Abstract
In Prunus, the self-incompatibility (S-) locus region is <70 kb. Two genes—the S-RNase, which encodes the functional female recognition component, and the SFB gene, which encodes the pollen recognition component—must co-evolve as a genetic unit to maintain functional incompatibility. Therefore, recombination must be severely repressed at the S-locus. Levels of recombination at genes in the vicinity of the S-locus have not yet been rigorously tested; thus it is unknown whether recombination is also severely repressed at these loci. In this work, we looked at variability levels and patterns at the Prunus spinosa SLFL1 gene, which is physically close to the S-RNase gene. Our results suggest that the recombination level increases near the SLFL1 coding region. These findings are discussed in the context of theoretical models predicting an effect of linked weakly deleterious mutations on the relatedness of S-locus specificities. Moreover, we show that SLFL1 belongs to a gene family of at least five functional genes and that SLFL1 pseudogenes are frequently found in the S-locus region.
FLOWERS that express self-incompatibility (SI) can achieve fertilization only when receiving pollen grains that express mating specificities different from their own pistils (de Nettancourt 1977). In species of Prunus, two closely linked S-locus genes are involved in the incompatibility recognition response: the S-RNase (which encodes the pistil component, a basic glycoprotein with ribonuclease activity; see review by Wang et al. 2003) and the SFB (the pollen component, a protein with an F-box motif; Entani et al. 2003; Ushijima et al. 2003).
To maintain functional incompatibility, the S-RNase and SFB genes must be in linkage disequilibrium, since they must co-evolve as a genetic unit. Nunes et al. (2006) showed that the evolutionary histories of these two genes are correlated, although not fully correlated. Evidence suggestive of rare recombination has been found at the S-RNase (Vieira et al.2003; Ortega et al. 2006) and SFB (Nunes et al. 2006; Vieira et al. 2008a) genes. Amino acid sites responsible for specificity differences are scattered throughout the S-RNase (Vieira et al. 2007) and SFB genes (Nunes et al. 2006; Vieira et al. 2008a). It is conceivable that short gene conversion tracts could cause the observed pattern without disrupting specificity recognition.
For a given specificity, the rate of successful fertilization is inversely related to the specificity frequency in the population (frequency-dependent selection; Wright 1939; Vekemans and Slatkin 1994; Schierup et al. 1998; Uyenoyama 2000). Under frequency-dependent selection, many specificities are maintained in populations, and in extant Prunus species this number can be as high as 33 (Vieira et al. 2008a). Specificities are also predicted to be maintained for long periods of time. In Prunus, the oldest specificities are estimated to be 15–20 million years old (Vieira et al. 2008b). The long-term maintenance of specificities is predicted to lead to high diversity at sites closely linked to the amino acid sites where selection acts (Nordborg et al. 1996; Charlesworth et al. 1997; Schierup et al. 2000; Innan and Nordborg 2003; Wiuf et al. 2004). Synonymous variability levels are similar at the S-RNase and SFB genes. The average per site synonymous divergence (Ks) is 0.241 for the S-RNase (Vieira et al. 2007) and 0.222 for SFB (Nunes et al. 2006).
The Prunus S-RNase gene is flanked by two functional genes, namely the SFB and SLFL1 genes. The latter gene is thought to define one of the boundaries of the S-haplotype-specific region (Ushijima et al. 2001, 2003). SLFL1 is expressed in male organs and also in the style, and it seems not directly involved in the gametophytic self-incompatibility specificity reaction since this gene is deleted in the functional Prunus avium S3 haplotype (Matsumoto et al. 2008). On the basis of six Prunus haplotypes, the physical distance between the S-RNase and SLFL1 gene varies from 6.7 kb to >30 kb (Entani et al. 2003; Ushijima et al. 2003). This variation is due in part to the presence of the SLFL1-related pseudogene and transposable element sequences located between the S-RNase and SLFL1 (Ushijima et al. 2001, 2003; Entani et al. 2003). SLFL1 seems to always have the same transcriptional orientation as the S-RNase gene (Ushijima et al. 2001, 2003; Entani et al. 2003).
The deduced SLFL1 amino acid sequence associated with two different Prunus dulcis specificities is 95.1% identical (Ks = 0.0795; Ushijima et al. 2003; data not shown). The same pattern is observed in Prunus mume, where the SLFL1 amino acid sequence associated with two distinct specificities has been shown to be 92.5% identical (Ks = 0.0722; Entani et al. 2003; data not shown). Ten other pairs of genes located in the vicinity of the S-locus region show >97.2% amino acid identities (Entani et al. 2003). P. mume nucleotide sequences are available for two genes in this region that are farther away from the S-RNase than SLFL1, namely SLFL2 and SLFL3. Ks values are 0.0263 (N = 3) and 0.0039 (N = 2), respectively (data not shown). These values are lower than that for SLFL1. Therefore, it is unclear whether there is recombination suppression at the SLFL1 gene and to what extent.
