Abstract
The frizzled signaling/signal transduction pathway controls planar cell polarity (PCP) in both vertebrates and invertebrates. Epistasis experiments argue that in the Drosophila epidermis multiple wing hairs (mwh) acts as a downstream component of the pathway. The PCP proteins accumulate asymmetrically in pupal wing cells where they are thought to form distinct protein complexes. One is located on the distal side of wing cells and a second on the proximal side. This asymmetric protein accumulation is thought to lead to the activation of the cytoskeleton on the distal side, which in turn leads to each cell forming a single distally pointing hair. We identified mwh as CG13913, which encodes a novel G protein binding domain–formin homology 3 (GBD–FH3) domain protein. The Mwh protein accumulated on the proximal side of wing cells prior to hair formation. Unlike planar polarity proteins such as Frizzled or Inturned, Mwh also accumulated in growing hairs. This suggested that mwh had two temporally separate functions in wing development. Evidence for these two functions also came from temperature-shift experiments with a temperature-sensitive allele. Overexpression of Mwh inhibited hair initiation, thus Mwh acts as a negative regulator of the cytoskeleton. Our data argued early proximal Mwh accumulation restricts hair initiation to the distal side of wing cells and the later hair accumulation of Mwh prevents the formation of ectopic secondary hairs. This later function appears to be a feedback mechanism that limits cytoskeleton activation to ensure a single hair is formed.
THE epidermis of many animals is polarized within the plane of the tissue. This tissue planar cell polarity (PCP) is dramatic in the adult cuticle of Drosophila, which is decorated with parallel arrays of hairs and sensory bristles. PCP in Drosophila is under the control of the frizzled (fz) signaling pathway (Gubb and García-Bellido 1982; Vinson and Adler 1987; Lawrence et al. 2007) and in recent years it has become clear that this pathway also controls planar polarity during vertebrate gastrulation, during the differentiation of stereocilia in the inner ear, and in the mammalian epidermis (Jessen et al. 2002; Wallingford et al. 2002; Curtin et al. 2003; Guo et al. 2004; Dabdoub and Kelley 2005; Montcouquiol et al. 2006; Wang et al. 2006; Ybot-Gonzalez et al. 2007).
In the fly wing fz pathway genes have been placed into three groups that represent both phenotypic and epistasis groups (Adler 1992; Wong and Adler 1993). These are the PCP (or core) genes, the planar polarity effector (PPE) genes, and the multiple-wing-hairs (mwh) gene. The PCP group includes fz, disheveled (dsh), prickle/spiny leg (pk/sple), Van Gogh (Vang) (aka strabismus), starry night (stan) (aka flamingo), and diego (dgo). The protein products of these genes accumulate asymmetrically on the distal (Fz, Dsh, and Dgo), proximal (Vang and Pk), or both distal and proximal (Stan) sides of wing cells (Usui et al. 1999; Axelrod 2001; Strutt 2001; Tree et al. 2002; Bastock et al. 2003; Das et al. 2004). These genes/proteins act as a functional group and are corequirements for the asymmetric accumulation of the others. Mutations in PCP genes result in the hair forming at a central location on the apical surface of wing cells and pointing in an abnormal direction (Wong and Adler 1993).
The PPE group includes inturned (in), fuzzy (fy), and fritz (frtz). Mutations in these genes differ from those in PCP genes as many mutant wing cells form two or three hairs with abnormal polarity. These hairs initiate at abnormal locations along the cell periphery. Several observations argue that the PPE genes function downstream of the PCP genes. First, loss-of-function mutations in PPE genes are epistatic to both gain and loss of functions in PCP genes (Lee and Adler 2002; Wong and Adler 1993). Next, the function of the PPE genes is not required for the asymmetric accumulation of PCP proteins (Usui et al. 1999; Axelrod 2001; Feiguin et al. 2001; Strutt 2001; Bastock et al. 2003). Finally, the In protein has been found to accumulate on the proximal side of wing cells under the direction of the PCP genes (Adler et al. 2004).
The multiple-wing-hairs (mwh) gene is the sole member of the third group. Mutations in mwh are epistatic to mutation in both the PCP and PPE genes (Lee and Adler 2002; Wong and Adler 1993) and mwh is not required for the asymmetric accumulation of PCP or PPE proteins (Usui et al. 1999; Axelrod 2001; Strutt 2001; Adler et al. 2004). Hence, mwh is thought to be the most downstream gene. A typical mwh wing cell produces three to five hairs with abnormal polarity and in this way differs from the upstream genes. Often some of the hairs produced by mwh cells are quite small. As is the case for PPE mutant cells, mwh cells form hairs at abnormal locations along the cell periphery (Wong and Adler 1993). Strikingly the abnormal polarity patterns seen in the wings of mutants are not random (Gubb and García-Bellido 1982; Wong and Adler 1993). Rather, the patterns are similar from one wing to another and similar for mutations in the PCP, PPE, and mwh genes providing additional evidence that these genes all function together in the generation of PCP (Taylor et al. 1998).
Studies on mwh have been limited due to the gene not having been characterized molecularly. We report here that mwh is CG13913, which encodes a novel G protein binding domain–formin homology 3 (GBD–FH3) domain protein. The expression of mwh mRNA increases prior to hair initiation (Ren et al. 2005) and we confirmed that this was also the case for Mwh protein. During this early period the protein accumulated on the proximal side of wing cells in a punctate manner. Overexpression of Mwh led to a delay in hair initiation, consistent with Mwh functioning as an antagonist of the cytoskeleton. Hence, we argue the proximal accumulation restricted hair initiation to the distal side of wing cells. Unlike other fz pathway proteins Mwh accumulated in growing hairs. Shift experiments with a temperature-sensitive allele showed that mwh functioned after hair initiation to inhibit the formation of secondary hairs. Our data point to mwh having two functions. We hypothesize an early Mwh function that controls hair polarity and a later one that prevents the formation of secondary hairs. We suggest that Mwh in and in the vicinity of growing hairs mediates the inhibition of secondary hair initiation.
