Abstract
The subspecies of honeybee indigenous to the Cape region of South Africa, Apis mellifera capensis, is unique because a high proportion of unmated workers can lay eggs that develop into females via thelytokous parthenogenesis involving central fusion of meiotic products. This ability allows pseudoclonal lineages of workers to establish, which are presently widespread as reproductive parasites within the honeybee populations of South Africa. Successful long-term propagation of a parthenogen requires the maintenance of heterozygosity at the sex locus, which in honeybees must be heterozygous for the expression of female traits. Thus, in successful lineages of parasitic workers, recombination events are reduced by an order of magnitude relative to meiosis in queens of other honeybee subspecies. Here we show that in unmated A. m. capensis queens treated to induce oviposition, no such reduction in recombination occurs, indicating that thelytoky and reduced recombination are not controlled by the same gene. Our virgin queens were able to lay both arrhenotokous male-producing haploid eggs and thelytokous female-producing diploid eggs at the same time, with evidence that they have some voluntary control over which kind of egg was laid. If so, they are able to influence the kind of second-division meiosis that occurs in their eggs post partum.
IN the honeybee, Apis mellifera, unfertilized eggs normally develop into haploid males by arrhenotokous parthenogenesis. Unfertilized eggs are produced by queens for the production of males and also by unmated queenless workers whose eggs also produce functional males (Dzierzon 1845). Very occasionally, however, a worker will lay an egg in which meiosis II is modified so that an unfertilized egg is able to restore diploidy and become female (Mackensen 1943; Tucker 1958), in a form of parthenogenesis known as thelytoky. Thelytoky is ubiquitous in workers of the South African subspecies A. m. capensis (hereafter Cape) (Onions 1912; Anderson 1963) and is thought to be controlled by a single gene, Th, which a mapping study has suggested may be homologous to Grainy Head of Drosophila melanogaster (Lattorff et al. 2005, 2007). In Cape workers, two haploid pronuclei of second-division meiosis fuse and produce a diploid zygote, which usually gives rise to a female that may be reared as a worker or a queen (Moritz et al. 1996; Jordan et al. 2008). Some Cape workers use this ability to produce female offspring and reproductively parasitize other colonies (Allsopp 1993; Neumann et al. 2001; Baudry et al. 2004; Dietemann et al. 2006; Jordan et al. 2008).
During this form of automictic (meiotic) thelytokous parthenogenesis there is a normal reduction division, bivalent formation and formation of chiasmata during meiosis I (Verma and Ruttner 1983). If a locus is distant from the centromere there will be multiple recombination events between the locus and the centromere, and the two pairs of alleles will become randomly placed on the four chromatids. Thus thelytokous parthenogenesis involving recombination means that for any locus heterozygous in the mother, there is a one of three chance that the offspring will be homozygous, whichever way the pronuclei combine (Table 1; Pearcy et al. 2006). This ratio arises because if we choose any one chromatid at random, two of the three remaining chromatids will carry the alternate allele.
TABLE 1.
Predicted effects of recombination events and different kinds of gamete fusion during thelytokous parthenogenesis on the probability, r, that a locus heterozygous in the mother will be homozygous in the offspring, and the consequences if the locus is the complementary sex determiner (csd)
| Recombination
|
||||
|---|---|---|---|---|
| Absent
|
Present
|
|||
| Mode of parthenogenesis | r | csd | r | csd |
| Terminal fusion | 1 | Inviable | 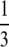 |
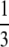 inviable inviable |
| Central fusion | 0 | Viable | 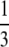 |
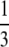 inviable inviable |
| Random fusion | 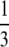 |
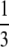 inviable inviable |
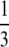 |
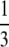 inviable inviable |
| Gamete duplication | 1 | Inviable | 1 | Inviable |
| Apomictic | 0 | Viable | 0 | Viable |
If there is interference to recombination or if loci are positioned close to the centromere and cannot recombine, the way in which the chromatids fuse determines what happens to the zygosity of offspring. During thelytokous parthenogenesis the products of meiosis II can fuse in one of three ways (Suomalainen et al. 1987; Pearcy et al. 2006). Let us assume that the four haploid pronuclei of meiosis II are aligned in a row as in A1A2B1B2. A1 and A2 were derived from nucleus A of meiosis I and B1 and B2 were derived from nucleus B. Under terminal fusion, terminal pronuclei fuse (i.e., A1 with A2 or B1 with B2). Under central fusion A2 fuses with B1, and in random fusion any of the pronuclei may fuse. Under terminal fusion without recombination, a locus heterozygous in the mother will become homozygous in the offspring. Under central fusion without recombination, a locus will remain heterozygous (Table 1). For completeness, other less likely scenarios for the fusion of gametes are given in Table 1.
When unmated Cape queens are stimulated to produce unfertilized eggs by exposure to carbon dioxide (Mackensen 1947), they too can produce diploid female offspring via thelytoky like queenless unmated workers (Crewe and Allsopp 1994). In contrast, when mated Cape queens lay unfertilized eggs, they produce males via arrhenotoky (Jordan et al. 2008). This indicates an extraordinary ability of Cape queens to manipulate the kind of parthenogenesis that occurs when they lay unfertilized eggs—thelytoky and arrhenotoky in unmated queens and arrhenotoky in mated queens. It also suggests that mated Cape queens could potentially produce daughters both sexually and asexually (Jordan et al. 2008).