Suppression of recombination leads to the accumulation of weak deleterious mutations in the S-locus region (reviewed in Uyenoyama 2005). Closely related specificities may share the same weak deleterious mutation due to recent common ancestry (Uyenoyama 1997). Therefore, it is predicted that in natural populations there should be a bias against closely related specificities. This effect should be more evident in species where suppression of recombination affects a large region around the S-locus. There are, however, other theoretical reasons to expect a bias against closely related specificities. For example, when a new specificity arises, it is expected that it will replace the specificity that gave origin to it, although it is conceivable that the original specificity may be brought back to the population by migration from another population (Uyenoyama et al. 2001). Thus far, in Prunus, the evidence for a bias against closely related specificities is ambiguous (Vieira et al. 2008b).
Within specificities, background selection against deleterious mutations at S-locus-linked genes leads to a reduction in effective population size (Charlesworth et al. 1993). As a consequence, little variability is expected within specificities, as it is observed in Prunus (Nunes et al. 2006; Ortega et al. 2006; Vieira et al. 2008a). Nevertheless, little variability is expected even in the absence of such an effect (Nunes et al. 2006).
Polymorphism data for genes located in the vicinity of the S-locus as well as data on reference loci, as reported in this work, can shed light on the size around the S-locus region where recombination is suppressed and to what extent it is suppressed. Only then is it possible to understand the impact of weakly deleterious S-locus-linked mutations on the evolution of the genes determining gametophytic self-incompatibility specificities in Prunus. Here, we estimate recombination levels at the SLFL1, S-RNase, and SFB genes, as well as in the region between the S-RNase and SLFL1 genes.
MATERIALS AND METHODS
Plant material and DNA extraction:
Prunus spinosa, a self-incompatible species (Salesses 1973; Nunes et al. 2006), is a dense shrub abundant in Europe (Halliday and Beadle 1983). Although P. spinosa has been described as being an allotetraploid species (Halliday and Beadle 1983), variations in ploidy levels have been reported (2n = 16, 24, 32, 40, 43, 44, 48, 53, 56, 59, or 64; Flora Iberica, http://www.rjb.csic.es/floraiberica/PHP/cientificos.php) even at the local population level (Baiashvili 1980).
Leaves were collected from the individuals of the P. spinosa population Rabal–Bragança (assigned as B) described by Nunes et al. (2006) and Vieira et al. (2008a). Genomic DNA was extracted from leaves of individual plants using the method of Ingram et al. (1997).
SLFL1 PCR amplification:
On the basis of the available SLFL sequences (Ushijima et al. 2001, 2003; Entani et al. 2003), primers 44F and 800R (supplemental Table 1) were designed to amplify the SLFL1 gene but not the SLFL2, SLFL3, and SFB genes. Genomic DNA of individuals B8, B10, B15, and B18 was used as template. Standard amplification conditions were 35 cycles of denaturation at 94° for 30 sec, primer annealing at 48° for 30 sec, and primer extension at 72° for 2 min. The 770-bp amplification product (the expected size) was cloned using the TA cloning kit (Invitrogen, Carlsbad, CA). For each individual, on average, the restriction pattern of the insert of 80 colonies was analyzed using RsaI and Sau3AI restriction enzymes. For each individual and restriction pattern, three colonies were sequenced to obtain a consensus sequence. The ABI PRISM BigDye cycle-sequencing kit (Perkin Elmer, Foster City, CA) and specific primers or the primers for the M13 forward and reverse priming sites of the pCR2.1 vector were used to prepare the sequencing reactions. Sequencing runs were performed by STABVIDA (Lisbon).
Nucleotide sequence chimeras can be obtained during the PCR reaction, and these could influence the interpretation of the results presented here. Nevertheless, care was taken to eliminate the chimeras by performing the entire procedure (including the PCR reaction) twice. Furthermore, 80 colonies were screened using restriction enzymes. Those colonies showing very rare restriction patterns were discarded. Finally, for each individual, all possible pairs of consensus sequences were inspected to make sure that nucleotide differences were not clustered, as expected, if one of the two sequences used in the comparison is a chimera.
SLFL4 PCR amplification:
Analysis of the cloned PCR fragments obtained in the previous section revealed two new SLFL genes (see results). Polymorphism studies were performed using genomic DNA from individuals B2, B4, B6, B10, B14, B15, and B19 and the SLFL4-specific primers B8-8/B18-4 (supplemental Table 1). We used the same PCR, cloning, and sequencing approaches as for SLFL1 (see above).
