Abstract
The ESC2 gene encodes a protein with two tandem C-terminal SUMO-like domains and is conserved from yeasts to humans. Previous studies have implicated Esc2 in gene silencing. Here, we explore the functional significance of SUMO-like domains and describe a novel role for Esc2 in promoting genome integrity during DNA replication. This study shows that esc2Δ cells are modestly sensitive to hydroxyurea (HU) and defective in sister chromatid cohesion and have a reduced life span, and these effects are enhanced by deletion of the RRM3 gene that is a Pif1-like DNA helicase. esc2Δ rrm3Δ cells also have a severe growth defect and accumulate DNA damage in late S/G2. In contrast, esc2Δ does not enhance the HU sensitivity or sister chromatid cohesion defect in mrc1Δ cells, but rather partially suppresses both phenotypes. We also show that deletion of both Esc2 SUMO-like domains destabilizes Esc2 protein and functionally inactivates Esc2, but this phenotype is suppressed by an Esc2 variant with an authentic SUMO domain. These results suggest that Esc2 is functionally equivalent to a stable SUMO fusion protein and plays important roles in facilitating DNA replication fork progression and sister chromatid cohesion that would otherwise impede the replication fork in rrm3Δ cells.
POST-TRANSLATIONAL modification, including phosphorylation, ubiquitination, and other types of covalent protein modification, is an important mechanism for rapidly altering protein stability, activity, or localization (Schwartz and Hochstrasser 2003). The process/pathway of SUMOylation, which is mechanistically analogous to ubiquitination, requires a distinct group of SUMO-specific enzymes to covalently attach SUMO to its protein targets (Muller et al. 2001; Seeler and Dejean 2003; Johnson 2004). In contrast to ubiquitination, SUMOylation usually enhances the stability of protein targets or the formation of protein complexes and therefore plays a role in regulating multiple cellular processes, including subcellular localization, signal transduction, cell–cycle progression, and genome stability.
Saccharomyces cerevisiae ESC2 (Establishment silent chromatin 2) was first identified as genes necessary for silencing at mating-type locus that, when mutated or deleted, give rise to a partial defect in gene silencing (Dhillon and Kamakaka 2000; Cuperus and Shore 2002; Andrulis et al. 2004). Sequence analysis showed that Esc2 includes two tandem C-terminal SUMO-like domains and an N-terminal polar low-complexity domain, and its domain architecture is conserved in fission yeast (Rad60) and humans (NIP45) (Novatchkova et al. 2005). Although ESC2 is not essential for growth, Schizosaccharomyces pombe rad60 is essential for growth and rad60 mutants are hypersensitive to DNA damaging agents (Morishita et al. 2002; Boddy et al. 2003). The essential function of S. pombe Rad60 may be to regulate homologous recombination at stalled or collapsed DNA replication forks or to prevent cell cycle progression in cells with DNA damage (Miyabe et al. 2006; Raffa et al. 2006). Thus, since the functions known for Esc2 and Rad60 appear to be largely disparities, additional studies are needed to determine the functional similarities and/or differences between S. cerevisiae Esc2 and S. pombe Rad60.
Tightly bound proteins or protein complexes and aberrant DNA structures can impede progression of the replication fork during S phase. Cells utilize several mechanisms, including DNA repair, DNA damage tolrerance, and DNA damage checkpoint pathways, to overcome such impediments and resume cell cycle progression (Cox et al. 2000; Barbour and Xiao 2003). Recent studies in yeast show that a Pif1-like 5′ to 3′ DNA helicase called Rrm3 facilitates restart of replication forks blocked by stable protein-DNA complexes (Ivessa et al. 2003). Cells that lack Rrm3 grow normally and are resistant to DNA damaging agents; however DNA replication is less processive due to frequent pausing, and an intra-S-phase/DNA damage checkpoint is activated in rrm3Δ cells (Torres et al. 2004; Azvolinsky et al. 2006). rrm3Δ is also a synthetic lethal with mrc1Δ, a claspin-like protein that is required for S-phase checkpoint activation and to stabilize stalled replication forks (Osborn and Elledge 2003; Torres et al. 2004; Szyjka et al. 2005). The replication function, but not the checkpoint function of Mrc1 is essential in rrm3Δ cells, because mrc1-AQ, which selectively inactivates Mrc1-dependent checkpoint activation, does not inhibit cell growth in rrm3Δ cells (Szyjka et al. 2005). These results suggest that Rrm3 and Mrc1 play distinct roles in rescuing stalled DNA replication forks.
This study characterized the in vivo function of the SUMO-like protein Esc2. The results demonstrate that deletion of both SUMO-like domains of Esc2 destabilizes and functionally inactivates Esc2, but one SUMO or one SUMO-like domain is sufficient to restore its stability. esc2Δ rrm3Δ double mutants show a severe growth defect and have a high rate of endogenous DNA damage and a defect in sister chromatid cohesion. The Esc2 SUMO-like domain is also likely to be involved in modulating the function of Mrc1 at stalled replication forks. These data suggest that Esc2 plays important roles not only in gene silencing but also in facilitating DNA replication fork progression and sister chromatid cohesion.
MATERIALS AND METHODS
Strains and plasmids:
All yeast strains used in this study are listed in Table 1. The 2.6-kb PstI–PstI genomic fragment containing ESC2 was cloned into pUC19. The esc2 mutants described below were obtained from pUC19-ESC2 by site-directed PCR mutagenesis. esc2Δsd1 was constructed by insertion of SpeI sites at the 195th and 296th amino acids, digestion with SpeI, and ligation. esc2Δsd2 and esc2ΔC were constructed by inserting a stop codon at the 375th or 195th amino acids, respectively. esc2ΔC-SUMO and esc2Δsd2-SUMO were constructed by inserting SpeI sites at the positions encoding the 195th and 375th amino acids, digesting with SpeI, and then ligating with the S. cerevisiae SUMO gene. The C-terminal four amino acids of full-length Smt3, including the Gly-Gly found at the C terminus of mature Smt3, were not included in these constructs to prevent covalent attachment of SUMO to other protein targets. Wild-type ESC2 and esc2 mutants were tagged with an HA cassette at the NH2 terminus. The PstI–PstI fragments of ESC2 and esc2 mutants containing the promoter and open reading frame were cloned into pRS306 or pRS426 for genome replacement and overexpression studies, respectively. pRS306-series plasmids were digested with SphI and integrated into the genome. The resultant strains were then plated onto SC plates containing 5-fluoroorotic acid to select for ura− cells. The Escherichia coli overexpression plasmids were constructed by ligating the NdeI–BamHI fragment of ESC2 and esc2 mutants tagged with the HA cassette into pET3a. pRS415-GFP-RAD52 was constructed by ligating the SalI–SalI RAD52 fragment of pSC52 (Hishida et al. 2002) into pRS415 and inserting a GFP cassette at its NH2 terminus.