MATERIALS AND METHODS
Fly genetics:
All flies were raised at 25° unless otherwise stated. Mutant stocks were obtained from the Drosophila Stock Center at Indiana University, generated in our lab, or were generous gifts from J. Axelrod, D. Strutt, T. Uemura, and T. Wolff. The FLP/FRT technology was used to generate genetics mosaics. To direct transgene expression, we used the Gal4/UAS system. For temperature-shift experiments the relevant animals were collected as white prepupae, placed into fresh food vials, and moved to the proper incubator. In line with FlyBase usage we use starry night instead of flamingo and Van Gogh instead of strabismus.
Immunostaining:
A standard staining procedure was used (Adler et al. 2004). Briefly, tissues were fixed with formaldehyde, rinsed, incubated with primary antibody, rinsed, incubated with flourescently labeled secondary antibody, and then mounted in ProLong (Invitrogen) Gold mountant.
Plasmid Constructs:
mwh cloning:
PCR, cloning, and sequencing used standard protocols. RACE was performed with a Marathon kit (Clontech). The extended mwh cDNA was PCR amplified with an Expand kit (Boehringer) and cloned into pCR2.1 (Clontech). pUASt-mwh was prepared by excising the mwh insert from pCR2.1 with EcoRI and cloning it into the EcoRI site of pUASt.
mwh subcloning for protein expression:
The mwh EST clone SD18376 was used as the template. The last exon of mwh with correct coding frame was amplified with primers GATE-mwh5: GGGGACAAGTTTGTACAAAAAAGCAGGCTTCTTTCTCAACACGTTCATTGAG-AGCG and GATE-mwh3: GGGGACCACTTTGTACAAGAAAGCTGGGTCTTAGTAGAGG-CCGGATGGCAGA. The PCR product was subcloned into pDORN 221 vector and then subcloned into pDEST 17 vector (Invitrogen Gateway technology).
Antibodies:
His-tagged truncated Mwh proteins (encoded by last exon) were expressed in Escherichia coli BL21-AI cells (Invitrogen) using the DEST 17 vector and purified from inclusion bodies (Novagen). Antibodies against Mwh were raised in rats and rabbits. Monoclonal anti-Armadillo and anti-Flamingo antibodies were obtained from Developmental Studies Hybridoma Bank at the University of Iowa. Alexa 488- and Alexa 568-conjugated secondary antibodies were purchased from Molecular Probes. Alexa 568 and 647 phalloidin were purchased from Molecular Probes.
RESULTS
CG13913 is mwh:
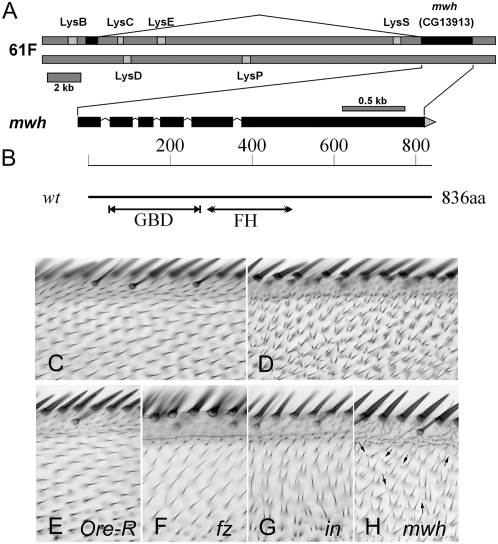
We identified mwh by deficiency mapping followed by sequencing of candidate genes. We identified mutations in CG13913 in four null alleles of mwh, all of which would truncate the protein (Table 1). These included two mutations in splice acceptor sites, one nonsense mutation, and one small deletion that altered the reading frame. For the mwh1 allele, which is a phenotypic null we were unable to amplify the 3′ end of the gene, suggesting it might be associated with a deletion, but we did not obtain definitive evidence on this point. By immunostaining we did not detect any Mwh protein in mwh1 mutant cells, consistent with it being a null allele. In our collection of mwh alleles there is a single hypomorphic allele (mwh6) and we found it contained a change of Cys 222 to Tyr. The significance of this change will be discussed further below. By RACE analysis we confirmed that CG13913 was internally spliced as predicted and that there was a noncoding exon encoded by sequences some 20 kb upstream of the CG13913 coding exons (Figure 1A). Several lysozyme genes exist in both orientations within the large CG13913 first intron.
TABLE 1.
Nature of mwh mutations
| Allele | Molecular change | Phenotype | Comment |
|---|---|---|---|
| mwh1 | Uncertain, unable to amplify 3′ end of gene | Null | Likely a deletion, no CRM |
| mwh2 | C1229633T | Null | Stop at codon 11 |
| mwh3 | G1230259T | Null | Eliminates exon 5 splice acceptor |
| mwh4 | 1230897–1230902 deletion | Null | Deletes exon 7 splice acceptor |
| mwh5 | 1230570–1230582 deletion | Null | Changes reading frame after codon 243 followed by 39 novel amino acids prior to a stop codon |
| mwh6a | G1230445A | Hypomorphic, ts | Cys 222 Tyr |
The temperature-sensitive (ts) mwh6 allele contained three amino acid substitutions compared to the reference wild-type sequence; however two of these were also found on another chromosome (from the same parental stock) that did not show the mwh6 phenotype and hence are likely neutral polymorphisms.
Figure 1.—
The mwh gene and protein. (A) A representation of the mwh gene. Other genes in the region and the experimentally determined splicing pattern are shown. (B) A representation of the Mwh protein. The relative location of the GBD and FH3 domains are shown along the 836 amino acid protein. (C) The dorsal surface of the anterior edge of a ap-Gal4/UAS-mwh; mwh wing. (D) The ventral surface of the same wing. ap-Gal4 drives expression of mwh only in dorsal cells providing rescue of the mutant phenotype in that cell layer. This rescue is seen in 100% of animals. (E–H) A small region from the anterior part of Ore-R, fz, in, and mwh wings. Note the hairs point distally in wild type but not in the mutants. The polarity phenotype is weaker in fz than in or mwh mutant wings in this region. Almost all of the cells in the in wing produce more than one hair and all of the cells in the mwh wing do so. One cell in the fz wing formed two hairs. The very small hairs seen in mwh wings (arrows) are not seen in any of the other genotypes.