In honeybees sex is determined by the combination of paternal and maternal alleles at a single locus, the complementary sex determiner (csd) locus (Beye et al. 2003). If the individual is heterozygous at the csd, it is female. If the individual is homozygous at the csd, a diploid male develops, but these are removed by workers at the first larval instar and are therefore inviable (Woyke 1963). If the individual is haploid and therefore hemizygous at the csd, it is male. The csd encodes an SR-type protein, which is enormously polymorphic (Hasselmann and Beye 2004) due to diversifying selection (Beye et al. 2003).
Sex determination via a single complementary sex locus has important consequences. In sexually producing populations we predict that selection will act to increase recombination rates at the csd because recombination increases the probability of heterozygosity at the csd. As expected, the region around the csd shows a sevenfold increase in recombination rate relative to other parts of the genome (Hasselmann and Beye 2006), presumably as a mechanism for maintaining heterozygosity. But what is expected in thelyotokous populations? In Table 1 we list the various kinds of gamete fusion that are possible and the consequences of the different forms on the probability that a locus heterozygous in the mother will become homozygous in diploid offspring. Table 1 shows that, in the absence of recombination, central fusion is favored over random fusion because heterozygosity is maintained. However, under all of terminal, random, and central fusion we expect a one-third reduction in heterozygosity at the sex locus if recombination occurs. Thus, in thelytokous populations with central fusion we expect reduced levels of recombination to evolve, at least on linkage group 3, which contains the sex locus. Studies of recombination rates in Cape workers show that they are at least an order of magnitude lower than in arrhenotokous queen meiosis (Moritz and Haberl 1994; Baudry et al. 2004), strongly suggesting that selection for reduced recombination has indeed occurred in thelytokous Cape workers.
Alternative means of parthenogenesis within the same species, and indeed the same individual, raise interesting questions concerning the mechanisms of gametogenesis in Cape queens. Gametogenesis in Cape queens is as yet undescribed, but is well understood for arrhenotokous populations. Queens in non-Cape populations store new eggs in their lateral oviducts (Dade 1977) with the maternal pronucleus arrested in metaphase I (Sasaki and Obaru 2002). Second-division meiosis occurs only after oviposition (Sasaki and Obaru 2002) when the diploid products of meiosis I align perpendicularly to the egg axis and undergo the second meiotic division. A single central nucleus becomes the maternal pronucleus, whereas the other three nuclei degenerate and become polar bodies (Petrunkewitsch 1901; Nachtsheim 1913; Yu and Omholt 1999). If the egg has been fertilized, one of the 6–10 sperm pronuclei present in the egg will fuse with the maternal pronucleus to produce a zygote and eventually a diploid female. If the egg has not been fertilized, the maternal nucleus continues to divide mitotically and will produce a haploid male by arrhenotokous parthenogenesis.
A detailed cytological description of thelytokous parthenogenesis in Cape worker-laid eggs is also available (Verma and Ruttner 1983). In thelytokous parthenogenesis by Cape workers the central (rather than the terminal or random) pronuclei fuse to produce the restored diploid nucleus, as if one of the central maternal pronuclei takes the place of a sperm pronucleus. A linkage study (Baudry et al. 2004) has confirmed the cytological evidence of central fusion.
Here we examine the sex, recombination rates, and the mode of gamete fusion in offspring of virgin queens of A. m. capensis that were treated with carbon dioxide to induce oviposition (Mackensen 1947). Jordan et al. (2008) showed that thelytokous reproduction is rare or absent in mated Cape honeybee queens, whereas it is normal in queens of the ant Catogyphos cursor (Pearcy et al. 2004, 2006). This investigation provides insights into the evolution of the widespread occurrence of thelytoky in the Cape worker and demonstrates that thelytoky is possible in the queen caste.
MATERIALS AND METHODS
Thelytokous and arrhenotokous reproduction in unmated queens:
In September 2006 in Stellenbosch, South Africa, we reared A. m. capensis queen pupae using standard methods (Harbo 1986; Laidlaw and Page 1997). Mature queen pupae were allowed to eclose in an incubator at 35°, and the virgins were then matured in the incubator in individual vials for 7 days, while being fed ad libitum on diluted honey. The queens were then anesthetized for 10 min with carbon dioxide to induce oviposition (Mackensen 1947) and introduced into nucleus colonies (Harbo 1986; Laidlaw and Page 1997) populated with A. m. scutellata workers and brood. To prevent mating, we clipped the wings of the queens, retaining the clippings for later genotyping. To limit the amount of worker reproduction in the nucleus colonies, and to aid the establishment of the virgin Cape queens, we used A. m. scutellata workers instead of A. m. capensis workers in the nucleus colonies. We anesthetized the queens at least once more 2 days after introduction and a third time if eggs were not seen. Until oviposition was observed, the queens were prevented from leaving their host colony by a grid of queen excluder material tacked over the entrance. Induction of oviposition in virgin queen honeybees from populations other than the Cape honeybee does not induce thelytokous parthenogenesis, but induces arrhenotokous parthenogenesis (Mackensen 1947).
As the first virgin-queen brood approached maturity, we collected brood from both worker and drone cells. To determine whether virgin Cape queens can produce both thelytokous and arrhenotokous progeny simultaneously, pupae were first sexed morphologically, and a sample of drone and worker progeny was then genotyped at microsatellite loci Am 059, Am 014, Am 107, and Am 061 (Solignac et al. 2003) to determine if they were sons of host workers, daughters of Cape workers foreign to the host colonies, or sons and daughters of the resident virgin queen. The queen genotype was obtained from tissue from the clipped wing of the queen. A progeny was rejected as being the offspring of the queen if it did not share at least one allele with the laying virgin queen at all four loci analyzed.