Characterization of extended haplotypes:
In both P. dulcis and P. mume, the SLFL1 and S-RNase genes have the same transcription direction (Entani et al. 2003; Ushijima et al. 2003). Therefore, we used the P. spinosa S-RNase sequences S1, S4, S7, S8, S9, S10, and S15 (GenBank accession nos. EF36467, EU878543, EU833958, DQ677587, DQ677588, DQ677589, and EF636468) present in individuals B8, B9, B10, B15, B18, and B19 (Nunes et al. 2006; Vieira et al. 2008a; supplemental Table 2) to design S-RNase-specific reverse primers (supplemental Table 1). Each specific S-RNase reverse primer was used in combination with the general SLFL1 forward primer 44F (or 104F in the case of the S10 haplotype). These PCR amplifications were performed using system 3 protocol of the Roche Expand long template PCR system (Roche). In all cases, the amplification product was cloned using the Topo XL PCR cloning kit (Invitrogen). To obtain consensus sequences, for each cloning experiment three colonies were sequenced using primers M13F, M13R (for the vector arms), 44F, and 800R (primers designed for the SLFL1 gene) and 42F and 93F (primers designed for the S-RNase gene; Nunes et al. 2006).
Testing associations between SLFL1 and SFB alleles:
The SFB alleles present in 20 different individuals from the P. spinosa Rabal–Bragança population have been reported by Vieira et al. (2008a). Therefore, to look for co-occurrence of SFB and SLFL1 alleles, specific primers were designed for SLFL1 alleles B18-2, B8-4, B15-3/B18-3, and B8-6/B15-4/B18-1 (supplemental Table 1). Standard PCR amplification conditions were used (see above).
Summary statistics:
DNA sequences were deposited in GenBank (accession nos. EU876704–EU876742). Amino acid sequences were aligned using ClustalX v. 1.64b (Thompson et al. 1997), and minor manual adjustments were performed using Proseq version 2.43 (http://helios.bto.ed.ac.uk/evolgen/filatov/proseq.html). Nucleotide sequences were aligned using the amino acid alignment as a guide. Analyses of DNA polymorphism, linkage disequilibrium, and the minimum number of recombination events were performed using DnaSP 4.1 (Rozas et al. 2003) and ProSeq version 2.43 software. Standard coalescent simulations constrained on the number of segregating sites and sample size were also performed using DnaSP 4.1 (Rozas et al. 2003).
Phylogenetic analyses of SLFL genes:
Due to computational burden, the phylogenetic relationship of the 35 SLFL sequences used was obtained using minimum evolution. The tree was obtained using complete deletion and Jukes–Cantor-corrected nucleotide distances, as implemented in the MEGA software (Kumar et al. 2004). Bootstrap values were obtained using 500 replicates.
Testing for selection at SLFL1:
Both the codeml software implemented in PAML 3.13 (Yang 1997) and the method of Wilson and McVean (2006) as implemented in the omegaMap v 0.5 software (http://www.danielwilson.me.uk) that uses a population genetics approximation to the coalescent with recombination were used.
When using PAML 3.13, 17 different Prunus SLFL1 sequences were used. The specified SLFL1 maximum-likelihood tree was obtained with PAUP (Swofford 2002) after using Modeltest (Posada and Crandall 1998) to find the simplest model of nucleotide sequence evolution that best fit the data, according to the Akaike information criterion (a GTR + G model with nucleotide frequencies A = 0.27750, C = 0.15360, G = 0.22540, and T = 0.34350, the substitution model A ↔ C = 1.5091, A ↔ G = 2.4386, A ↔ T = 0.6374, C ↔ G = 1.7718, C ↔ T = 1.5168, G ↔ T = 1.0000, and a gamma distribution with a shape parameter α of 0.5812). Simple models that allow for positive selection [Yang's (1997) M2 (three categories, one of them with a Ka/Ks > 1) and M3 models (three categories)] are as likely as a model not allowing for positive selection (model M1).