Additional proof that CG13913 was mwh came from experiments where we found that a UAS-CG13913 transgene provided rescue of a null mwh allele when driven by either apterous-Gal4 (ap-Gal4) or engrailed-Gal4 (Figure 1, C and D). Almost complete rescue was obtained using the actin-Gal4 driver, which drives expression at a somewhat lower level. In contrast, little rescue was obtained using patched-Gal4 (ptc-Gal4) and this was restricted to very proximal parts of the ptc domain (data not shown). As is described later, this is presumably due to ptc-Gal4 not being effective in driving mwh expression just prior to and during hair morphogenesis.
Independent evidence that CG1913 was mwh came from our finding that three separate transformants (41513, 41514, and 45265) from the Vienna Drosophila RNAi Center that encoded double-stranded RNA from CG13913 produced a mwh mutant phenotype when expressed by ap-GAL4. These data provided compelling evidence that CG13913 encoded mwh. Henceforth we refer to CG13913 as mwh.
The Mwh protein:
The predicted Mwh ORF encodes an 836 residue protein with a formin FH3 and GBD (GTPase binding domain) domains (Figure 1B). These two domains are frequently found together in proteins such as the Diaphanous family formins. In some recent papers these “sequence domains” have been renamed to reflect “structural domains” as a GBD (smaller than previous GBD domain), a Dad interacting domain (DID), corresponding to part of both the older GBD and FH3 domains), and a dimerization domain (DD), corresponding to part of the older FH3 domain) domain (Goode and Eck 2007). These domains correspond to the regulatory region of Diaphanous family formins.
We identified Mwh homologs in other arthropods, including the lepidopteran Bombyx mori, the coleopteran Tribolium castaneum, the hemipteran Pediculus humanus, the hymenopteran Apis melifera, and even the crustacean Daphnia pulex (see alignments in supplemental Figure S1). However it was not detected in the unassembled sequence traces from the arachnid Ixodes scapularis nor in nematodes or vertebrates. The distribution of Mwh across distantly related arthropod orders suggested it was part of an ancient morphogenesis network. Notable was the distinctive and highly conserved C-terminal KVTDLPSGLY motif. An FH3 domain was identified in our original Blast searches and additional psi-Blast searches and multiple sequence alignments (Rivero et al. 2005) identified vertebrate genes that were similar to an ∼430 amino acid region of Mwh that comprised the amino terminal regulatory region of Diaphanous family formins and included both GBD and FH3 domains (supplemental Figures S2 and S3). There are other Drosophila genes that are more similar to the identified vertebrate genes, thus, the vertebrate genes are not orthologs of mwh. Mwh does not contain the actin binding FH1 and FH2 domains that are characteristic of true formins, thus while related, Mwh is not a formin.
In the Diaphanous family formins the GBD domain binds a Rho GTPase and this displaces the autoinhibitory C-terminal DAD domain (Watanabe et al. 1999; Alberts 2001) from the DID. This leads to the activation of Diaphanous and its stimulation of actin polymerization. The residue changed in mwh6 is within the GBD domain and conserved in other Mwhs. Hence this region is of functional significance. On the basis of similarity to Diaphanous, this residue is predicted to be part of the α-8 helix that interacts directly with the G protein (Rose et al. 2005). By analogy, we suggest that the Mwh GBD domain will bind a small G protein in a regulatory interaction.
Mwh accumulation and localization is dynamic:
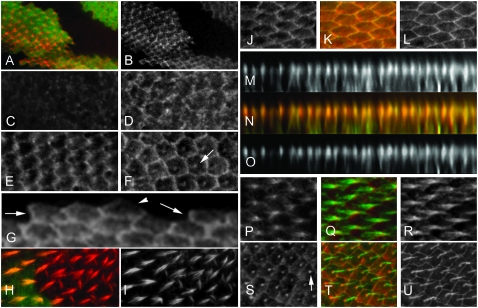
We raised a polyclonal anti-Mwh antibody that was able to specifically detect the endogenous Mwh protein in pupal wings and used it to localize Mwh (Figure 2, A and B). We did not detect Mwh in wing disc or pupal wing cells by immunostaining prior to ∼30 hr after puparium formation (apf) (Figure 2, C–F). By the time of hair initiation strong Mwh staining was detected.
Figure 2.—
Mwh localization is dynamic. (A and B) Confocal images of a mwh clone marked by a loss of GFP in a pupal wing stained with anti-GFP (green) and anti-Mwh (red) antibodies. A shows the merged image. B (as well as other single channel sections) is shown in grayscale as this provides better contrast. (C–F) Pupal wings stained with anti-Mwh antibody at 28 hr, 30 hr, 32 hr, and 36 hr after puparium formation (apf), respectively. The arrow points to accumulation of Mwh at the base of the hair in the 36-hr wing. (G) Mwh immunostaining at the boundary between expressing and nonexpressing cells. The arrows point to proximal edges that show accumulation of the protein. The arrowhead points to the distal edge of an expressing cell that does not accumulate Mwh. (H and I) A mwh clone marked by the loss of GFP and stained with phalloidin. Note the multiple hair cell phenotype is limited to the mutant cells. (J–L) Pupal wing cells stained with anti-Armadillo (green) and anti-Mwh (red) antibodies. K is a merge. (M–O) Z section reconstructions from the wing shown in J–L. Note that Mwh and Armadillo are colocalized near the apical surface of wing cells. (P–R) The accumulation of Mwh (red) and F-actin (green) in growing hairs. Note that Mwh is preferentially localized in proximal regions of the hair, but not extensively in the actin rootlet that extends into the cell. (S–U) Pupal wing cells aged ∼36 hr apf. Note the accumulation of Mwh at the base of the hairs (arrow in G3).
As noted earlier we found only very weak rescue of mwh using ptc-Gal4 UAS-mwh. This could be due to ptc-Gal4 not driving the needed level of mwh expression at the appropriate time. Consistent with this explanation we could easily detect a long stripe of Mwh by immunostaining of ptc-Gal4; UAS-mwh third instar wing discs and 24-hr pupal wings (supplemental Figure S4A). In 27-hr pupal wings Mwh staining was bright only in the center of the ptc domain (where ptc-Gal4 activity is highest) in the proximal half of the wing (supplemental Figure S4B). In 32- to 34-hr apf pupal wings, during and after the start of hair growth we detected increased Mwh immunostaining only in very proximal regions in the center of the ptc domain (supplemental Figure S4, C and D). Using the decline in the intensity of staining we were able to follow the decline in Mwh levels and this allowed us to estimate that the half-life of the Mwh protein was at most 2–3 hr in young pupal wings (supplemental Figure S5).