Recombination rates during thelytokous reproduction:
For this question we focused genotyping effort on the offspring of queen 1. This queen produced large numbers of worker brood in worker cells and no drone progeny. Pupae from worker cells or newly emerged callow workers (n = 44) were genotyped at 28 microsatellite loci on linkage groups 1 and 3 (which contains the csd). These loci were all heterozygous in the queen. Microsatellite loci and PCR primers were obtained from the microsatellite-based map Solignac_3 generated from 2008 microsatellite and other PCR-based markers segregating in the worker progeny of two hybrid queens (Solignac et al. 2007). This level of coverage provides accurate estimation of map distances between marker loci.
Under thelytokous parthenogenesis with central fusion, the expected recombination rate between a locus and the centromere is 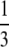 (see above and Table 1). Exceptions will occur when loci are situated <100 cM from the centromere, if distortions are caused by lethal allelic combinations at the sex locus, or by any other distorter of fair meiosis. The recombination fraction between a locus and its centromere, θ, can be estimated as the proportion of offspring that are homozygous in offspring (assuming the locus is heterozygous in the mother) (Baudry et al. 2004). Assuming no distortions to fair meiosis, the map distance, D, between a locus and the centromere can be calculated from
(see above and Table 1). Exceptions will occur when loci are situated <100 cM from the centromere, if distortions are caused by lethal allelic combinations at the sex locus, or by any other distorter of fair meiosis. The recombination fraction between a locus and its centromere, θ, can be estimated as the proportion of offspring that are homozygous in offspring (assuming the locus is heterozygous in the mother) (Baudry et al. 2004). Assuming no distortions to fair meiosis, the map distance, D, between a locus and the centromere can be calculated from 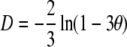 (Rizet and Engelmann 1949; Baudry et al. 2004). This relationship assumes that the probability of a chiasmata forming is Poisson distributed and corrects for the occurrence of double crossovers. Rizett and Engelmann's equation can also be used to calculate the map distance between any two pairs of loci, in which case θ is the proportion of individuals that are heterozygous at one locus and homozygous at the second (Baudry et al. 2004). Similarly, the inverse
(Rizet and Engelmann 1949; Baudry et al. 2004). This relationship assumes that the probability of a chiasmata forming is Poisson distributed and corrects for the occurrence of double crossovers. Rizett and Engelmann's equation can also be used to calculate the map distance between any two pairs of loci, in which case θ is the proportion of individuals that are heterozygous at one locus and homozygous at the second (Baudry et al. 2004). Similarly, the inverse 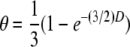 can be used to convert map distances from the Solignac_3 map to the expected recombination fraction between two loci or a locus and the centromere in thelytokously produced progeny under the assumption of fair meiosis. We used these equations to determine if patterns of recombination observed in the progeny of our queen differed from expectations under a model of thelyotokous parthenogenesis with central fusion or if they were more compatible with alternative modes of gamete fusion given in Table 1. We also used them to compare recombination rates in thelytokous reproduction observed here with recombination rates reported in Baudry et al. (2004).
can be used to convert map distances from the Solignac_3 map to the expected recombination fraction between two loci or a locus and the centromere in thelytokously produced progeny under the assumption of fair meiosis. We used these equations to determine if patterns of recombination observed in the progeny of our queen differed from expectations under a model of thelyotokous parthenogenesis with central fusion or if they were more compatible with alternative modes of gamete fusion given in Table 1. We also used them to compare recombination rates in thelytokous reproduction observed here with recombination rates reported in Baudry et al. (2004).
DNA extraction and microsatellite genotyping:
Tissue was obtained from the hind legs of worker and drone pupae and newly emerged callows and from the clipped wings of the virgin queens. DNA was extracted by grinding tissue in 500 μl of 5% Chelex solution followed by 10 min boiling (Walsh et al. 1991). Standard PCR conditions (Estoup et al. 1994) were used to amplify microsatellite loci (Solignac et al. 2003). PCR products (1.2 μl) from each multiplex reaction were added to 10 μl formamide and 100 nl LIZ DNA size standard (Applied Biosystems, Foster City, CA). Samples were run on a 3130xl Genetic Analyser (Applied Biosystems), with capillary length 36 cm and injection time of 15 sec at 1200 V, for 41 min. Resultant data files were analyzed using Genemapper software (Applied Biosystems) and genotypes for each individual were constructed.
RESULTS
Thelytokous and arrhenotokous reproduction in the same queens:
Both drone and worker brood were observed in all four colonies (Table 2). Workers were active contributors to egg laying in most colonies, reducing the number of queen-laid progeny we sampled. Nonetheless we were able to confirm thelytokous reproduction by queens in all four colonies. In three colonies queens laid both arrhenotokous and thelytokous offspring (Table 2). There is also evidence that queens preferentially laid eggs in the correct cell size, with thelytokous workers mostly reared in worker-sized cells and arrhenotokous drones in drone-sized cells. In all, 185 queen-laid individuals were retrieved from the correct cells and 10 from incorrect cells. This deviates significantly from random (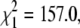 P < 0.001). These samples were taken as soon as the first queen progeny began to emerge and are therefore not thelytokous granddaughters of the virgin queens.
P < 0.001). These samples were taken as soon as the first queen progeny began to emerge and are therefore not thelytokous granddaughters of the virgin queens.
TABLE 2.