TABLE 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| W1588-4A | W303-1A (MATa), RAD5 | McDonald et al. (1997) |
| W1588-4B | W303-1B (MATα), RAD5 | McDonald et al. (1997) |
| YTO571 | W1588-4A, HA-ESC2 | This study |
| YTO665 | W1588-4A, HA-esc2Δsd1 | This study |
| YTO575 | W1588-4A, HA-esc2Δsd2 | This study |
| YTO613 | W1588-4A, HA-esc2ΔC | This study |
| YTO708 | W1588-4A, HA-esc2Δsd2-SUMO | This study |
| YTO610 | W1588-4A, HA-esc2ΔC-SUMO | This study |
| YTO13 | W1588-4A, esc2Δ∷kanMX4 | This study |
| YLS586 | W303-1B, hmrΔB∷ADE2 | Chi and Shore (1996) |
| YTO549 | YLS586, esc2Δ∷kanMX4 | This study |
| YTO670 | YLS586, esc2Δsd1 | This study |
| YTO550 | YLS586, esc2Δsd2 | This study |
| YTO731 | YLS586, esc2ΔN | This study |
| YTO619 | YLS586, esc2ΔC | This study |
| YTO620 | YLS586, esc2ΔC-SUMO | This study |
| YTO642 | W1588-4A, esc2Δ∷HIS3 | This study |
| YTO646 | W1588-4A, sir2Δ∷kanMX4 | This study |
| YTO647 | W1588-4A, esc2Δ∷HIS3 sir2Δ∷kanMX4 | This study |
| YTO533 | W1588-4A, rrm3Δ∷ADE2 | This study |
| YTO655 | W1588-4A, rrm3Δ∷URA3 | This study |
| YTO739 | W1588-4A, sir2Δ∷kanMX4 rrm3Δ∷URA3 | This study |
| YTO541 | W1588-4A, esc2Δ∷kanMX4 rrm3Δ∷ADE2 | This study |
| YTO164 | W1588-4A, esc2Δsd2 | This study |
| YTO543 | W1588-4A, esc2Δsd2 rrm3Δ∷ADE2 | This study |
| YTO51 | W1588-4A, rad51Δ∷URA3 | This study |
| YTO693 | W1588-4A, mrc1Δ∷kanMX4 | This study |
| YTO695 | W1588-4A, esc2Δ∷HIS3 mrc1Δ∷kanMX4 | This study |
| YPH1444 | MATaade2 his3 trp1 ura3 leu2 can1 lacI-NLS-GFP∷HIS3 lacO∷URA3∷CEN15 | Xu et al. (2004) |
| YTO536 | YPH1444, esc2Δ∷kanMX4 | This study |
| YTO534 | YPH1444, rrm3Δ∷ADE2 | This study |
| YTO535 | YPH1444, esc2Δ∷kanMX4 rrm3Δ∷ADE2 | This study |
| YTO540 | YPH1444, sir2Δ∷kanMX4 | This study |
| YTO701 | YPH1444, mrc1Δ∷kanMX4 | This study |
| YTO702 | YPH1444, esc2Δ∷LEU2 mrc1Δ∷kanMX4 | This study |
| YTO703 | YPH1444, rrm3Δ∷ADE2 sir2Δ∷kanMX4 | This study |
| TH500 | W1588-4A, RAD53-myc.kanMX4 | This study |
| YTO675 | TH500, esc2Δ∷HIS3 | This study |
| YTO676 | TH500, esc2Δsd2 | This study |
| YTO728 | TH500, esc2Δsd2-SUMO | This study |
| YTO721 | TH500, rrm3Δ∷ADE2 | This study |
| YTO678 | TH500, esc2Δ∷HIS3 rrm3Δ∷ADE2 | This study |
| YTO679 | TH500, esc2Δsd2 rrm3Δ∷ADE2 | This study |
| YTO729 | TH500, esc2Δsd2-SUMO rrm3Δ∷ADE2 | This study |
| YTO740 | TH500, sir2Δ∷HIS3 | This study |
| YTO741 | TH500, sir2Δ∷HIS3 rrm3Δ∷ADE2 | This study |
Yeast two-hybrid assay:
Gal4-based Matchmaker Two-Hybrid System 3 (Clontech) was used for the yeast two-hybrid assay. The Sir2 protein was fused to the Gal4 activation domain in pGADT7 vector and the Esc2 protein and several esc2 mutant proteins were fused to the Gal4 DNA-binding domain in pGBKT7, and expressed in S. cerevisiae tester strain AH109.
Preparation of yeast extracts and Western blotting:
Total protein extract was prepared from 5 × 106 cells from logarithmically growing culture using the trichloroacetic acid (TCA) method described by Pellicioli et al. (1999). Proteins were analyzed by SDS–PAGE, transferred to PVDF membranes, and probed with anti-HA or -Myc monoclonal antibody (Roche). Detection was performed with HRP-conjugated secondary antibodies followed by treatment using the ECL advance Western blot detection kit (BD Biosciences).
Immunoprecipitation:
Cell cultures (1–2 × 107 cells/ml) were collected by centrifugation and washed once with lysis buffer (10% glycerol, 50 mm Tris-HCl at pH 7.5, 150 mm NaCl, 1 mm EDTA, 0.1% NP-40, 1 mm PMSF, Protease Inhibitor Cocktail) (Sigma). Cells were resuspended in the same volume of lysis buffer, and 2 volumes of glass beads were added. Cells were disrupted using a Bead Beater (BIOSPEC) for 1 min, four times. After centrifugation, the supernatant fraction was collected and used for immunoprecipitation. For immunoprecipitation, 40 μl of anti-HA agarose conjugate beads (Sigma) were used for 1 ml of cell lysate and the mixture was rotated for 2 hr at 4°. The beads were washed four times, resuspended in sample buffer, and boiled for 3 min. Samples were analyzed by SDS–PAGE and signals were detected by Western blot analysis (BD Biosciences).