We found a complex pattern for Mwh localization in pupal wing cells (Figure 2). Mwh accumulated in a zigzag pattern at the cell periphery of wing cells between 30 and 34 hr apf (Figure 2, E, and J–L). The accumulation was quite uneven. In z-sections it was located at the same location as Armadillo (β-catenin), a key component of adherens junctions (Figure 2, M–O). Both the uneven zigzag staining and the accumulation near the adherens junctions are also properties of the PCP and PPE proteins. The Mwh staining pattern was not nearly as sharp as that seen with PCP proteins such as Stan or In as some Mwh was seen near but not at the proximal plasma membrane. Indeed, Mwh did not completely colocalize with Stan or In (Figure 3, A–F). The subcellular distribution of Mwh was dynamic. It accumulated in both emerging (e.g., from 30 to 32 hr apf) (Figure 2E) and growing wing hairs (e.g., from 32 to 34 hr apf) (Figure 2, F, and P–R). Compared to F-actin it appeared to accumulate preferentially in the proximal part of the hair. Later in hair morphogenesis Mwh preferentially localized to the base of the hairs (e.g., after 36 hr apf) (Figure 2, F, and S–U). At later stages we also saw Mwh along the cell periphery, but the zigzag pattern was no longer distinct and the staining was relatively uniform. Other fz pathway proteins do not localize in growing hairs (Usui et al. 1999; Axelrod 2001; Strutt 2001; Tree et al. 2002; Bastock et al. 2003; Adler et al. 2004; Das et al. 2004).
Figure 3.—
(A–C) A 30-hr pupal wing stained with both anti-Mwh (red) and anti-Stan (green) antibodies. As in Figure 2 single-channel images are shown in grayscale to improve contrast. B is the merged image. Note the Stan staining is more precisely localized at the plasma membrane and the two proteins are not tightly colocalized. (D–F) A 30-hr pupal wing stained with both anti-Mwh (red) and anti-In (green) antibodies. In immunostaining is very sensitive to fixation so only very weak fixation was used in this experiment and this led to the poor anti-Mwh staining. (I and K) A wing bristle where the endogenous Mwh protein is immunostained (red). F-actin is in green. (J and L) Higher magnification images of the proximal region of the bristle. Note that Mwh accumulates along the large actin bundles and is preferentially in the proximal part of the bristle. M–O) A piece of larval body wall muscle where mwh expression was driven using Tubulin-Gal4. Mwh is shown in red and F-actin in green (Alexa 488 phalloidin). Note the strong Mwh staining in the gap between regions of F-actin staining.
To determine if Mwh was localized at the proximal or distal or both sides of cells, we immunostained pupal wings that contained mwh mutant clones. The staining pattern of cells located at clone boundaries showed that Mwh accumulated at the proximal sides of wing cells (Figure 2G). This pattern is also seen for Pk, Vang, and In.
The regulation of hair initiation and the actin cytoskeleton by mwh:
mwh has long been used as a cuticular marker in genetic mosaics and thought to act cell autonomously. We confirmed this by examining marked clones in pupal wings where obvious changes in hair formation and the actin cytoskeleton were restricted to mutant cells (Figure 2, H and I).
The adult wing phenotype of mwh is similar to those of PCP and PPE genes in that loss-of-function mutations result in similar abnormal polarity phenotypes (Figure 1, E–H). For example, in a fz, in, or mwh mutant, hairs in the most anterior region of the wing point toward the anterior margin as opposed to distally as in wild type (Figure 1, E–H). In this region of the wing the fz phenotype is weaker than that of in or mwh. The mwh mutant phenotype differs from upstream genes due to most mwh cells forming three to five hairs instead of the one formed by the vast majority of fz cells and the one to three formed by in cells. Often, one or two of the hairs produced by a mwh cell were very small and such tiny hairs are rarely seen in mutants of PCP or PPE genes (Figure 1H).
That loss of mwh function led to extra hairs, suggested that Mwh served as an inhibitor to restrict hair initiation. However, the increase in mwh expression just prior to hair initiation suggested that Mwh might serve to activate the cytoskeleton. To attempt to distinguish between these hypotheses we examined the consequences of expressing mwh precociously. We saw no change in the actin cytoskeleton in wing discs expressing mwh and we saw no change in the timing of hair initiation or the appearance of the cytoskeleton in pupal wings where mwh was precociously expressed by ptc-Gal4. Thus, Mwh did not show any evidence of activating hair initiation. A limitation of this experiment is that as noted earlier, at the normal time of hair initiation ptc-Gal4 activity is low and does not result in mwh overexpression. To get around this limitation we directed a higher level of Mwh expression by using a combination of two Gal4 drivers (e.g., ptc-Gal4/+; Tub-Gal4/UAS-mwh). In such flies, we observed a weak gain-of-function phenotype that consisted of occasional small hairs, cells that failed to form a hair, multiple hair cells, and regions of slightly altered polarity (Figure 4, A and B). These phenotypes were primarily seen in the proximal part of the ptc domain, in the vicinity of the posterior cross vein and in the proximal part of the anteriormost region of the wing. This phenotype while weak showed complete penetrance. In 32-hr pupal wings of this genotype we observed that hair initiation was delayed over a substantial part of the ptc domain and also near the posterior cross vein (Figure 4, D–F). Since the adult phenotype was seen in a much smaller region of the wing than the pupal phenotype we concluded that most of the cells that were delayed in hair initiation still produced adult hairs of normal morphology. The ability of cells with delayed hair initiation to “catch up” and produce normal adult hairs was previously seen for clones of not (non-stop) (Ren et al. 2005). We concluded that Mwh acted as a negative regulator of hair initiation. The lack of an actin phenotype when overexpressed in wing discs or in loss-of-function mutations in many cell types (data not shown) indicated that Mwh was not a general regulator of the cytoskeleton, but rather a cell type/developmental stage-specific regulator.