Numbers of thelytokous and arrhenotokous progeny produced by unmated Cape queens and the number of queen-laid progeny found in incorrect cells
| Drones
|
Workers
|
|||||
|---|---|---|---|---|---|---|
| Queen | No. genotyped | No. produced arrhenotokously by the virgin queen | No. laid incorrectly in worker cells by the virgin queen | No. genotyped | No. produced thelytokously by the virgin queen | No. laid incorrectly in drone cells by the virgin queen |
| 1 | 47 | 0 | 0 | 99 | 99 | 0 |
| 2 | 76 | 17 | 10 | 4 | 4 | 0 |
| 3 | 48 | 48 | 0 | 4 | 4 | 0 |
| 4 | 19 | 19 | 0 | 32 | 4 | 0 |
| Total | 190 | 84 | 10 | 139 | 111 | 0 |
Genotypes of individual bees used to compile this table are given in the supplemental material.
Mode of thelytokous reproduction in a virgin Cape queen:
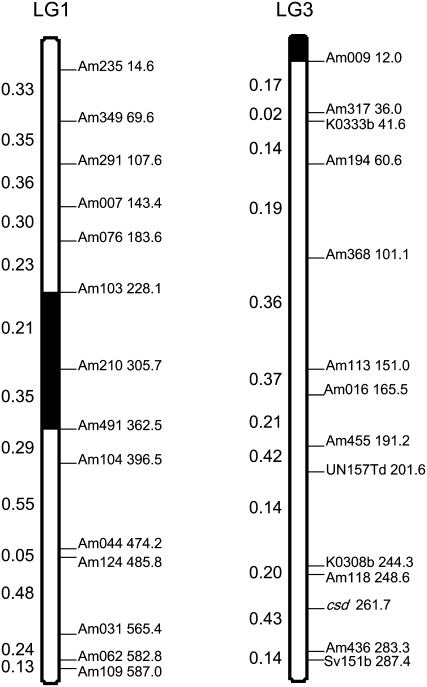
In the absence of centromeric interference, expected recombination fractions between all pairs of loci, θexp, calculated from the map distances from the Solignac_3 map using the Rizet and Engelmann (1949) correction are universally 0.33. The observed recombination fractions, θobs, between pairs of loci are given in Figure 1.
Figure 1.—
Representation of linkage groups 1 and 3 of the honeybee derived from the genetic map Solignac_3 (Solignac et al. 2007) showing the loci studied here. The cumulative map distance from one telomere is given after the name of each locus. The observed recombination fraction between pairs of loci, θobs, estimated as the proportion of bees homozygous at one locus and heterozygous at the second is given on the left-hand side of each linkage group. The expected recombination fraction between all pairs of loci is 0.33 after the Rizet and Engelmann correction. The solid areas on the chromosomes are thought to encompass the centromeres (Baudry et al. 2004). The location of the complementary sex determiner (csd) locus is indicated on linkage group 3.
On linkage group 1, loci Am 103, 210, and 491 were expected to show reduced recombination rates because they lie within or close to the centromeric region (Baudry et al. 2004) (Figure 1). We confirm a reduced recombination rate between loci Am 103 and Am 210, but the region between Am 210 and Am 491 showed a θobs of 0.35 (Figure 1), suggesting that the centromere is >100 cM distant from Am 491. On the other hand, the region between Am 076 and Am 103 showed a θobs of only 0.23, suggesting that the centromere of linkage group 1 may be slightly more telomeric than suggested by Baudry et al. Excluding loci Am 076, 103, and 210, the average number of recombinant workers per locus on linkage group 1, 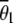 , was 0.33 (SE ± 0.014). This is not significantly different from the expected 0.33 on the assumption of automictic parthenogenesis with central, random, or terminal fusion of gametes (P > 0.05, one-sample t-test with 10 d.f.). However,
, was 0.33 (SE ± 0.014). This is not significantly different from the expected 0.33 on the assumption of automictic parthenogenesis with central, random, or terminal fusion of gametes (P > 0.05, one-sample t-test with 10 d.f.). However, 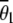 deviated significantly (P < 0.001) from 0 homozygotes expected under apomixis and 100% homozygotes expected under gamete duplication.
deviated significantly (P < 0.001) from 0 homozygotes expected under apomixis and 100% homozygotes expected under gamete duplication.
On linkage group 3, loci Am 009, Am 317, Am 194, and K0333b are within 100 cM of the terminal centromere and were expected to show reduced recombination rates (Baudry et al. 2004; Figure 1). As expected, these loci showed lower recombination rates than most other loci on this chromosome and no recombinants at all were seen at locus Am 009. Excluding these four loci, 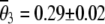 , which is marginally significantly different from 0.33% (t9 = 1.9, P = 0.05). This reduction in expected homozygosity is expected due to the effects of csd, which may have caused selection inviability of some homozygotes, especially near locus K0338 (Figure 2). Nonetheless, the proportion of homozygous individuals observed for noncentromeric loci on linkage group 3 differed significantly (P < 0.001) from that expected under both gamete duplication (100% homozygosity) and apomixis (no homozygotes) on the basis of one-sample t-tests with 9 d.f..
, which is marginally significantly different from 0.33% (t9 = 1.9, P = 0.05). This reduction in expected homozygosity is expected due to the effects of csd, which may have caused selection inviability of some homozygotes, especially near locus K0338 (Figure 2). Nonetheless, the proportion of homozygous individuals observed for noncentromeric loci on linkage group 3 differed significantly (P < 0.001) from that expected under both gamete duplication (100% homozygosity) and apomixis (no homozygotes) on the basis of one-sample t-tests with 9 d.f..