Silencing assay:
HMR∷ADE2 silencing was performed as described previously (Chi and Shore 1996; Dhillon and Kamakaka 2000). Isogenic strains were grown in YPD culture to early logarithmic phase (2–5 × 106 cells/ml) at 30°, and serial dilutions were plated on YPD plates to obtain well-dispersed, discrete colonies. Plates were incubated at 30° for 3 days and then 4° for 7 days until colony color (red, white, pink, or sector) could be distinguished and then photographed.
Senescence analysis:
Senescence was analyzed by counting the number of replicative cycles before cessation of cell division, as described previously (Kennedy et al. 1994).
Other materials and methods:
Fluorescence-activated cell sorting (FACS), microscopy, and sister chromatid cohesion assay were performed as described previously (Hishida et al. 2002, 2006; Xu et al. 2004).
RESULTS
Construction of esc2 truncation mutants:
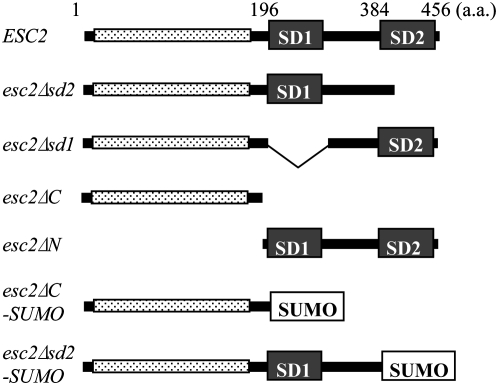
Esc2 has a N-terminal low complexity polar region enriched in positively and negatively charged residues and a C-terminal globular region with two SUMO-like domains, SD1 and SD2 (Figure 1). To examine the biological functions of Esc2 and the specific roles of SD1 and SD2, expression constructs for a series of domain truncation mutants of Esc2 were generated. Two domain substitution mutants were also generated, in which a SUMO domain replaced SD2 or SD1/2 of Esc2. These truncated forms of Esc2 are shown schematically in Figure 1.
Figure 1.—
Schematic of Esc2 truncation mutants. The domains of Esc2 are shown schematically. The N-terminal low complexity region with polar and charged residues is represented by the stippled bar; the C-terminal globular region has two SUMO-like domains, indicated as SD1 and SD2. The SUMO domain is encoded by SMT3 gene.
Interaction between Sir2 and Esc2 truncation mutants:
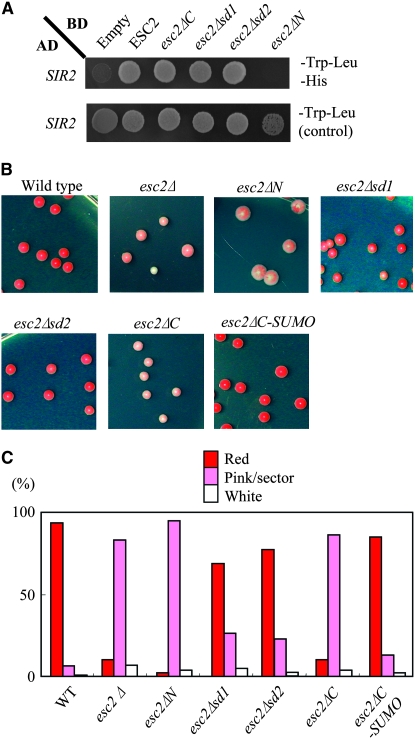
Previous two-hybrid studies showed that Esc2 interacts with Sir2, implicating Esc2 in gene silencing (Cuperus and Shore 2002). Here, the physical interaction between Esc2 and Sir2 was mapped by performing yeast two-hybrid assays in which the “bait” domain included truncated derivatives of Esc2 fused to the Gal4 DNA binding domain (Gal4 BD). These fusion proteins were expressed in yeast on a multi-copy plasmid from an ADH1 promoter and detected in crude extract by Western blot analysis (data not shown). The two-hybrid assay demonstrated that Esc2ΔC lacking both SD1 and SD2 still interacts with Sir2, but Esc2ΔN lacking the N-terminal domain does not (Figure 2A), indicating that the N-terminal domain of Esc2 is responsible for its interaction with Sir2.
Figure 2.—
N-terminal region of Esc2 is required for gene silencing. (A) Two-hybrid assays were conducted with wild-type Esc2 or truncated versions of Esc2 and Sir2, as described in materials and methods. Positive interactions were detected by growth on SC −Trp −Leu −His plates. (B) A colorimetric assay was used to evaluate HMR∷ADE2 silencing in isogenic strains bearing either wild type, esc2Δ, esc2ΔN, esc2Δsd1, esc2Δsd2, esc2ΔC, or esc2ΔC-SUMO. Cells were grown to log phase, serially diluted, and plated on YPD plates. Plates were incubated at 30° for 3 days and at 4° for 7 days. (C) The colonies were scored as solid red (silenced), red with white sectors (reduced silencing), or white (no silencing). More than 200 individual colonies were scored for each strain.
Domains of Esc2 required for silencing at the HMR locus:
To next determine which domains of Esc2 are required for silencing, a functional screen for ESC2 was developed on the basis of an ADE2 reporter gene at HMR. In this screen, wild-type ESC2 silences the ADE2 reporter at HMR, giving rise to red colonies, and null or partial esc2 function yields white, pink, and/or sectored colonies (Dhillon and Kamakaka 2000; Cuperus and Shore 2002). esc2 mutants were integrated into the genome at the ESC2 locus under control of the native promoter and screened for function. The results indicate strong silencing of ADE2 and mostly red colonies in cells expressing wild-type ESC2 and truncated esc2 lacking SD1 or SD2 (i.e., esc2Δsd1 or esc2Δsd2) (Figure 2, B and C). In contrast, expression of esc2ΔN, esc2ΔC, or esc2Δ led to a large increase in the fraction of pink and white colonies and a large decrease in the fraction of red colonies (Figure 2, B and C). Interestingly, the level of ADE2 silencing and fraction of red colonies was very similar in cells expressing ESC2 and esc2ΔC-SUMO, in which SD1/2 was replaced with one SUMO protein. These results suggest that the N-terminal region of Esc2 and at least one SUMO-like or SUMO domain are required for Esc2-mediated silencing at HMR.