When using omegaMap, since it uses a population genetics approximation to the coalescent with recombination, we used only P. spinosa SLFL1 sequences (N = 15). A total of 250,000 iterations and a burn-in of 25,000 were used in all analyses. All codons were assumed to be at equal frequencies. Ten random sequence orders were used. The parameters to be estimated were the selection parameter (ω = Ka/Ks), the population recombination rate (ρ), the rate of synonymous transversion (μ), the transition–transversion ratio (κ), and the insertion/deletion rate (ϕ;Wilson and McVean 2006). The first two parameters may vary along the sequence. A block of 30 codons (∼10% of the sequence size) is used when estimating both ω and ρ. One objective and one subjective approach to prior specification were used. First, inverse distributions were used as priors for ω and ρ, and improper inverse distributions were used for the other parameters (μ, κ, and ϕ). The bounds for ω were 0.01–1000 and for ρ, 0.00000001–1000. Thus the posterior density outside this range should be about zero. When the bound for ω was 0.0001–10 rather than 0.01–1000, similar parameter estimates were obtained (data not shown). Starting values for μ, κ, and ϕ were, respectively, 0.1, 1, and 1. In the second approach to prior specification, exponential distributions were used for all parameters (starting values were μ = 0.1, κ = 1, ω = 1, ρ = 0.001, and ϕ = 1). Similar parameter estimates were obtained regardless of the approach used. Strong evidence for positively selected sites (posterior probability values >95%) was never obtained.
Amino acid variability levels along the SLFL1 protein:
Normed variability indices for each site of the SLFL1gene were calculated as in Kheyr-Pour et al. (1990). Thus, for each site, information on both the number of different amino acids as well as their frequencies was used.
Estimating the population recombination rate at the SLFL1 gene:
The Wilson and McVean (2006) method allows co-estimation of the ratio Ka/Ks and the population recombination rate. Estimates are given for every codon, but we used information on codon positions 31–217 only. The first 30 and last 30 codons (the size of the block used) were discarded since estimates at the edges of the sequence can be inaccurate when using a block approach (Wilson and McVean 2006). For the interval considered, the two runs using different prior specification approaches (see above) converged to the same estimate of the population recombination rate. Therefore we performed a joint analysis of the two runs to obtain a point estimate for the population recombination rate at SLFL1 as well as the 95% credibility intervals.
Estimating the relative importance of recombination and mutation at the SLFL1, S-RNase, and SFB genes:
The following Prunus data sets were used (every DNA sequence in the data sets is unique): 17 SLFL1 sequences, the 88 S-RNase sequences used by Vieira et al. (2007), and the 70 SFB sequences used by Vieira et al. (2008a). To infer the number of independent recombination events implied by each DNA sequence data set, the recombination detection program RDP (Martin et al. 2005) was used. The following methods, with default options, were selected: RDP, Chimaera, BootScan, 3Seq, GeneConv, MaxChi, and SiScan. A sequence was taken as recombinant if at least one of the methods used identified a recombination tract in that sequence with a probability <0.05. The number of inferred independent recombination events was often smaller than the number of sequences showing evidence for recombination tracts, since inferred recombination events may be old and thus may be apparent in several descendant sequences. For each data set, the total number of synonymous mutations implied by the data was inferred using Yang's (1997) methodology under the appropriate model [M0 (this work), M3 (Vieira et al. 2007), and M3 (Vieira et al. 2008a) for the SLFL1, S-RNase, and SFB data sets, respectively].
Estimating the population recombination rate between SLFL1 and the S-locus:
Estimates of the population recombination rate between SLFL1 and the S-locus were obtained using the formulas given by Kamau and Charlesworth (2005).
RESULTS
P. spinosa SLFL1-like genes:
For individuals B8, B10, B15, and B18, a 770-bp amplification product obtained with primers 44F and 800R that support the amplification of the SLFL1 gene (covering 63% of this gene) was cloned. For these individuals, the amplification product revealed 9, 4, 4, and 7 SLFL1-like sequences, respectively. Although ploidy levels vary in P. spinosa, no more than 6 alleles at the S-locus have been described for these individuals (supplemental Table 2; Nunes et al. 2006; Vieira et al. 2008a). Thus the amplification of >6 SLFL1-like sequences in two individuals (B8 and B18) indicates that other SLFL genes are being amplified. The 24 sequences define 16 different nucleotide sequences (Table 1). Blastn searches revealed that 12 of them show >87% nucleotide identity with Prunus SLFL1 sequences. On the other hand, the remaining four sequences (B8-3, B8-7/B18-5, B8-8/B18-4, and B10-4) showed ∼78% nucleotide identity with previously described Prunus SLFL1 sequences. Specific primers were designed for sequences B8-3 and B8-8/B18-4 (supplemental Table 1). For both primer sets, an amplification product with the expected size was obtained when genomic DNA from each of the 20 different P. spinosa individuals of the Bragança natural population (for which SFB alleles are known; Vieira et al. 2008a) was used. Specificity of the PCR reaction was confirmed by sequencing the amplification products (data not shown). Since these SLFL1-related sequences are amplified in all individuals, it is unlikely that they are SLFL1 alleles, as this would imply that all individuals have the same two divergent SLFL1 alleles. Since both sequences are present in all individuals analyzed, and the synonymous nucleotide divergence between both types is high (Ks = 0.2901), it is likely that they represent two different genes. Therefore, we named SLFL4 the B8-8/B18-4 type of sequence and SLFL5 the B8-3 type of sequence.