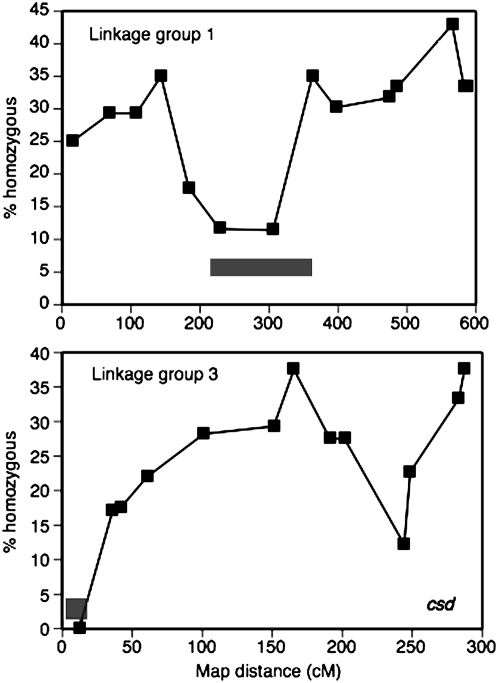
Figure 2.—
Proportion of individuals homozygous at a locus plotted against the Solignac_3 (Solignac et al. 2007) genetic maps for linkage groups 1 and 3. Centromeric regions determined by Baudry et al. (2004) are indicated by the bars. The location of the complementary sex determiner locus is indicated on linkage group 3.
The noncentromeric loci provide strong evidence that automictic parthenogenesis is more likely than gamete duplication or apomixis. The centromeric loci, where there is a reduction in recombination, can be used to determine whether random, central, or terminal fusion of gametes during automixis is more likely. Under central fusion with recombination we expect an increase in the proportion of individuals that are homozygous away from the centromere toward the chromosomal arms. Under terminal fusion we expect the reverse polarity (Baudry et al. 2004). In Figure 2 we plotted the proportion of individuals that are homozygous against the map distances obtained from the Solignac_3 genetic maps. Linkage group 1 shows two gradients of increasing homozygosity away from the metacentric centromere. Linkage group 3 shows a gradient of increasing homozygosity away from the terminal centromere. These patterns (especially that on linkage group 1) are consistent with central fusion and are inconsistent with terminal fusion, random fusion, or gamete duplication.
Baudry et al. (2004) calculated the map distance between loci Am 062 and Am 031 in the sexually produced progeny of an A. m. capensis queen and between Am 062 and Am 109 in the progeny of an arrhenotokous A. m. mellifera worker. This allows us to make a direct comparison of recombination rates in a normal A. m. capensis queen meiosis, an A. m. mellifera arrhenotokous worker meiosis, and a thelytokous A. m. capensis queen meiosis (Table 3). Our calculated map distances (Table 3) are larger than those estimated from other progenies, including that of normal queen meiosis in A. m. mellifera, suggesting that there is no reduction in recombination rates in the thelytokous meiosis of the A. m. capensis queen. Furthermore, we can directly compare the recombination frequency between loci Am 031 and Am 062 in thelytokous workers from Figure 3 of Baudry et al. (2004) and compare this directly to the recombination rate between these same loci in the thelytokous progeny of a virgin queen (this study). In the Baudry et al. study, 3 of 108 individuals showed recombination between these two loci, whereas in our study 10 of 42 individuals were recombinant, showing that there is a highly significant reduction in worker thelytokous parthenogenesis compared to that observed in the virgin queen (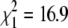 , P < 0.001).
, P < 0.001).
TABLE 3.
Linkage distances, D, in centimorgans estimated between two pairs of loci on linkage group 1 from various progeny
| Locus pair
|
||
|---|---|---|
| D estimated from progeny of | Am 062-031 | Am 062-109 |
| A. m. capensis queen (normal meiosis)a | 22.4 | — |
| A. m. capensis queen (thelytokous parthenogenesis)b | 56.5 | 32.4 |
| A. m. mellifera worker (arrhenotokous parthenogenesis)a | — | 6.5 |
| A. m. mellifera queen (normal meiosis)c | 17.4 | 4.2 |
Table 2 of Baudry et al. (2004), using the Haldane correction.
This study, calculated using the Rizet and Engelmann (1949) correction from data in Figure 1.
Derived from the Solignac-3 map (Solignac et al. 2007).
DISCUSSION
Warmelo (1912, p. 786) remarked that “… it would seem contrary to all the laws of nature that the African worker bee produce her progeny in a wholly different manner from the queen which is essentially a worker bee with fully developed reproductive organs.” Our study has shown that Warmelo was only half right with respect to thelytokous parthenogenesis in Cape queens and workers. In both castes, it appears that diploidy is restored by central fusion rather than terminal fusion of meiotic products or other possible mechanisms of gamete fusion listed in Table 1. However, the massively reduced rates of recombination observed in thelytokous parthenogenesis of the Cape worker (Moritz and Haberl 1994; Baudry et al. 2004) are apparently absent when a virgin Cape queen reproduces thelytokously.