Figure 4.—
The overexpression of mwh inhibits hair initiation. A and B are from ptc-GAL4/+; Tub-Gal4/UAS-mwh flies. A is from the dorsal wing surface just distal to the anterior cross vein. Several double hair cells are shown (arrows) and a weak polarity phenotype is seen. B is from the ventral surface just distal to the posterior cross vein. In this region many cells from very small hairs (or no hairs) (arrowheads) and others form double hair cells (arrows). (C) From a ptc-GAL4/+; Tub-GAL4/+ fly. A high level of GAL4 expression can induce the formation of a small number of multiple hair cells (arrow). This phenotype is much weaker than that seen with ptc-GAL4/+; Tub-Gal4/UAS-mwh wings. The number of multiple hair cells is about fourfold higher in this latter genotype. (D) A ptc-GAL4/+; Tub-Gal4/UAS-mwh pupal wing stained for F-actin. (E) An equivalent Ore-R control wing. They are centered on the middle of the ptc domain ventral surface. Note the delayed hair formation in the central part of the ptc domain in D (marked with an asterisk). (F) A ptc-GAL4/+; Tub-Gal4/UAS-mwh showing the region around the posterior cross vein. Arrows point to cells forming multiple hairs. The arrowhead points to a cell that has not yet begun to form a hair.
The multiple hair cell phenotype and the accumulation of Mwh in growing hairs suggested that Mwh might bind to the actin cytoskeleton. To test this possibility we examined the distribution of Mwh in other cell types. mwh mutations do not result in a mutant phenotype in bristles, however by immunostaining we found the endogenous protein colocalized with the large bundles of actin filaments found around the periphery of growing bristles (Figure 3, G–L) (Tilney et al. 1995). We extended these experiments to look at the localization of Mwh in larval body muscle and salivary gland cells where it is not normally expressed. The well-characterized sarcomeric structure of larval body wall muscle provided a scaffold to test the hypothesis that Mwh could bind to F-actin as opposed to an alternative cytoskeletal component. In larval muscle Mwh accumulated in a striated pattern (Figure 3, M–O) that did not coincide with F-actin staining and instead appeared to be localized to the M line. This suggested the possibility that Mwh interacted with a protein that regulated the activity or assembly of myosin. The lateral membranes of salivary gland cells stained strongly for F-actin and there were also bright staining actin “bars” in the basalmost one-third of these cells. When we expressed mwh in salivary gland cells, Mwh also accumulated at both of these locations (data not shown). We concluded that Mwh could bind to a constituent of the actin cytoskeleton but likely not F-actin itself.
mwh has two temporally separate functions:
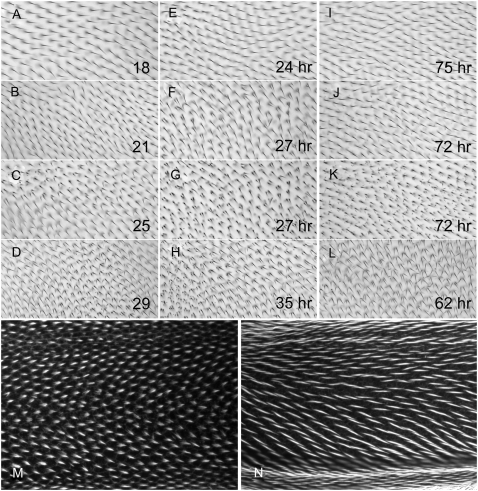
The two locations for Mwh accumulation were temporally distinct suggesting they reflected separate functions. We used a series of temperature shifts with the temperature-sensitive mwh6 allele to test this hypothesis. When mwh6 flies were grown at 18° they showed only a very weak phenotype (Figure 5A). In contrast when grown at 29° they showed a strong hypomorphic phenotype (Figure 5D).
Figure 5.—
mwh has two functions. (A–L) mwh6 adult wings after various temperature regimes. A–D are wings from flies raised completely at 18°, 21°, 25°, and 29°, respectively. Note how the phenotype becomes more severe as the temperature increased. E–H show wings from pupae that were shifted from 29° to 18° at 24 hr, 27 hr (shown at two focal planes), and 35 hr apf, respectively. Note that a shift at 27 hr produces a relatively strong polarity phenotype but only a small number of short hairs. I–L show wings from pupae shifted from 18° to 29° at 75 hr, 72 hr (two focal planes), and 62 hr apf. Note the many very small hairs in K. (M and N) mwh6 70-hr pupal wings that show short- and medium-length hairs (as discussed in the text).
To facilitate the interpretation of shift experiments we first examined the timing of hair initiation in this stock at the permissive and restrictive temperatures. When this stock was grown at 29° we found 0 of 4 24-hr apf wings contained hairs, 3 of 5 27-hr apf wings contained hairs (when detected the hairs were quite short and were not seen in the proximalmost part of the wing), and 5 of 5 31-hr apf wings contained long hairs. We concluded that hair initiation was ∼27 hr apf at 29°. When the stock was grown at 18°, 0 of 5 64-hr wings had hairs, 6 of 7 68- to 69-hr apf wings had hairs (in 5 of the 6 the hairs were short) and 13 of 13 70- to 72-hr apf wings had hairs (for 12/13 medium-length hairs were seen in all regions of the wing (see Figure 5, M and N). We concluded that hair initiation was ∼67–69 hr apf at 18°. Initial temperature-shift experiments showed that the temperature-sensitive period was around the time of hair initiation.