The SUMO-like domain is required for Esc2 stability:
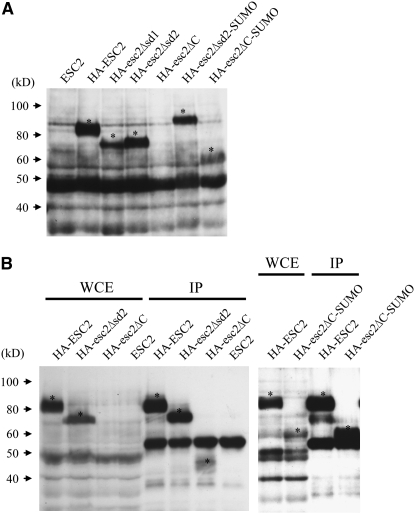
The above results suggest that different domains of Esc2 are required for interacting with Sir2 and for functional silencing of ADE2 at HMR. However, these data could also reflect differential levels of expression or stability of esc2 truncation mutants. This possibility was addressed by quantifying expression of genomic esc2 truncation mutants carrying an N-terminal HA epitope tag. The results of this analysis are shown in Figure 3. Note that wild-type HA-Esc2 had an apparent electrophoretic mobility of 85 kDa, which is larger than its predicted size of 60 kDa. Similar results were observed when HA-Esc2 was expressed in E. coli (data not shown) and therefore may be due to the high density of charged amino acids in the Esc2 N-terminal domain. Wild-type Esc2 and all of the Esc2 derivatives were stable and were expressed at a similar level except Esc2ΔC, whose expression was not detected in crude-cell extracts (Figure 3A) or was weakly detected with a mobility of ∼45 kDa in immunoprecipitates of whole-cell extracts (Figure 3B). In contrast to Esc2ΔC, Esc2ΔC-SUMO expression was detected in both crude extract and immunoprecipitates of whole-cell extracts although the relative expression level was slightly reduced in crude extracts as compared with other HA-tagged Esc2 proteins (Figure 3, A and B). These data suggest that Esc2ΔC is intrinsically unstable and that one C-terminal SUMO or SUMO-like domain is sufficient to stabilize Esc2ΔC. This is consistent with the observation above that esc2ΔC-SUMO silenced ADE2 at HMR as effectively as ESC2.
Figure 3.—
Expression of HA-tagged Esc2 truncation mutants. (A) Protein extracts were prepared from the indicated strains. Samples were analyzed by SDS–PAGE followed by Western blotting with anti-HA antibody. (B) Crude extracts were immunoprecipitated and Western blotted with anti-HA antibody as described in materials and methods. HA-tagged Esc2 proteins are indicated by an asterisk.
Esc2 is involved in cell life span:
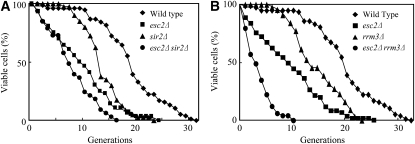
Previous studies show that sir2Δ cells have a shorter life span and reach senescence faster than wild-type cells (Kaeberlein et al. 1999). Here, the effect of esc2Δ on yeast life span was measured by counting the number of daughter cells generated from an individual mother cell (Kennedy et al. 1994). The results show that the mean life span for esc2Δ cells is 9.8 generations, while the mean life span of wild-type yeast is 19.0 generations (Figure 4A), indicating that mutation of ESC2 causes a shortening of life span relative to wild type. sir2Δ cells also have a short life span (mean of 13.5), but young esc2Δ cells have higher mortality than sir2Δ cells (Figure 4A). esc2Δ sir2Δ cells have an even shorter mean life span of 5.2 generations (Figure 4A), suggesting that esc2 and sir2 are not epistatic with respect to life span. These results suggest that Esc2, like Sir2, plays a role in yeast senescence, but that these genes act independently on life span.
Figure 4.—
esc2Δ cells show the reduced life span. (A) Life span was measured for wild-type, esc2Δ sir2Δ, and esc2Δ sir2Δ cells. Mean number of replicative cycles is as follows: Wild type, 19.0; esc2Δ, 9.8; sir2Δ, 13.5; esc2Δ sir2Δ, 5.2. (B) Life span was measured for wild-type, esc2Δ rrm3Δ, and esc2Δ rrm3Δ cells. The data of wild-type and esc2Δ cells are the same as in Figure 4A. Mean number of replicative cycles is as follows: Wild type, 19.0; esc2Δ, 9.8; rrm3Δ, 13.5; esc2Δ rrm3Δ, 2.3.
Additional roles for Esc2 in yeast:
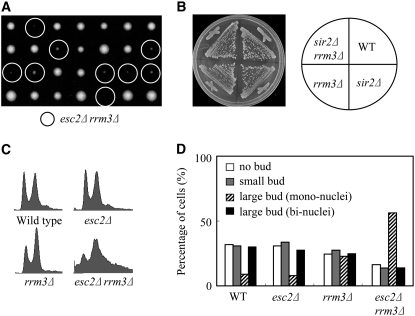
Rrm3 is a member of the Pif1 family of DNA helicases, a family that is highly conserved from yeasts to humans (Boule and Zakian 2006) and its helicase activity is required for efficient replication past specific, particularly stable chromatin-associated complexes (Torres et al. 2004; Azvolinsky et al. 2006). This study and previous studies have shown that esc2Δ rrm3Δ double mutants have a severe growth defect (Figure 5A) (Tong et al. 2004). In contrast to esc2Δ rrm3Δ cells, we found that sir2Δ rrm3Δ cells have no growth defect (Figure 5B), suggesting that severe growth defect of esc2Δ rrm3Δ cells is not due to a silencing defect. Moreover, the esc2Δ rrm3Δ double mutant has a significant reduction in mean life span (2.3 generations) compared to either single mutant (Figure 4B). The majority of esc2Δ rrm3Δ cells cease cell division as large-budded cells in G2 at the end of their life span, whereas the terminal phenotype of wild-type or esc2Δ sir2Δ cells are usually as unbudded G1 cells (data not shown). In addition, a comparison of the mortality rates of the early generations (∼10 generations) shows that esc2Δ rrm3Δ cells have a significantly increased mortality compared to esc2Δ cells, even though rrm3Δ cells have similar mortality rates as wild type (Figure 4B). In contrast, sir2Δ mutation has no apparent effect on the mortality rates of the early generations of esc2Δ cells (Figure 4A). Thus, the synergistic reduction in the life span of esc2Δ rrm3Δ mutants supports the notion that Esc2 have an additional function other than its silencing function.