TABLE 1.
Nucleotide identity of the 16 different P. spinosa SLF1-like sequences and P. avium SLFL1-S4 allele
| Sequence types | % nucleotide identity with the P. avium SLFL1-S4 haplotype (AB280953) |
|---|---|
| B8-1/B10-3 | 97 |
| B15-1 | 95 |
| B8-2 | 95 |
| B18-6 | 95 |
| B8-6/B15-4/B18-1 | 97 |
| B8-9 | 96 |
| B15-3/B18-3 | 96 |
| B10-1 | 95 |
| B8-5/B10-2 | 89 |
| B18-7/B15-2 | 94 |
| B8-4 | 88 |
| B18-2 | 88 |
| B8-3 | 78 |
| B8-7/B18-5 | 78 |
| B10-4 | 78 |
| B8-8/B18-4 | 78 |
S-locus region SLFL1 pseudogenes:
Two SLFL1 sequence types (B18-6 and B18-7/B15-2) have multiple in-frame stop codons. SLFL1 pseudogenes have been described in the P. mume S1 haplotype (Entani et al. 2003), although the corresponding nucleotide sequences are not available in the public databases. One of the pseudogenes is located between the SLFL1 and the S-RNase gene (Entani et al. 2003). To estimate how frequent this situation is in Prunus, we determined the sequence of the SLFL1-like gene that is closest to the S-RNase, as well as a fragment of the S-RNase gene (data not shown), for seven P. spinosa S haplotypes (S1, S4, S7, S8, S9, S10, and S15; see materials and methods and supplemental Table 2).
For all S haplotypes analyzed here, with the exception of the S9 haplotype, the SLFL1 primers used allow amplification of a region that is ∼770 bp long. The SLFL1-like sequence amplified from the S9 haplotype is longer (1183 bp). Putative splicing sites can be found around the 413-bp insertion. When the putative intron is removed, the protein encoded by this gene is six amino acids longer than all other SLFL1 proteins. Nevertheless, SLFL1 has been described as being an intronless gene (Entani et al. 2003; Ushijima et al. 2003). Therefore, it is likely a pseudogene. The SLFL1 sequence obtained from the S4, S10, and S15 haplotypes show multiple in-frame stop codons. Thus, in four of the seven cases here studied, the neighbor of the S-RNase gene is an SLFL1 pseudogene rather than the SLFL1 functional gene.
The SLFL1 sequence from the S4 haplotype is identical to one of the sequences obtained from B15 and B18 individuals. These individuals have been shown to have the S4 haplotype (supplemental Table 2).
Phylogenetic analyses:
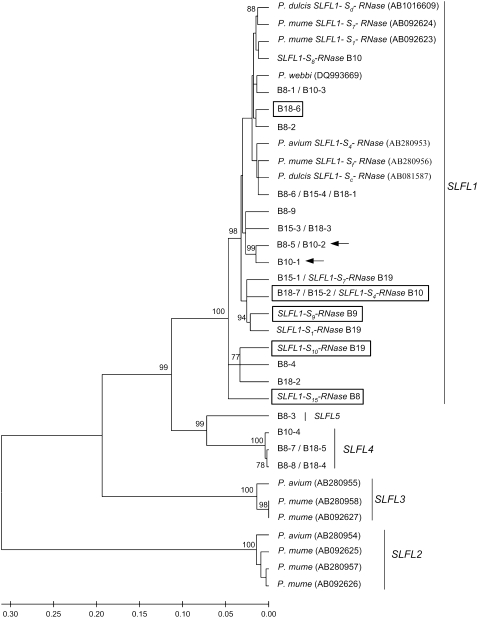
The phylogenetic relationship of Prunus SLFL1, SLFL2, SLFL3, SLFL4, and SLFL5 gene sequences is presented in Figure 1. All Prunus SLFL1 sequences cluster together with high bootstrap value (Figure 1). SLFL1 pseudogenes are found mingled with functional SLFL1 sequences. In two cases, SLFL1 pseudogenes cluster with functional SLFL1 sequences with high bootstrap value (>91%). The SLFL4 and SLFL5 genes are more closely related than either to the other SLFL genes. SLFL4/SLFL5 are more closely related to SLFL1 than to SLFL2 and SLFL3 (Figure 1). SLFL2 is shown as an out-group to the other SLFL genes.