Reduced rates of recombination are essential for the maintenance of genetic diversity in a parthenogen propagating thelytokously with central fusion (Belshaw and Quicke 2003; Baudry et al. 2004). In the case of honeybees where there is a single sex-determining locus that must be heterozygous for the expression of the female sex, maintenance of heterozygosity is essential, at least at the csd. Absence of reduced rates of recombination in the queen suggests that reduced recombination in the worker is under separate genotypic control from the control of thelytoky itself. Lattorff et al. (2007, 2005) showed that in the Cape worker, thelytoky is controlled by a single locus. This locus also influences two other traits related to worker reproduction pleiotropically: ovary activation and the production of a queen-like pheromonal bouquet (Lattorff et al. 2007). However, our results suggest that this locus may not be responsible for reduced rates of recombination, which is likely to be under separate genetic control. Clonal worker lineages [of which there are probably many (Jordan et al. 2008)] that do not successfully evolve reduced rates of recombination will be at a strong selective disadvantage against lineages that can do so and are likely to go extinct due to increasing homozygosity at the csd.
We have confirmed genetically the remarkable ability of unmated Cape queens to produce both thelytokous and arrhenotokous eggs during the same period (Crewe and Allsopp 1994). Our data suggest that virgin Cape queens have at least partial control over which kind of meiosis their eggs undergo. Where queens produced both male and female offspring, these were mostly (but not always) found in the correct cells. This suggests that Cape queens can to a large extent choose the ploidy of their eggs. An alternative explanation is that virgin queens lay thelytokous and arrhenotokous eggs at random in worker and drone cells, but that the workers selectively rear only those eggs that are laid in the appropriate cells. However, as workers readily rear larvae of any sex in both drone cells and worker cells without selection (Calderone and Kuenen 2001), it seems much more likely that virgin Capensis queens can influence whether they lay diploid or haploid eggs rather than workers removing the larvae that are located in the wrong cell type.
How could the ability to lay arrhenotokous or thelytokous eggs be advantageous to Cape queens? The ability to lay thelytokous eggs allows queens to effectively clone themselves. It has been argued that such an ability should be at a selective advantage during reproductive swarming, as the queen need not share the genome of her gyne offspring with her mating partners (Pearcy et al. 2004; Fournier et al. 2005; Jordan et al. 2008). When producing workers, Cape queens can produce haploid eggs and fertilize them with their stored sperm. As workers are mostly sterile, the queen pays little or no fitness cost by sharing her genome with her mating partners (Pearcy et al. 2004) and may increase her fitness by generating a genetically variable worker progeny (Jones et al. 2004; Mattila and Seeley 2007; Oldroyd and Fewell 2007; Seeley and Tarpy 2007). The ability to produce males that will potentially mate with other queens is also advantageous. The optimal strategy, if it is biologically possible, is to do all these things.
Jordan et al. (2008) reported that when Cape colonies undergo reproductive swarming, queens occasionally lay eggs in queen cells that are parthenogenetic offspring of themselves, suggesting that indeed, mated queens may have the ability to produce clonal queen offspring during reproductive swarming. Intriguingly, however, although two of these three individuals were shown by either morphological or genetic means to be female, they were homozygous at multiple loci that were heterozygous in their mother. Thus these offspring were presumably not produced by the same kind of thelytokous reproduction as observed here. The degree of homozygosity would suggest that these offspring were the products of the terminal fusion of two pronuclei or perhaps that the mothers of these eggs had some kind of ability to eliminate sperm pronuclei, yet maintain heterozygosity at the sex locus. Although such a mechanism seems unlikely, a reciprocal situation is known to occur in the little fire ant Wasmannia auropunctata, where the maternal genome is eliminated in eggs destined to be queens, thus allowing the male mating partners of queens to be genetically reincarnated as queens (Fournier et al. 2005).
The mechanism by which a queen might choose (or at least influence) the ploidy of her unfertilized eggs is difficult to envisage. In arrhenotokous populations queens have complete voluntary control over whether or not a particular egg they lay is fertilized (Ratnieks and Keller 1998). If the queen encounters a drone-sized cell [which she measures with her front tarsi (Koeniger 1970)], she refrains from releasing sperm onto the egg as it is laid. The egg then develops arrhenotokously as a male (Winston 1987). If she encounters a worker-sized cell she releases a minute amount of sperm onto the surface of the egg as it is laid, and these eggs develop as females (Harbo 1979). The process is remarkably accurate, and queens rarely make mistakes (Ratnieks and Keller 1998). No such mechanical option is available to Cape queens. To be able to choose the ploidy of her egg a queen must be able to influence the second-division meiosis that occurs in her egg after it has been laid, presumably by some signal encoded as the egg is laid. If she desires to lay a female-producing egg in a worker cell (or a queen cell), she must cause the two central pronuclei to fuse post partum. If she desires to lay a male-producing egg in a drone cell, she must cause all but one of the four pronuclei to degenerate, while the remaining pronucleus begins to divide mitotically, again post partum. And she must be able to switch between the two kinds of parthenogenesis depending on the kind of cell she is laying in.
In many insect species mitotic division of the zygote is stimulated by the presence of sperm in the cytoplasm of the egg (Sander 1985). In the Hymenoptera, however, an alternative mechanism is required because unfertilized eggs can develop by arrhenotokous parthenogenesis. In honeybee queens this stimulus is the physical squeezing of the egg as it is laid (Sasaki and Obaru 2002), so division occurs whether the egg is fertilized or not. Perhaps the queen goes through the same physical motion as she would to release sperm onto the egg, and this somehow stimulates the central pronuclei to fuse rather than to die, perhaps by a secretion from the accessory gland of the spermatheca.