Figure 5.—
Cells lacking Esc2 and Rrm3 exhibit a severe growth defect. (A) Tetrads of heterozygous diploid cells formed by the crosses indicated were dissected and grown on YPD at 30° for 3 days. (B) Yeast strains of indicated genotypes were streaked out on YPD plate and grown at 30° for 3 days. (C) S/G2 cell cycle delay of esc2Δ rrm3Δ cells. FACS analysis of the DNA content of log-phase cells. Cells were grown to early logarithmic phase at 30° and DNA content of asynchronous cultures of the indicated strains was measured by FACS. (D) Mutants were grown to early logarithmic phase and stained with DAPI. Cells were analyzed microscopically for the presence of single cells, small-budded cells, and large-budded (mononucleated or binucleated) cells.
Cell cycle progression in esc2Δ rrm3Δ cells was evaluated by analyzing the DNA content of early logarithmic phase asynchronous cultures grown at 30°. The results indicated a normal cell cycle progression in wild-type and esc2Δ cells, a modest increase in rrm3Δ cells with 2C DNA content, and significant accumulation of esc2Δ rrm3Δ cells with 2C DNA content (Figure 5C). Furthermore, the fraction of large-budded cells was ∼40, 40, 49, and 70% in wild-type, esc2Δ, rrm3Δ, and escΔ rrm3Δ cells, respectively (Figure 5D). Most of the large-budded esc2Δ rrm3Δ cells had one nucleus at the single bud neck (Figure 5D). These results suggest that cell cycle progression through S/G2 is delayed in esc2Δ rrm3Δ cells.
Accumulation of DNA damage and activation of the S/G2 checkpoint in esc2Δ rrm3Δ cells:
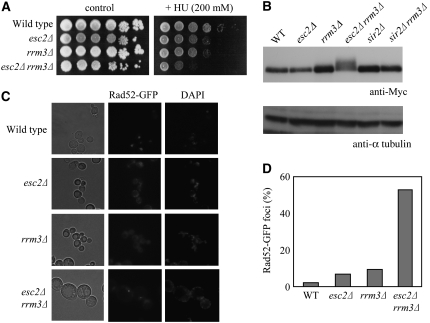
Cells with defects in S-phase progression are often hypersensitive to HU, which inhibits DNA replication. Thus, the relative sensitivity of esc2Δ, rrm3Δ, and esc2Δ rrm3Δ cells to HU was evaluated. The results showed that esc2Δ and rrm3Δ cells were modestly sensitive to HU, but rrm3Δ esc2Δ cells were hypersensitive to HU (Figure 6A). Rad53 phosphorylation was also evaluated as a marker for induction of the DNA damage/replication checkpoint. An electrophoretic mobility shift assay showed that a low level of slow migrating phosphorylated Rad53 accumulates in rrm3Δ cells as reported previously (Torres et al. 2004), but a higher level of hyperphosphorylated Rad53 accumulates in rrm3Δ esc2Δ cells (Figure 6B), suggesting a higher-than-normal level of spontaneous DNA damage in these cells. In addition, slow-migrating hyperphosphorylated Rad53 was not detected in rrm3Δ sir2Δ cells (Figure 6B). These data suggest that Esc2 is involved in tolerating spontaneous DNA replication problems such as stalled replication forks.
Figure 6.—
Constitutive hyperphosphorylation of Rad53 and accumulation GFP-Rad52 foci in esc2Δ rrm3Δ cells. (A) esc2Δ rrm3Δ cells are sensitive to HU. Serial dilutions of the indicated strains were spotted onto selective media containing 200 mm HU and incubated at 30° for 4 days. (B) Phosphorylation of Rad53 increases in esc2Δ rrm3Δ cells. Cells were grown to early logarithmic phase (2–5 × 106 cells/ml). The indicated strains were harvested and protein extracts were prepared. Rad53 protein phosphorylation was analyzed by 6% SDS–PAGE followed by Western blotting using anti-Myc antibody. α-Tubulin was used as a loading control. (C) The number of GFP-Rad52 foci increases in esc2Δ rrm3Δ cells. Wild-type, esc2Δ rrm3Δ, and esc2Δ rrm3Δ cells containing pGFP-RAD52 were grown to early logarithmic phase and then examined by fluorescence microscopy. (D) The number of GFP-RAD52 foci were counted. At least 100 cells were examined for each strain.
The Rad53 activation suggests that esc2Δ rrm3Δ cells accumulate spontaneous DNA damage. To test this possibility, we examined GFP-Rad52 foci formation in esc2Δ rrm3Δ cells because Rad52 forms discrete foci at sites of DNA damage in replicating cells (Lisby et al. 2001), and these foci can be readily visualized by fluorescence microscopy in cells expressing GFP-Rad52. GFP-Rad52 functions normally in vivo, since it fully complements the repair deficiency of a RAD52 deletion strain (data not shown). In a logarithmically growing culture, very few Rad52 foci are visible in wild type. Mutations of ESC2 or RRM3 cause a slight increase of spontaneous Rad52 foci (Figure 6, C and D). However, a significant number of Rad52 foci are detected in esc2Δ rrm3Δ cells (Figure 6, C and D), and most of the foci occur in large-budded cells with a single nucleus, while only a few Rad52 foci occur in unbudded cells (data not shown). These results indicate that esc2Δ rrm3Δ cells accumulate DNA damage in S/G2 and spontaneously activate the DNA damage checkpoint.
ESC2 interacts genetically with MRC1:
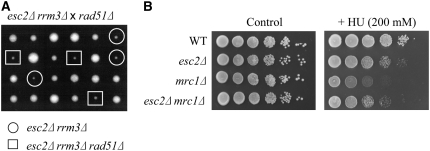
Previous studies have shown that the synthetic lethality of rrm3Δ sgs1Δ and rrm3Δ srs2Δ cells is suppressed by deletion of RAD51 or RAD52 (Schmidt and Kolodner 2004; Torres et al. 2004), suggesting that toxic recombination intermediates accumulate and cause cell death in these cells. However, a different mechanism may lead to poor growth in esc2Δ rrm3Δ cells, because deletion of RAD51 does not suppress their severe growth defect (Figure 7A). Like esc2Δ rrm3Δ cells, deletion of RAD51 also fails to suppress the synthetic lethality of mrc1Δ rrm3Δ mutants (Torres et al. 2004). We, therefore, examined the genetic interaction between esc2Δ and mrc1Δ. Interestingly, esc2Δ mrc1Δ double mutants are not severely defective for growth, and deletion of ESC2 does not exacerbate the HU sensitivity of mrc1Δ cells, but rather suppresses it to the same level as esc2Δ cells (Figure 7B). These results suggest that ESC2 interacts genetically with MRC1 and Esc2 may modulate Mrc1 function(s) during DNA replication.
