale, 2007. cAMP signalling is involved in growth, germination, mycoparasitism and secondary metabolism in Trichoderma virens. Microbiology 153 1734–1742. [DOI] [PubMed] [Google Scholar]
- Neiman, A. M., 2005. Ascospore formation in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69 565–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas, K. B., H. B. Nicholas and D. W. Deerfield II, 1997. GeneDoc: analysis and visualization of genetic variation. EMBNEW. NEWS 4: 14.
- Nolting, N., and S. Pöggeler, 2006. A STE12 homologue of the homothallic ascomycete Sordaria macrospora interacts with the MADS box protein MCM1 and is required for ascosporogenesis. Mol. Microbiol. 62 853–868. [DOI] [PubMed] [Google Scholar]
- Nowrousian, M., and P. Cebula, 2005. The gene for the lectin-like protein is transcriptionally activated during sexual development, but is not essential for fruiting body formation in the filamentous fungus Sordaria macrospora. BMC Microbiol. 5 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrousian, M., S. Masloff, S. Pöggeler and U. Kück, 1999. Cell differentiation during sexual development of the fungus Sordaria macrospora requires ATP citrate lyase activity. Mol. Cell. Biol. 19 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrousian, M., C. Würtz, S. Pöggeler and U. Kück, 2004. Comparative sequence analysis of Sordaria macrospora and Neurospora crassa as a means to improve genome annotation. Fungal Genet. Biol. 41 285–292. [DOI] [PubMed] [Google Scholar]
- Nowrousian, M., C. Ringelberg, J. C. Dunlap, J. J. Loros and U. Kück, 2005. Cross-species microarray hybridization to identify developmentally regulated genes in the filamentous fungus Sordaria macrospora. Mol. Genet. Genomics 273 137–149. [DOI] [PubMed] [Google Scholar]
- Nowrousian, M., S. Frank, S. Koers, P. Strauch, T. Weitner et al., 2007. a The novel ER membrane protein PRO41 is essential for sexual development in the filamentous fungus Sordaria macrospora. Mol. Microbiol. 64 923–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowrousian, M., M. Piotrowski and U. Kück, 2007. b Multiple layers of temporal and spatial control regulate accumulation of the fruiting body-specific protein APP in Sordaria macrospora and Neurospora crassa. Fungal Genet. Biol. 44 602–614. [DOI] [PubMed] [Google Scholar]
- Overton, M. C., S. L. Chinault and K. J. Blumer, 2005. Oligomerization of G-protein-coupled receptors: lessons from the yeast Saccharomyces cerevisiae. Eukaryot. Cell 4 1963–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsley, T. B., G. C. Segers, D. L. Nuss and A. L. Dawe, 2003. Analysis of altered G-protein subunit accumulation in Cryphonectria parasitica reveals a third Gα homologue. Curr. Genet. 43 24–33. [DOI] [PubMed] [Google Scholar]
- Pfaffl, M. W., G. W. Horgan and L. Dempfle, 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 30 e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöggeler, S., and U. Kück, 2000. Comparative analysis of mating-type loci from Neurospora crassa and Sordaria macrospora: identification of novel transcribed ORFs. Mol. Gen. Genet. 263 292–301. [DOI] [PubMed] [Google Scholar]
- Pöggeler, S., and U. Kück, 2001. Identification of transcriptionally expressed pheromone receptor genes in filamentous ascomycetes. Gene 280 9–17. [DOI] [PubMed] [Google Scholar]
- Pöggeler, S., and U. Kück, 2004. A WD40 repeat protein regulates fungal cell differentiation and can be replaced functionally by the mammalian homologue striatin. Eukaryot. Cell 3 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöggeler, S., and U. Kück, 2006. Highly efficient generation of signal transduction knockout mutants using a fungal strain deficient in the mammalian ku70 ortholog. Gene 378 1–10. [DOI] [PubMed] [Google Scholar]
- Pöggeler, S., M. Nowrousian, S. Jacobsen and U. Kück, 1997. An efficient procedure to isolate fungal genes from an indexed cosmid library. J. Microbiol. Methods 29 49–61. [Google Scholar]
- Pöggeler, S., M. Nowrousian and U. Kück, 2006. a Fruiting-body development in ascomycetes, pp. 325–355 in The Mycota I, edited by U. Kües and R. Fischer. Springer-Verlag, Berlin, Heidelberg, Germany.
- Pöggeler, S., M. Nowrousian, C. Ringelberg, J. J. Loros, J. C. Dunlap et al., 2006. b Microarray and real-time PCR analyses reveal mating type-dependent gene expression in a homothallic fungus. Mol. Genet. Genomics 275 492–503. [DOI] [PubMed] [Google Scholar]
- Rosén, S., J. H. Yu and T. H. Adams, 1999. The Aspergillus nidulans sfaD gene encodes a G protein β subunit that is required for normal growth and repression of sporulation. EMBO J. 18 5592–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, G., and M. L. Pall, 1979. Properties of two cyclic nucleotide-deficient mutants of Neurospora crassa. J. Bacteriol. 137 1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., and D. W. Russel, 2001. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Schrick, K., B. Garvik and L. H. Hartwell, 1997. Mating in Saccharomyces cerevisiae: the role of the pheromone signal transduction pathway in the chemotropic response to pheromone. Genetics 147 19–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchuk, N. A., A. V. Bryksin, Y. A. Nusinovich, F. C. Cabello, M. Sutherland et al., 2004. Construction of long DNA molecules using long PCR-based fusion of several fragments simultaneously. Nucleic Acids Res. 32 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, M. I., M. P. Strathmann and N. Gautam, 1991. Diversity of G proteins in signal transduction. Science 252 802–808. [DOI] [PubMed] [Google Scholar]
- Skiba, N. P., H. Bae and H. E. Hamm, 1996. Mapping of effector binding sites of transducin α-subunit using Gαt/Gαi1 chimeras. J. Biol. Chem. 271 413–424. [DOI] [PubMed] [Google Scholar]
- Slessareva, J. E., S. M. Routt, B. Temple, V. A. Bankaitis and H. G. Dohlman, 2006. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein α subunit at the endosome. Cell 126 191–203. [DOI] [PubMed] [Google Scholar]
- Springer, M. L., 1993. Genetic control of fungal differentiation: the three sporulation pathways of Neurospora crassa. BioEssays 15 365–374. [DOI] [PubMed] [Google Scholar]
- Terenzi, H. F., M. M. Flawia and H. N. Torres, 1974. A Neurospora crassa morphological mutant showing reduced adenylate cyclase activity. Biochem. Biophys. Res. Commun. 58 990–996. [DOI] [PubMed] [Google Scholar]
- Thompson, J. D., D. G. Higgins and T. J. Gibson, 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versele, M., K. Lemaire and J. M. Thevelein, 2001. Sex and sugar in yeast: two distinct GPCR systems. EMBO Rep. 2 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]