To analyze the phenotypes of adult wings we examined qualitatively the polarity of hairs in the adult wing (Figure 5), and quantitatively the fraction of cells that formed a single hair and the average number of medium and long hairs per cell (within our test region) (Table 2). When pupae raised at the restrictive temperature (29°) were shifted to the permissive temperature (18°) prior to hair initiation (at 24 hr apf) the phenotype was indistinguishable from those grown continually at 18° (Figure 5, A and E). This was true for the abnormal polarity of hairs, the fraction of cells that formed a single hair (100%), and the average number of hairs/cell (1 per cell) (Table 2). When the shift was made a few hours later (27 hr apf, around the time of hair initiation), the wings were different from those grown entirely at either the permissive or restrictive temperature. They showed a moderate hair-polarity phenotype (Figure 5, F and G); 51% of cells formed a single hair and on average 1.63 hairs per cell (Table 2). There were few of the very small hairs that are common in mwh but not in fy or frtz mutants. When pupae were shifted several hours after hair formation (35 hr apf) there was no rescue and the wings resembled those from animals that were kept at 29°. They showed a strong polarity phenotype, only 4% of the cells formed a single hair and on average they contained 2.56 hairs per cell (Figure 5H and Table 2). Different results were seen with the complementary shifts. Shifting from 18° to 29° well after hair formation (75 hr apf) had no effect (Figure 5I and Table 2), while shifting pupae several hours prior to hair initiation (62/64 hr apf) resulted in a completely restrictive phenotype (Figure 5L and Table 2). Once again an intermediate phenotype was seen when pupae were shifted right around the time of hair initiation (68 hr apf) (Table 2). A novel result was obtained when animals were shifted several (≈4) hours after hair initiation (72 hr apf). With this shift most wing cells produced a single relatively normal length distally pointing hair (Figure 5J and Table 2). These phenotypes were similar to those seen when no shift was made. In addition, most of these cells produced several very small secondary hairs that could best be seen when the microscope was focused close to the surface of the wing (Figure 5K). We concluded from these observations that mwh functioned prior to hair initiation to control the number and polarity of medium/long hairs and after hair initiation to block the formation of small extra hairs. mwh is unique among fz pathway genes in having a function in the wing after hair initiation. Shifting temperature-sensitive alleles of fz or in after the time of hair initiation had no effect on hair number or polarity (Adler et al. 1994a,b). Further, inducing the expression of fz pathway components after hair initiation also failed to alter hair number or polarity (Krasnow and Adler 1994; Strutt and Strutt 2002, 2007).
TABLE 2.
Effects of temperature shifts on the phenotype of mwhts
| Allele | Original temperature | Time of shifta | Fraction of single-hair cellsb | P vs. 18° | P vs. 29° | Mean hairs/cellc | P vs. 18° | P vs. 29° |
|---|---|---|---|---|---|---|---|---|
| mwhts | 18° | None | 1.00 (0) | NR | 0.002 | 1.00 (0.002) | NR | <0.001 |
| mwhts | 21° | None | 0.87 (0.013) | 0.005 | 0.002 | 1.14 (0.014) | <0.001 | <0.001 |
| mwhts | 29° | None | 0.055 (0.015) | 0.002 | NR | 2.47 (0.028) | <0.001 | NR |
| mwhts | 18° | 72 hr | 1.00 (0) | 1.00 | 0.002 | 1.00 (0.002) | 1.0 | <0.001 |
| mwhts | 18° | 69 hr | 1.00 (0) | 1.00 | 0.002 | 1.00 (0.002) | 1.0 | <0.001 |
| mwhts | 18° | 68 hr | 0.78 (0.095) | 0.015 | 0.002 | 1.26 (0.022) | <0.001 | <0.001 |
| mwhts | 18° | 66 hr | 0.052 (0.0095) | 0.002 | 0.699 | 2.48 (0.029) | <0.001 | 0.94 |
| mwhts | 29° | 24 hr | 1.00 (0) | 1.00 | 0.002 | 1.00 (0.002) | 1.0 | <0.001 |
| mwhts | 29° | 25 hr | 0.995 (0.0034) | 0.394 | 0.002 | 1.01 (0.0033) | 0.842 | <0.001 |
| mwhts | 29° | 27 hr | 0.51 (0.14) | 0.015 | 0.002 | 1.63 (0.030) | <0.001 | <0.001 |
| mwhts | 29° | 31 hr | 0.043 (0.011) | 0.002 | 0.55 | 2.53 (0.0285) | <0.001 | 0.09 |
| mwh1 | 21° | None | 0.012 (0.0048) | 0.002 | 0.041 | 2.78 (0.030) | <0.001 | <0.001 |
P, probability; NR, not relevant.
Animals were shifted either from 18° to 29° or from 29° to 18°.
The fraction of cells from our assay region forming a single hair was determined for six wings of each genotype and these values were compared. Only long- and medium-length hairs were considered. The Mann–Whitney rank sum test was used to test for significance, as the data points did not show equal variance.
The distribution of hair-cell types (single, double, etc.) was determined for 100 cells from each of six wings. The 600 hair-cell populations from each genotype were compared. Only long- and medium-length hairs were considered. The Mann–Whitney rank sum test was used to test for significance, as the data points did not show equal variance.
DISCUSSION
mwh has two functions in hair morphogenesis:
The subcellular localization of Mwh was dynamic and unique for a protein involved in PCP. Mwh preferentially localized to the proximal side of pupal wing cells prior to hair formation (e.g., 30–32 hr apf), in emerging hairs (at 32–34 hr), and later at the base of mature hairs (e.g., after 36 hr apf) (Figure 2). Our temperature-shift experiments argued that mwh had discrete functions before and after hair initiation. We hypothesize that the early proximal accumulation of Mwh leads to hair initiation at the distal side of wing cells. This likely represents mwh's function as a downstream component in the fz pathway. We further suggest that the inhibition of small ectopic hair formation that was detected by temperature shifts after hair initiation is mediated by Mwh that accumulates in the growing hair and in the base of the hair.
Our observation that the overexpression of mwh around the time of hair initiation can lead to a delay in hair initiation and a small and multiple hair phenotype in adult wings argues that Mwh acts as a negative regulator of hair initiation. This is consistent with Mwh being localized to the proximal side of wing cells when hairs initiate on the distal side, and Mwh being localized to the hair when it functions to prevent the formation of small hairs after the normal hair has begun to grow.
How is Mwh recruited to the hair?
The accumulation of Mwh in the hair is presumably due to its interacting with one or more hair components. The hair contains both peripheral actin filaments and centrally localized microtubules. Because of the small size of the hair it is difficult to distinguish between Mwh accumulating with either or both of these cytoskeletons. We therefore examined the localization of Mwh in other cell types. In both bristles and salivary gland cells we found that Mwh colocalized with the actin cytoskeleton. Although Mwh contained a GBD–FH3 domain that is also found in formins it lacked the FH1 and FH2 domains that mediate formin binding to F-actin (Goode and Eck 2007). This suggested Mwh would not interact directly with actin. Consistent with this hypothesis we found that in larval muscle Mwh accumulated in a striated pattern that was offset from the bands of F-actin. From this we concluded that Mwh did not bind directly to F-actin. The localization to what appeared to be the M line in muscle suggested Mwh interacted with a protein that regulated myosin assembly or activity in striated muscle (Mercer et al. 2006; Musa et al. 2006) and perhaps with a similar protein in epidermal cells.