Figure 7.—
ESC2 interacts genetically with MRC1 (A) The rad51Δ mutation does not suppress the growth defect of esc2Δ rrm3Δ cells. Tetrads of diploid cells formed by the crosses indicated were dissected and grown on YPD at 30° for 3 days. (B) esc2 mutation is epistatic to mrc1 mutation with regard to HU sensitivity. Serial dilutions of the indicated strains were spotted onto selective media containing the 200 mm HU and incubated at 30° for 3 days.
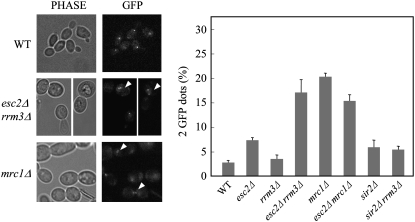
esc2Δ cells have a defect in sister chromatid cohesion:
Mrc1 is known to be required for sister chromatid cohesion (Xu et al. 2004), raising the possibility that the interaction between Esc2 and Mrc1 could modulate this function of Mrc1. Here, a quantitative assay for chromatid cohesion was performed in strain background containing a lac operator array and expressing LacI-GFP (Xu et al. 2004); lac operator repeats are integrated near the centromere of chromosome XV in the yeast genome. The results showed that esc2Δ cells have a modest defect in sister chromatid cohesion, while mrc1Δ cells have a more severe defect in chromatid cohesion (Figure 8). Interestingly, the sister chromatid cohesion defect of mrc1Δ is partially suppressed by esc2Δ. In contrast, esc2Δ rrm3Δ cells but not sir2Δ rrm3Δ cells have a severe defect in sister chromatid cohesion, even though rrm3Δ cells do not have a defect in sister chromatid cohesion (Figure 8), indicating that the severe sister chromatid cohesion defect of esc2Δ rrm3Δ cells is not due to a silencing defect. These data suggest that Esc2 and Mrc1 act cooperatively during sister chromatid cohesion and that Rrm3 might play a cryptic role in sister chromatid cohesion in the absence of Esc2.
Figure 8.—
Esc2 is required for sister chromatid cohesion. Sister chromatid cohesion assays. Sister chromatid cohesion was analyzed in the indicated strains, counting at least 300 cells for each genotype. One GFP dot was observed in normal G2/M phase cells. When sister chromatid cohesion is defective, the proportion of cells with two GFP dots increases. The results represent the average of three independent measurements.
SUMO-like domain is required for S-phase progression in rrm3Δ cells:
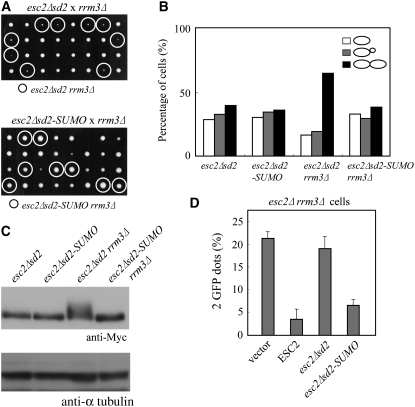
The role of the SUMO-like domain of Esc2 during DNA replication-associated stress was examined by comparing cell growth in esc2Δsd2 rrm3Δ and esc2Δ rrm3Δ cells. The results demonstrated an equally severe growth defect in esc2Δsd2 rrm3Δ and esc2Δ rrm3Δ, and that normal growth was restored in esc2Δsd2-SUMO rrm3Δ cells (Figure 9A). We also confirmed that cell cycle delay in late S/G2, aberrant phosphorylation of Rad53 and a defect in sister chromatid cohesion occur in esc2Δsd2 rrm3Δ cells, as demonstrated above in esc2Δ rrm3Δ cells (Figure 9, B–D). In addition, esc2Δsd2-SUMO restored the normal cell cycle progression to wild-type levels and suppressed the aberrant checkpoint activation (Figure 9, B and C). In addition, esc2Δsd2-SUMO, but not esc2Δsd2, complemented the defect in sister chromatid cohesion in esc2Δ rrm3Δ cells (Figure 9D). Thus, SD2 of Esc2 appears to be essential for normal growth and sister chromatid cohesion in rrm3Δ cells and be functionally substituted by the authentic SUMO.
Figure 9.—
SUMO can functionally substitute for the SUMO-like domain of ESC2. (A) Tetrads of heterozygous diploid cells formed by the crosses indicated were dissected and grown on YPD at 30° for 3 days. (B) Mutants were grown to early logarithmic phase and analyzed microscopically for the presence of single cells, small-budded cells, and large-budded cells. (C) esc2Δsd2-SUMO suppresses the hyperphosphorylation of Rad53 in rrm3Δ cells. Cells were treated as described in the legend for Figure 6B. (D) Sister chromatid cohesion defect in esc2Δsd2 rrm3Δ cells. esc2Δ rrm3Δ cells were transformed with pRS415 (vector), pESC2, pESC2Δsd2, and pESC2Δsd2-SUMO. Cells were assayed as described in the legend for Figure 8.
DISCUSSION
This study explored the functional significance of the SUMO-like domains of Esc2. We demonstrated that truncated Esc2ΔC protein, which lacks SD1/2, interacts with Sir2 in a yeast two-hybrid assay, but is defective for silencing a reporter gene at HMR. However, the silencing defect of esc2ΔC is probably due to the fact that it is expressed at a very low level and appears to be unstable in yeast cells, even though it is soluble and relatively stable in E. coli (data not shown). Fusion of one SUMO domain to the C terminus of esc2ΔC (esc2ΔC-SUMO) increases the steady state protein level in vivo and restores functional gene silencing at HMR. These results suggest that the N-terminal domain of Esc2 interacts with Sir2 and plays a role in gene silencing and that one SUMO or SUMO-like domain is at least required for the stability of Esc2 in vivo. It should be noted that the Esc2ΔC protein is not aberrantly folded because the Esc2ΔC protein still interacts with Sir2 when overproduced and exists as a soluble protein when expressed in E. coli. Therefore, the SUMO-like domains might act as the stabilizer in yeast to protect degradation. The previous study has shown that the SUMO-like domain of fission yeast Rad60, an ortholog of ESC2, is required to form a homodimer through the interaction with putative SUMO-binding motifs of its own (Raffa et al. 2006). Therefore, the inability of Esc2ΔC to form a homodimer might affect its stability in vivo. Alternatively, a protein complex formation with other partners and/or subnuclear localization of Esc2 via the SUMO-like domains might contribute to the stability of Esc2.