Pearcy et al. (2006) explored the population genetics of thelytoky in the ant C. cursor. They showed that as with A. m. capensis, thelytoky is achieved by central fusion of automictic products. In C. cursor, colonies are established by parthenogenetic daughters of queens, which are generally highly inbred (Pearcy et al. 2004). Workers, in contrast, are produced sexually, and workers may become the mothers of a replacement queen if their queen dies (Pearcy et al. 2004). This ant system differs from that of the Cape bee, where queens almost always produce both daughter queens and workers sexually (Jordan et al. 2008). A parthenogenetic queen lineage will eventually have a high rate of homozygosity, even if recombination is constrained, and will be uncompetitive with more heterozygous queens laid by workers. This may explain why asexual reproduction, which we have shown is possible in Cape honeybee queens, is rarely used for the production of daughter queens. Perhaps the method of sex determination differs between C. cursor and the honeybee, so that inbreeding is less of a problem in the ant.
Acknowledgments
We thank Christian Fransman for his help in the field and Theunis Engelbrecht of Douglas Bee Farms for lending us the A. m. scutellata colonies. This work was supported by Australian Research Council grants to B.P.O. and M.B. and a University of Sydney grant to M.B. Our manuscript was improved by the comments of Nathan Lo and Sharoni Shafir and two anonymous reviewers.
References
- Allsopp, M. H., 1993. Summarized overview of the Capensis problem. S. Afr. Bee J. 65 127–136. [Google Scholar]
- Anderson, R. H., 1963. The laying worker in the Cape honeybee Apis mellifera capensis. J. Apic. Res. 2 85–92. [Google Scholar]
- Baudry, E., P. Kryger, M. Allsopp, N. Koeniger, D. Vautrin et al., 2004. Whole genome scan in thelytokous-laying workers of the Cape honey bee (Apis mellifera capensis): central fusion, reduced recombination rates and centromere mapping using half-tetrad analysis. Genetics 167 243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belshaw, R., and D. L. J. Quicke, 2003. The cytogenetics of thelytoky in a predominantly asexual parasitoid wasp with covert sex. Genome 46 170–173. [DOI] [PubMed] [Google Scholar]
- Beye, M., M. Hasselmann, M. K. Fondrk, R. E. Page and S. W. Omholt, 2003. The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell 114 419–429. [DOI] [PubMed] [Google Scholar]
- Calderone, N. W., and L. P. S. Kuenen, 2001. Effects of Western honey bee (Hymenoptera: Apidae) colony, cell type, and larval sex on host acquisition by female Varroa destructor (Acdari: Varroidae). J. Econ. Entomol. 95 1022–1030. [DOI] [PubMed] [Google Scholar]
- Crewe, R., and M. Allsopp, 1994. Sex and the single queen: recent experiments with capensis and scutellata queens. S. Afr. Bee J. 66 58–62. [Google Scholar]
- Dade, H. A., 1977. Anatomy and Dissection of the Honeybee. International Bee Research Association, London.
- Dietemann, V., J. Pflugfelder, S. Härtel, P. Neumann and R. Crewe, 2006. Social parasitism by honeybee workers (Apis mellifera capensis Esch.): evidence for pheromonal resistance to host queen's signals. Behav. Ecol. Sociobiol. 60 785–793. [Google Scholar]
- Dzierzon, J., 1845. Gutachten über die von Herrn Direktor Stöhr im ersten und zweiten Kapitel des General-Gutachtens aufgestellten Fragen. Eichstädter Bienenzeitung 1 109–113, 119–121. [Google Scholar]
- Estoup, A., M. Solignac and J.-M. Cornuet, 1994. Precise assessment of the number of patrilines and of genetic relatedness in honey bee colonies. Proc. R. Soc. Lond. Ser. B 258 1–7. [Google Scholar]
- Fournier, D., A. Estoup, R. M. Orivel, J. Foucaud, H. Jourdan et al., 2005. Clonal reproduction by males and females in the little fire ant. Nature 435 1230–1234. [DOI] [PubMed] [Google Scholar]
- Harbo, J. R., 1979. The rate of depletion of spermatozoa in the queen honeybee spermatheca. J. Apic. Res. 18 204–207. [Google Scholar]
- Harbo, J. R., 1986. Propagation and instrumental insemination, pp. 361–389 in Bee Genetics and Breeding, edited by T. E. Rinderer. Academic Press, Orlando, FL.
- Hasselmann, M., and M. Beye, 2004. Signatures of selection among sex determining alleles of the honey bee. Proc. Natl. Acad. Sci. USA 101 4888–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmann, M., and M. Beye, 2006. Pronounced differences of recombination activity at the sex determination locus of the honeybee, a locus under strong balancing selection. Genetics 174 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, J., M. Myerscough, S. Graham and B. P. Oldroyd, 2004. Honey bee nest thermoregulation: diversity promotes stability. Science 305 402–404. [DOI] [PubMed] [Google Scholar]
- Jordan, L. A., M. H. Allsopp, B. P. Oldroyd, T. C. Wossler and M. Beekman, 2008. Cheating honeybee workers produce royal offspring. Proc. R. Soc. Lond. Ser. B 275 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeniger, N., 1970. Factors determining the laying of drone and worker eggs by the queen honey bee. Bee World 51 166–169. [Google Scholar]
- Laidlaw, H. H., and R. E. J. Page, 1997. Queen Rearing and Bee Breeding. Wicwas Press, Cheshire, UK.