Mwh, G proteins, and formins:
The sequence similarity between Mwh and the regulatory domain of formins suggested several possible biochemical mechanisms that could be functionally important. In Diaphanous family formins the GBD binds Rho1, which results in the activation of the formin to promote actin polymerization (Goode and Eck 2007). Rho1 has been implicated in fly planar polarity, making it a strong candidate for interacting with Mwh (Strutt et al. 1997). In preliminary studies we have obtained both genetic and biochemical evidence for such an interaction but the interpretation of the data is complicated by our finding that Rho1 has multiple functions in wing hair development. The presence of the DD domain also suggests the possibility that Mwh might dimerize with a true formin and regulate the actin cytoskeleton in that way. An obvious candidate for such a function is the DAAM formin, which in Xenopus was found to bind to Dsh (Habas et al. 2001). However, studies on fly DAAM failed to reveal any function in planar polarity or hair morphogenesis (Matusek et al. 2006). The diaphanous gene is another potential candidate. This gene has been well studied but there are no reports on its role in the differentiation of the adult epidermis. Intriguingly, Diaphanous localizes to growing embryonic denticles (Price et al. 2006), which like hairs are actin-containing extensions of epidermal cells. As is the case for epidermal hairs, mwh mutations result in the formation of extra denticles (Dickinson and Thatcher 1997) suggesting Dia as a possible partner for Mwh. None of the other fly formins have been found to function in wing hair development or wing planar polarity but several have not been well studied and are expressed in pupal wings (e.g., CG10990, Formin 3, and Fhos) (Ren et al. 2005). One of these could function with mwh.
A model for Mwh in wing tissue polarity:
There are several possible models as to how Mwh located on the proximal side of wing cells resulted in distal hair formation. One is that proximal Mwh stimulated hair initiation at the juxtaposed distal side of neighboring cells. However, mwh acts cell autonomously so we can rule out this model. An alternative is that Mwh, In and other PPE proteins formed a protein complex that organized the cytoskeleton to direct intracellular transport of hair-building components to the distal side leading to hair initiation there. This is plausible but no changes in the cytoskeleton prior to hair initiation have been seen in these mutants. A third possible model and one we prefer is that proximal Mwh locally inhibits hair initiation by regulating the cytoskeleton (Figure 6). This would lead to the preferential activation of the cytoskeleton on the distal side of the cell where Mwh levels were lowest. The cytoskeleton would self assemble to refine the area for hair initiation to a small region near the distal vertex. As the cytoskeleton is activated distally and a new hair emerges, Mwh would be recruited to this region, perhaps by binding to a hair component and/or a protein that interacted with a cellular myosin. Mwh would once again function locally to inhibit new hair initiation events and hence ensure that only a single unbranched hair forms. We view this latter Mwh function as a type of feedback inhibition that limits the activation of the cytoskeleton.
Figure 6.—
A model for mwh function. In 32-hr pupal wings Mwh accumulates on the proximal side of wing cells. As differentiation proceeds, activators of the cytoskeleton accumulate. Local inhibition by Mwh leads to the cytoskeleton being activated in the distalmost part of the cell. In 34-hr pupal wings hair morphogenesis has begun. The growing hair contains both F-actin and microtubules and associated bound proteins. Mwh is recruited to the proximal part of the hair. At this location it inhibits the cytoskeleton from initiating new hair outgrowth centers.
Acknowledgments
We gratefully acknowledge the provision of laboratory space by M. Ashburner. We thank Jeannette Charlton for technical help. The NCBI, EBI, FlyBase, and the Kyoto University GenomeNet supplied online bioinformatics services used in this study. We thank the reviewers for helpful comments. This work was supported by a grant from the National Institute of General Medical Science to P.N.A. and a grant from the United Kingdom Medical Research Council to Michael Ashburner.
References
- Adler, P. N., 1992. The genetic control of tissue polarity in Drosophila. BioEssays 14 735–741. [DOI] [PubMed] [Google Scholar]
- Adler, P. N., J. Charlton, K. H. Jones and J. Liu, 1994. a The cold-sensitive period for frizzled in the development of wing hair polarity ends prior to the start of hair morphogenesis. Mech. Dev. 46 101–107. [DOI] [PubMed] [Google Scholar]
- Adler, P. N., J. Charlton and W. J. Park, 1994. b The Drosophila tissue polarity gene inturned functions prior to wing hair morphogenesis in the regulation of hair polarity and number. Genetics 137 829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler, P. N., C. Zhu and D. Stone, 2004. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr. Biol. 14 2046–2051. [DOI] [PubMed] [Google Scholar]
- Alberts, A. S., 2001. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J. Biol. Chem. 276 2824–2830. [DOI] [PubMed] [Google Scholar]
- Axelrod, J. D., 2001. Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signaling. Genes Dev. 15 1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock, R., H. Strutt and D. Strutt, 2003. Strabismus is asymmetrically localised and binds to Prickle and Dishevelled during Drosophila planar polarity patterning. Development 130 3007–3014. [DOI] [PubMed] [Google Scholar]
- Curtin, J. A., E. Quint, V. Tsipouri, R. M. Arkell, B. Cattanach et al., 2003. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr. Biol. 13 1129–1133. [DOI] [PubMed] [Google Scholar]
- Dabdoub, A., and M. W. Kelley, 2005. Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. J. Neurobiol. 64 446–457. [DOI] [PubMed] [Google Scholar]
- Das, G., A. Jenny, T. J. Klein, S. Eaton and M. Mlodzik, 2004. Diego interacts with Prickle and Strabismus/Van Gogh to localize planar cell polarity complexes. Development 131 4467–4476. [DOI] [PubMed] [Google Scholar]