Figure 1.—
Linearized rooted minimum evolution tree showing the relationship of Prunus SLFL genes. Bootstrap values >70% are shown. Sequences that present in-frame stop codons or that have intron-like insertions are boxed. These sequences are believed to be pseudogenes; arrows indicate sequences that show a significant Tajima's relative rate test.
Individuals B8 and B10 have two identical SLFL1 sequences (B8-1/B10-3 and B8-5/B10-2), but they share allele SFB22 only. Although the two types of sequences do not show in-frame stop codons in the region analyzed, it is likely that one of the sequence types is from a pseudogene (see S-locus region SLFL1 pseudogenes). Pseudogenes are known to evolve faster than functional copies. To test this hypothesis, we performed Tajima's relative rate tests using as the out-group one SLFL4 sequence and, as one of the in-groups, the P. avium SLFL1 sequence. Significant results were obtained for sequences B8-5/B10-2. We extended these analyses to all other SLFL1 sequences with no in-frame stop codons. Only when sequence B10-1 was used was a significant result obtained. B10-1 and B8-5/B10-2 sequences cluster together with 100% bootstrap support (Figure 1).
Evidence for historical recombination at SLFL1:
Table 2 shows the per-site synonymous polymorphism for Prunus SLFL1 gene sequences as well as for P. spinosa, P. mume, and P. dulcis sequences. All putative SLFL1 pseudogene sequences have been removed from the analyses. For comparison, levels of polymorphism at the P. spinosa SLFL4 gene are also shown. There is no evidence to support the view that patterns of polymorphism at SLFL4 are influenced by the S-locus, and thus this gene is used here as a reference locus. In P. mume, Entani et al. (2003) did not find this gene when sequencing a region of ∼32 and 22 kb at the left and right of the S-locus, respectively. For P. spinosa, the synonymous variability level is about four times higher at SLFL1 than at SLFL4. This observation suggests that variability patterns at SLFL1 are being influenced by the neighboring S-RNase–SFB genes. When using all individuals of the Bragança population and specific primers for four SLFL1 alleles (B18-2, B8-4, B15-3/B18-3, and B8-6/B15-4/B18-1; supplemental Table 1), complete co-occurrence of SFB and SLFL1 alleles was indeed observed (Table 3). Furthermore, when using long PCR, a specific primer for a given S-RNase allele and a SLFL1 general primer, a fragment with the same size was obtained from all individuals of the Bragança population known to have that particular S-RNase allele (Table 3).
TABLE 2.
DNA sequence variation summary
|
SLFL1
|
SLFL4: | ||||
|---|---|---|---|---|---|
| All Prunus (N = 22) | P. spinosa (N = 15) | P. mume (N = 3) | P. dulcis (N = 2) | P. spinosa (N = 12) | |
| Silent π JC | 0.09340 | 0.10532 | 0.05893 | 0.07880 | 0.02639 |
| Rm | 16 | 14 | — | — | 2 |
| 4GT | 673/15931 | 599/11325 | — | — | 6/72 |
| LD | 1108 (1)/15931 | 663 (0)/11325 | — | — | 5 (1)/15 |
| ZnS | 0.1113 | 0.1744 | — | — | 0.3466 |
| φ-test | P < 5 × 10−7 | P < 5 × 10−9 | — | — | P = 0.085 |
N, number of sequences used; π, average number of pairwise nucleotide differences per base pair Jukes–Cantor corrected (Nei 1987); Rm, minimum number of recombination events (Hudson and Kaplan 1985); 4GT, number of pairwise comparisons presenting the four gametic types over the total number of all pairwise comparisons; LD, pairs of sites showing significant linkage disequilibrium using Fisher's exact test (in parentheses after Bonferroni correction for multiple comparisons) over the total number of all pairwise comparisons ; ZnS, average of R2 values over all pairwise comparisons (Kelly 1997); φ-test, probability of observing the inferred nucleotide homoplasies under the assumption of no recombination, as implemented in SplitsTree4 (Huson and Bryant 2006).
TABLE 3.
Amplification products that show associations with a particular SFB allele
| SLFL1 allele or haplotype | Individuals that show the expected amplification product | SFB allele |
|---|---|---|
| B18-2 | B13, B14, B16, B18, B26 | SFB2 |
| B8-4 | B8 | SFB15 or SFB16 or SFB17 or SFB18a |
| B15-3/B18-3 | B14, B15, B18, B22, B26 | SFB5 |
| B8-6/B15-4/B18-1 | B5, B7, B8, B13, B15, B17, B26 | SFB24 |
| SLFL1-S8-RNaseB10 | B6, B10, B16, B19, B24 | SFB8 |
| SLFL1-S1-RNaseB19 | B15, B16, B19, B21, B22, B24, B25, B28 | SFB1 |
These four SFB alleles appear only in the B8 individual (Nunes et al. 2006; Vieira et al. 2008a).