- Lattorff, H. M. G., R. F. A. Moritz and S. Fuchs, 2005. A single locus determines thelytokous parthenogenesis of laying honeybee workers (Apis mellifera capensis). Heredity 94 533–537. [DOI] [PubMed] [Google Scholar]
- Lattorff, H. M. G., R. F. A. Moritz, R. M. Crewe and M. Solignac, 2007. Control of reproductive dominance by the thelytoky gene in honeybees. Biol. Lett. 3 292–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackensen, O., 1943. The occurrence of parthenogenetic females in some strains of honey-bees. J. Econ. Entomol. 36 465–467. [Google Scholar]
- Mackensen, O., 1947. Effect of carbon dioxide on initial oviposition of artificially inseminated and virgin queen honey bees. J. Econ. Entomol. 40 344–349. [DOI] [PubMed] [Google Scholar]
- Mattila, H. R., and T. D. Seeley, 2007. Genetic diversity in honey bee colonies enhances productivity and fitness. Science 317 362–364. [DOI] [PubMed] [Google Scholar]
- Moritz, R. F. A., and M. Haberl, 1994. Lack of meiotic recombination in thelytocous parthenogenesis of of laying workers of Apis mellifera capensis (the Cape honeybee). Heredity 73 98–102. [Google Scholar]
- Moritz, R. F. A., P. Kryger and M. H. Allsopp, 1996. Competition for royalty in bees. Nature 384 31. [Google Scholar]
- Nachtsheim, H., 1913. Cytologische studien über die geschlechtsbestimmung bie der honigbiene (Apis mellifera L.). Arch. Zellforsch. 11 119–241. [Google Scholar]
- Neumann, P., S. E. Radloff, R. F. A. Moritz, H. R. Hepburn and S. L. Reece, 2001. Social parasitism by honeybee workers (Apis mellifera capensis Escholtz): host finding and resistance of hybrid host colonies. Behav. Ecol. 12 419–428. [Google Scholar]
- Oldroyd, B. P., and J. H. Fewell, 2007. Genetic diversity promotes homeostasis in insect colonies. Trends Ecol. Evol. 22 408–413. [DOI] [PubMed] [Google Scholar]
- Onions, G. W., 1912. South African ‘fertile worker bees’. Agric. J. Union S. Afr. 1 720–728. [Google Scholar]
- Pearcy, M., S. Aron, C. Doums and L. Keller, 2004. Conditional use of sex and parthenogenesis for worker and queen production in ants. Science 306 1780–1783. [DOI] [PubMed] [Google Scholar]
- Pearcy, M., O. Hardy and S. Aron, 2006. Thelytokous parthenogenesis and its consequences on inbreeding in an ant. Heredity 96 377–382. [DOI] [PubMed] [Google Scholar]
- Petrunkewitsch, A., 1901. Die Richt ungskörper und ihr Schicksal im befruchteten und ubenfruchteten Bienenei. Zool. Jahrb. Abt. Anat. Ontog. Tiere. 14 573–608. [Google Scholar]
- Ratnieks, F. L. W., and L. Keller, 1998. Queen control of egg fertilization in the honey bee. Behav. Ecol. Sociobiol. 44 57–61. [Google Scholar]
- Rizet, G., and C. Engelmann, 1949. Contribution à l'étude génétique d'un Ascomycète tétrasporé: Podospora anserina (Ces.) Rehm. Rev. Cytol. Biol. Veg. 11 201–304. [Google Scholar]
- Sander, K., 1985. Fertilization and egg cell activation in insects, pp. 409–430 in Biology of Fertilization, edited by C. B. Metz and A. Monroy. Academic Press, Orlando, FL.
- Sasaki, K., and Y. Obaru, 2002. Egg activation and timing of sperm acceptance by an egg in honeybees (Apis mellifera L.). Insect Soc. 49 234–240. [Google Scholar]
- Seeley, T. D., and D. R. Tarpy, 2007. Queen promiscuity lowers disease within honeybee colonies. Proc. R. Soc. Lond. Ser. B 274 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solignac, M., D. Vautrin, A. Loiseau, F. Mougel, E. Baudry et al., 2003. Five hundred and fifty microsatellite markers for the study of the honeybee (Apis mellifera L.) genome. Mol. Ecol. Notes 3 307–311. [Google Scholar]
- Solignac, M., F. Mougel, D. Vautrin, M. Monnerot and J.-M. Cornuet, 2007. A third-generation microsatellite-based linkage map of the honey bee, Apis mellifera, and its comparison with the sequence-based physical map. Genome Biol. 8 R66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomalainen, E., A. Saura and J. Lokki, 1987. Cytology and Evolution in Parthenogenesis. CRC Press, Boca Raton, FL.
- Tucker, K. W., 1958. Automictic parthenogenesis in the honey bee. Genetics 43 299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, L. R., and F. Ruttner, 1983. Cytological analysis of the thelytokous parthenogenesis in the Cape honeybee (Apis mellifera capensis Escholtz). Apidologie 14 47–57. [Google Scholar]
- Walsh, P. S., D. A. Metzger and R. Higuchi, 1991. Chelex (R)100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10 507. [PubMed] [Google Scholar]
- Warmelo, D. S., 1912. South African fertile-worker bees and parthenogenesis. Agric. J. Union S. Afr. 3 786–789. [Google Scholar]
- Winston, M. L., 1987. The Biology of the Honey Bee. Harvard University Press, Cambridge, MA.
- Woyke, J., 1963. What happens to diploid drone larvae in a honeybee colony? J. Apic. Res. 2 73–75. [Google Scholar]
- Yu, R., and S. W. Omholt, 1999. Early developmental processes in the fertilized honeybee (Apis mellifera) oocyte. J. Insect Physiol. 45 763–767. [DOI] [PubMed] [Google Scholar]