- Dickinson, W. J., and J. W. Thatcher, 1997. Morphogenesis of denticles and hairs in Drosophila embryos: involvement of actin-associated proteins that also affect adult structures. Cell Motil. Cytoskeleton 38 9–21. [DOI] [PubMed] [Google Scholar]
- Feiguin, F., M. Hannus, M. Mlodzik and S. Eaton, 2001. The ankyrin repeat protein Diego mediates Frizzled-dependent planar polarization. Dev. Cell 1 93–101. [DOI] [PubMed] [Google Scholar]
- Goode, B. L., and M. J. Eck, 2007. Mechanisms and function of formins in the control of actin assembly. Ann. Rev. Biochem. 76 593–627. [DOI] [PubMed] [Google Scholar]
- Gubb, D., and A. García-Bellido, 1982. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 68 37–57. [PubMed] [Google Scholar]
- Guo, N., C. Hawkins and J. Nathans, 2004. From the cover: Frizzled6 controls hair patterning in mice. Proc. Natl. Acad. Sci. USA 101 9277–9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas, R., Y. Kato and X. He, 2001. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel formin homology protein Daam1. Cell 107 843–854. [DOI] [PubMed] [Google Scholar]
- Jessen, J. R., J. Topczewski, S. Bingham, D. S. Sepich, F. Marlow et al., 2002. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol. 4 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnow, R. E., and P. N. Adler, 1994. A single frizzled protein has a dual function in tissue polarity. Development 120 1883–1893. [DOI] [PubMed] [Google Scholar]
- Lawrence, P. A., G. Struhl and J. Casal, 2007. Planar cell polarity: One or two pathways? Nat. Rev. Genet. 8 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H., and P. N. Adler, 2002. The function of the frizzled pathway in the Drosophila wing is dependent on inturned and fuzzy. Genetics 160 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matusek, T., A. Djiane, F. Jankovics, D. Brunner, M. Mlodzik et al., 2006. The Drosophila formin DAAM regulates the tracheal cuticle pattern through organizing the actin cytoskeleton. Development 133 957–966. [DOI] [PubMed] [Google Scholar]
- Mercer, K. B., R. K. Miller, T. L. Tinley, S. Sheth, H. Qadota et al., 2006. Caenorhabditis elegans UNC-96 is a new component of M-lines that interacts with UNC-98 and paramyosin and is required in adult muscle for assembly and/or maintenance of thick filaments. Mol. Biol. Cell 17 3832–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montcouquiol, M., E. B. Crenshaw and M. W. Kelley, 2006. Noncanonical Wnt signaling and neural polarity. Annu. Rev. Neurosci. 29 363–386. [DOI] [PubMed] [Google Scholar]
- Musa, H., S. Meek, M. Gautel, D. Peddie, A. J. Smith et al., 2006. Targeted homozygous deletion of M-band titin in cardiomyocytes prevents sarcomere formation. J. Cell Sci. 119 4322–4331. [DOI] [PubMed] [Google Scholar]
- Price, M. H., D. M. Roberts, B. M. McCartney, E. Jezuit and M. Peifer, 2006. Cytoskeletal dynamics and cell signaling during planar polarity establishment in the Drosophila embryonic denticle. J. Cell Sci. 119 403–415. [DOI] [PubMed] [Google Scholar]
- Ren, N., C. Zhu, H. Lee and P. N. Adler, 2005. Gene expression during Drosophila wing morphogenesis and differentiation. Genetics 171 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero, F., T. Muramoto, A.-K. Meyer, H. Urushihara, T. Uyeda et al., 2005. A comparative sequence analysis reveals a common GBD/FH3–FH1-FH2-DAD architecture in formins from Dictyostelium, fungi and metazoa. BMC Genomics 6 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, R., M. Weyand, M. Lammers, T. Ishizaki, M. R. Ahmadian et al., 2005. Structural and mechanistic insights into the interaction between Rho and mammalian Dia. Nature 435 513–518. [DOI] [PubMed] [Google Scholar]
- Strutt, D., and H. Strutt, 2007. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev. Biol. 302 181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt, D. I., 2001. Asymmetric localization of Frizzled and the establishment of cell polarity in the Drosophila wing. Mol. Cell 7 367–375. [DOI] [PubMed] [Google Scholar]
- Strutt, D. I., U. Weber and M. Mlodzik, 1997. The role of RhoA in tissue polarity and Frizzled signalling. Nature 387 292–295. [DOI] [PubMed] [Google Scholar]
- Strutt, H., and D. Strutt, 2002. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev. Cell 3 851–863. [DOI] [PubMed] [Google Scholar]
- Taylor, J., N. Abramova, J. Charlton and P. N. Adler, 1998. Van Gogh: a new Drosophila tissue polarity gene. Genetics 150 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney, L., M. Tilney and G. Guild, 1995. F actin bundles in Drosophila bristles. I. Two filament cross-links are involved in bundling. J. Cell Biol. 130 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tree, D. R. P., J. M. Shulman, R. Rousset, M. P. Scott, D. Gubb et al., 2002. Prickle mediates feedback amplification to generate asymmetric planar cell polarity signaling. Cell 109 371–381. [DOI] [PubMed] [Google Scholar]
- Usui, T., Y. Shima, Y. Shimada, S. Hirano, R. W. Burgess et al., 1999. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell 98 585–595. [DOI] [PubMed] [Google Scholar]
- Vinson, C. R., and P. N. Adler, 1987. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329 549–551. [DOI] [PubMed] [Google Scholar]
- Wallingford, J. B., S. E. Fraser and R. M. Harland, 2002. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev. Cell 2 695–706. [DOI] [PubMed] [Google Scholar]
- Wang, Y., N. Guo and J. Nathans, 2006. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J. Neurosci. 26 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe, N., T. Kato, A. Fujita, T. Ishizaki and S. Narumiya, 1999. Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1 136–143. [DOI] [PubMed] [Google Scholar]
- Wong, L., and P. Adler, 1993. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. J. Cell Biol. 123 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybot-Gonzalez, P., D. Savery, D. Gerrelli, M. Signore, C. E. Mitchell et al., 2007. Convergent extension, planar-cell-polarity signalling and initiation of mouse neural tube closure. Development 134 789–799. [DOI] [PMC free article] [PubMed] [Google Scholar]