This study also demonstrated that esc2 mutants have a shorter life span than wild-type or sir2Δ cells, that esc2Δ sir2Δ double mutants have a shorter life span than esc2Δ or sir2Δ single mutants, and that esc2Δ appears to differentially increase mortality in young cells (as indicated by the shape of the curve shown in Figure 4). Previous studies in sgs1 mutants suggested that high mortality in young sgs1 cells (the early part of the curve) reflected an increased frequency of DNA damage-induced cell cycle arrest, while mortality of older cells (the later part of the curve) reflected age-related cell death (McVey et al. 2001). Therefore, these results suggest that Esc2 and Sir2 may act independently of life span, and it is possible that the effect of esc2Δ on life span reflects increased genome instability during DNA replication, as discussed below.
Mutations in ESC2 caused a severe defect in cell growth when combined with rrm3Δ, whereas the mutation in SIR2 did not, suggesting that the severe growth defect of esc2Δ rrm3Δ cells is not due to a silencing defect. FACS and morphological analysis showed that esc2Δ rrm3Δ cells fail to progress normally through the cell cycle and accumulate in late S/G2 primarily as large-budded cells with a single nucleus. In addition, Rad53 is constitutively hyperphosphorylated in rrm3Δ esc2Δ cells, and the number of Rad52 foci is significantly higher than in wild-type, rrm3Δ, and esc2Δ cells in the absence of exogenous DNA damage. These data suggest that spontaneous DNA damage accumulates in rrm3Δ esc2Δ cells, causing constitutive activation of a DNA damage checkpoint. esc2Δ cells also have a defect in sister chromatid cohesion, which is greatly enhanced by rrm3Δ. This defect is fully complemented by expressing wild-type ESC2 but not by esc2Δsd2. esc2Δsd2 is expressed at a similar level as wild-type ESC2 and is proficient in gene silencing. Thus, it appears that SD2 may play a role in replication fork progression and/or the rescue of stalled replication forks in the absence of Rrm3. In this regard, Esc2 may be at least partially redundant with Rrm3 in facilitating replication fork progression. In particular, in cells lacking Esc2, Rrm3 may prevent breakage of stalled replication forks, suppress activation of the S-phase checkpoint response, and promote sister chromatid cohesion.
Defects in homologous recombination suppress the severe growth defect of sgs1Δ rrm3Δ and srs2Δ rrm3Δ cells (Schmidt and Kolodner 2004; Torres et al. 2004). This result can be explained as follows: in rrm3 mutants, stalled or broken replication forks are substrates for Rad51-dependent homologous recombination, which generates toxic recombination intermediates in sgs1 or srs2 cells. However, the growth defect of esc2Δ rrm3Δ cells is not suppressed by rad51Δ, suggesting that there is a defect upstream of Rad51-dependent homologous recombination. Like esc2Δ rrm3Δ cells, the mrc1Δ rrm3Δ lethality is also not suppressed by rad51Δ (Torres et al. 2004). In addition, mrc1Δ mutants have a defect in sister chromatid cohesion and have a partial loss of silencing at HMR or telomeric loci (Hu et al. 2001; Xu et al. 2004). Given the phenotypic similarities between esc2Δ and mrc1Δ, it is likely that Esc2 and Mrc1 are epistatic. This is consistent with the observation that esc2Δ does not enhance the HU-hypersensitivity and defect in sister chromatid cohesion in mrc1Δ cells, but rather partially suppresses both phenotypes. These data suggest that Esc2 might modulate the replication function of Mrc1 in the presence of replication stress. Thus, deregulation of Mrc1 function might explain the partial defect in sister chromatid cohesion in esc2Δ mutants and the growth defect of esc2Δ rrm3Δ double mutants.
Our data suggest that Esc2 plays important roles not only in gene silencing but also in facilitating DNA replication fork progression and sister chromatid cohesion. Although there is currently no evidence for a direct interaction between Esc2 and Mrc1, it remains possible that Esc2 may interact with Mrc1 transiently or modulate the replication function of Mrc1 indirectly through its interactions with other proteins involved in replication fork progression. Furthermore, our findings unveiled a novel function for Rrm3 in sister chromatid cohesion and yeast life span. Additional studies to more precisely elucidate the roles of Esc2, Rrm3, and Mrc1 during replication fork progression will provide insight into the mechanisms of crosstalk between proteins involved in DNA replication, sister chromatid cohesion, transcriptional silencing, and aging.
Acknowledgments
We thank S. Holmes and H. Klein for strains, and N. Haruta and Y. Tsutsui for helpful comments. This work was supported by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists (to T.O.) and Grants-in-Aid for Scientific Research from The Ministry of Education, Science, Sports and Culture of Japan.