Variability levels at P. spinosa SLFL1 (the larger sample) are on the same order as divergence between Prunus species from the Prunus and Amygdalus subgenera (Table 4). Species of these two subgenera shared a common ancestor ∼2.5 million years ago (Vieira et al. 2008b). Therefore, this observation indicates that SLFL1 alleles are on average ∼2.5 million years old. That most variability predates Prunus speciation is indicated by the high number of shared variants and the low number of fixed differences between Prunus species (Table 4).
TABLE 4.
Synonymous divergence (Jukes–Cantor corrected) at the SLFL1 gene (above the diagonal) and number of fixed/shared polymorphisms (below the diagonal)
| P. spinosa | P. mume | P. dulcis | |
|---|---|---|---|
| P. spinosa | — | 0.08665 | 0.08850 |
| P. mume | 0/10 | — | 0.06474 |
| P. dulcis | 1/12 | 0/7 | — |
The high variability at the SLFL1 gene compared to SLFL4, taken above as evidence for restricted recombination between SLFL1 and the S-locus, could be due to diversifying selection acting on the SLFL1 gene. We tested for this possibility using two different approaches (Yang 1997; Wilson and McVean 2006; see materials and methods). Both the phylogenetic and population genetics approach present potential problems that can affect the identification of sites under positive selection (see Vieira et al. 2007).
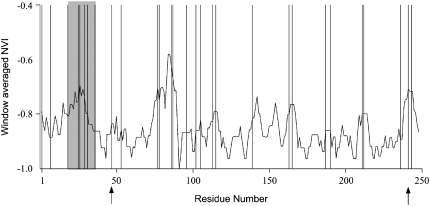
When using Yang's (1997) approach for detecting amino acid sites under positive selection, of all models tested, the simplest model that fits the data is model M1 that does not consider a positively selected class (see materials and methods). Two amino acid positions (47 and 247; at these sites there are four and five different amino acids, respectively; Figure 2) have, on average, posterior probabilities of selection >50% when using omegaMap. Nevertheless, under no condition did any of these sites show strong evidence for positive selection (posterior probability values >95%). Therefore, the hypothesis that diversifying selection is acting on the SLFL1 gene itself cannot be ruled out, although it seems very unlikely.
Figure 2.—
Window-averaged plot of normed variability index along the SLFL1 gene. The shadowed region indicates a variable region. Vertical lines indicate the location of the 10% most variable amino acid sites. Amino acid sites 47 and 241 showing four and five different amino acids, respectively, are indicated with arrows.
Despite the evidence for specific associations between SLFL1 and S-RNase–SFB genes as well as the old age of SLFL1 alleles, there is ample evidence suggestive of recombination at the SLFL1 gene (Table 2). For example, the minimum number of recombination events (Hudson and Kaplan 1985) implied by the 22 SLFL1 sequences is 16. Furthermore, despite the relatively small sample size, 4.2% of all pairwise comparisons show all four gametic types and only 6.9% of all possible pairs of sites show significant linkage disequilibrium (only one pair gives a significant result if the sequential Bonferroni correction is applied). The overall linkage disequilibrium, as measured by Kelly's (1997) ZnS statistic, is relatively low (varying from 0.1113 to 0.1744). Nevertheless, for P. spinosa (the larger sample), standard coalescent simulations show that it is likely to obtain the observed value under the assumption of no recombination (P > 0.05) although the simulations performed do not incorporate the effect of the neighboring S-RNase–SFB genes. The phylogenetic φ-test for recombination (Huson and Bryant 2006) gives a strong indication for recombination (Table 2), as do other tests for recombination (see results, The relative importance of recombination and mutation at the SLFL1, S-RNase, and SFB genes).
The 10 Prunus SLFL1 alleles known to be associated with a given S-RNase allele (6 in P. spinosa, 2 in P. mume, and 2 in P. dulcis) can be used to test whether the evolutionary histories of the two genes are correlated. To test this prediction, per-site synonymous (Ks) values were calculated for the S-RNase pairwise comparisons and for the corresponding SLFL1 pairwise comparisons. A nonsignificant correlation was obtained between synonymous divergence values at the two genes (r = 0.026; P > 0.05 Spearman nonparametric correlation). Therefore, it seems likely that, historically, the region where the SLFL1 gene is located has experienced non-negligible levels of recombination. Nevertheless, when the partition-homogeneity test was performed using only variable sites (to correct for a possible effect of different variability levels at the SLFL1 and S-RNase genes; Cunningham 1997), as implemented in PAUP (1000 replicates), a P-value of 0.049 was obtained. This value is too high to safely conclude that the SLFL1 and S-RNase tree topologies are significantly different (Cunningham 1997). This, however, can be the result of a small sample size and lack of definition of the two topologies.