References
- Andrulis, E. D., D. C. Zappulla, K. Alexieva-Botcheva, C. Evangelista and R. Sternglanz, 2004. One-hybrid screens at the Saccharomyces cerevisiae HMR locus identify novel transcriptional silencing factors. Genetics 166 631–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azvolinsky, A., S. Dunaway, J. Z. Torres, J. B. Bessler and V. A. Zakian, 2006. The S. cerevisiae Rrm3p DNA helicase moves with the replication fork and affects replication of all yeast chromosomes. Genes Dev. 20 3104–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour, L., and W. Xiao, 2003. Regulation of alternative replication bypass pathways at stalled replication forks and its effects on genome stability: a yeast model. Mutat. Res. 532 137–155. [DOI] [PubMed] [Google Scholar]
- Boddy, M. N., P. Shanahan, W. H. McDonald, A. Lopez-Girona, E. Noguchi et al., 2003. Replication checkpoint kinase Cds1 regulates recombinational repair protein Rad60. Mol. Cell. Biol. 23 5939–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boule, J. B., and V. A. Zakian, 2006. Roles of Pif1-like helicases in the maintenance of genomic stability. Nucleic Acids Res. 34 4147–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi, M. H., and D. Shore, 1996. SUM1–1, a dominant suppressor of SIR mutations in Saccharomyces cerevisiae, increases transcriptional silencing at telomeres and HM mating-type loci and decreases chromosome stability. Mol. Cell. Biol. 16 4281–4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, M. M., M. F. Goodman, K. N. Kreuzer, D. J. Sherratt, S. J. Sandler et al., 2000. The importance of repairing stalled replication forks. Nature 404 37–41. [DOI] [PubMed] [Google Scholar]
- Cuperus, G., and D. Shore, 2002. Restoration of silencing in Saccharomyces cerevisiae by tethering of a novel Sir2-interacting protein, Esc8. Genetics 162 633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon, N., and R. T. Kamakaka, 2000. A histone variant, Htz1p, and a Sir1p-like protein, Esc2p, mediate silencing at HMR. Mol. Cell 6 769–780. [DOI] [PubMed] [Google Scholar]
- Hishida, T., T. Ohno, H. Iwasaki and H. Shinagawa, 2002. Saccharomyces cerevisiae MGS1 is essential in strains deficient in the RAD6-dependent DNA damage tolerance pathway. EMBO J. 21 2019–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishida, T., T. Ohya, Y. Kubota, Y. Kamada and H. Shinagawa, 2006. Functional and physical interaction of yeast Mgs1 with PCNA: impact on RAD6-dependent DNA damage tolerance. Mol. Cell. Biol. 26 5509–5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, F., A. A. Alcasabas and S. J. Elledge, 2001. Asf1 links Rad53 to control of chromatin assembly. Genes Dev. 15 1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivessa, A. S., B. A. Lenzmeier, J. B. Bessler, L. K. Goudsouzian, S. L. Schnakenberg et al., 2003. The Saccharomyces cerevisiae helicase Rrm3p facilitates replication past nonhistone protein-DNA complexes. Mol. Cell 12 1525–1536. [DOI] [PubMed] [Google Scholar]
- Johnson, E. S., 2004. Protein modification by SUMO. Annu. Rev. Biochem. 73 355–382. [DOI] [PubMed] [Google Scholar]
- Kaeberlein, M., M. McVey and L. Guarente, 1999. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13 2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, B. K., N. R. Austriaco, Jr. and L. Guarente, 1994. Daughter cells of Saccharomyces cerevisiae from old mothers display a reduced life span. J. Cell Biol. 127 1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisby, M., R. Rothstein and U. H. Mortensen, 2001. Rad52 forms DNA repair and recombination centers during S phase. Proc. Natl. Acad. Sci. USA 98 8276–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, J. P., A. S. Levine and R. Woodgate, 1997. The Saccharomyces cerevisiae RAD30 gene, a homologue of Escherichia coli dinB and umuC, is DNA damage inducible and functions in a novel error-free postreplication repair mechanism. Genetics 147 1557–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey, M., M. Kaeberlein, H. A. Tissenbaum and L. Guarente, 2001. The short life span of Saccharomyces cerevisiae sgs1 and srs2 mutants is a composite of normal aging processes and mitotic arrest due to defective recombination. Genetics 157 1531–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyabe, I., T. Morishita, T. Hishida, S. Yonei and H. Shinagawa, 2006. Rhp51-dependent recombination intermediates that do not generate checkpoint signal are accumulated in Schizosaccharomyces pombe rad60 and smc5/6 mutants after release from replication arrest. Mol. Cell. Biol. 26 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita, T., Y. Tsutsui, H. Iwasaki and H. Shinagawa, 2002. The Schizosaccharomyces pombe rad60 gene is essential for repairing double-strand DNA breaks spontaneously occurring during replication and induced by DNA-damaging agents. Mol. Cell. Biol. 22 3537–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, S., C. Hoege, G. Pyrowolakis and S. Jentsch, 2001. SUMO, ubiquitin's mysterious cousin. Nat. Rev. Mol. Cell. Biol. 2 202–210. [DOI] [PubMed] [Google Scholar]
- Novatchkova, M., A. Bachmair, B. Eisenhaber and F. Eisenhaber, 2005. Proteins with two SUMO-like domains in chromatin-associated complexes: the RENi (Rad60-Esc2–NIP45) family. BMC Bioinformatics 6 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn, A. J., and S. J. Elledge, 2003. Mrc1 is a replication fork component whose phosphorylation in response to DNA replication stress activates Rad53. Genes Dev. 17 1755–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicioli, A., C. Lucca, G. Liberi, F. Marini, M. Lopes et al., 1999. Activation of Rad53 kinase in response to DNA damage and its effect in modulating phosphorylation of the lagging strand DNA polymerase. EMBO J. 18 6561–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa, G. D., J. Wohlschlegel, J. R. Yates, III and M. N. Boddy, 2006. SUMO-binding motifs mediate the Rad60-dependent response to replicative stress and self-association. J. Biol. Chem. 281 27973–27981. [DOI] [PubMed] [Google Scholar]
- Schmidt, K. H., and R. D. Kolodner, 2004. Requirement of Rrm3 helicase for repair of spontaneous DNA lesions in cells lacking Srs2 or Sgs1 helicase. Mol. Cell. Biol. 24 3213–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, D. C., and M. Hochstrasser, 2003. A superfamily of protein tags: ubiquitin, SUMO and related modifiers. Trends Biochem. Sci. 28 321–328. [DOI] [PubMed] [Google Scholar]
- Seeler, J. S., and A. Dejean, 2003. Nuclear and unclear functions of SUMO. Nat. Rev. Mol. Cell. Biol. 4 690–699. [DOI] [PubMed] [Google Scholar]
- Szyjka, S. J., C. J. Viggiani and O. M. Aparicio, 2005. Mrc1 is required for normal progression of replication forks throughout chromatin in S. cerevisiae. Mol. Cell 19 691–697. [DOI] [PubMed] [Google Scholar]
- Tong, A. H., G. Lesage, G. D. Bader, H. Ding, H. Xu et al., 2004. Global mapping of the yeast genetic interaction network. Science 303 808–813. [DOI] [PubMed] [Google Scholar]
- Torres, J. Z., S. L. Schnakenberg and V. A. Zakian, 2004. Saccharomyces cerevisiae Rrm3p DNA helicase promotes genome integrity by preventing replication fork stalling: viability of rrm3 cells requires the intra-S-phase checkpoint and fork restart activities. Mol. Cell. Biol. 24 3198–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H., C. Boone and H. L. Klein, 2004. Mrc1 is required for sister chromatid cohesion to aid in recombination repair of spontaneous damage. Mol. Cell. Biol. 24 7082–7090. [DOI] [PMC free article] [PubMed] [Google Scholar]