When using the Wilson and McVean (2006) approach and the P. spinosa random sample of 15 sequences, an average (and standard deviation) per-codon point estimate of 0.198 ± 0.063 is obtained for the population recombination rate at SLFL1. The lower and higher 95% credible intervals are, respectively, 0.082 ± 0.043 and 0.505 ± 0.150. The SLFL1 protein is ∼409 amino acids long. Therefore, the SLFL1 gene is expected to experience ∼81 recombination events per generation (0.07 recombination events between adjacent nucleotides per generation). Nevertheless, the approach here used assumes a panmictic population. Patterns of variability at the SLFL1 gene may be influenced by the S-locus. Thus, patterns of variability at SLFL1 may look like those expected for a subdivided population. Therefore, the recombination estimate presented here may be an overestimate.
The relative importance of recombination and mutation at the SLFL1, S-RNase, and SFB genes:
The number of inferred independent recombination events is 3, 9, and 15 for the SLFL1, S-RNase, and SFB data sets used here, respectively. Furthermore, 136, 679.2, and 1367.9 synonymous mutations are implied by the SLFL1, S-RNase, and SFB data sets, respectively. Therefore, there are 0.022, 0.013, and 0.011 recombination events per synonymous mutation for the SLFL1, S-RNase, and SFB genes, respectively. This calculation suggests that the recombination rate at the SLFL1 gene may be only twofold higher than that at the S-RNase and SFB genes. Nevertheless, the power to detect recombinant sequences may depend on sample size and variability levels.
Estimating the population recombination rate between SLFL1 and the S-RNase:
For P. spinosa, using a simplified model that does not explicitly incorporate the effect of selection at the neighboring S-locus but rather approximates it by assuming two alleles held at intermediate frequencies (Kamau and Charlesworth 2005), an estimate of 0.33 was obtained for the population recombination rate between SLFL1 and the S-RNase. Nevertheless, for P. spinosa, when a model that explicitly models selection at the neighboring S-locus is used, a much higher estimate (9.04) is obtained for the population recombination rate between SLFL1 and the S-RNase. For the calculations, we used SLFL4 as a reference locus (Table 2), assumed 33 specificities in the P. spinosa Bragança population (Vieira et al. 2008a) and an f-value (the multiple by which variability at the selected locus is increased compared with the population average variability value) of 9.13 (obtained considering that the average synonymous variability level at the Prunus S-RNase gene is 0.241; Vieira et al. 2007). Ideally, the average of synonymous variability levels observed at several reference loci should be used but such data are not available for any Prunus species. Standard coalescent simulations suggest that when a per-site synonymous variability value of 0.026 is observed at SLFL4 (Table 2), the true value could be as low as 0.018. When this value is used rather than the 0.026 value, an estimate of 6.15 is obtained for the population recombination rate between SLFL1 and the S-RNase (the f-value is now 13.4). The physical distance between the SLFL1 and the S-RNase gene varies (Entani et al. 2003; Ushijima et al. 2003). Assuming an average distance of 20 kb between the two genes, between 1.65 × 10−5 to 4.57 × 10−4 recombination events between adjacent nucleotides are expected per generation, depending on the method used.
The approach used here assumes that each specificity is mutually exchangeable and that there is no dominance among specificities and similar frequencies for all specificities. There is no theoretical expectation regarding isoplethy in polyploids. Recently, it has been shown (Vieira et al. 2008a) that in the P. spinosa population studied here, specificity frequencies may be unequal. The deviation is due to an apparent excess of both high- and low-frequency specificities. This type of deviation is similar to that observed in wild cherry populations, where a significant departure from the isoplethic distribution is also observed when standard tests are used (Stoeckel et al. 2008). These authors have, however, shown that the observed allele-frequency distribution is compatible with genetic drift and a model of subdivided populations and moderate migration between demes. Furthermore, for polyploid species, such as P. spinosa, it is conceivable that all chromosome pairings are not equally likely during meiosis. Nevertheless, in the polyploid Prunus cerasus, this is not the case (Hauck et al. 2006). Moreover, in Prunus, heteroallelic pollen retains its SI phenotype (Hauck et al. 2006). Therefore, it may be appropriate to use the formula of Kamau and Charlesworth (2005).
DISCUSSION
The SLFL4 and SLFL5 genes identified here are more closely related to SLFL1 than to SLFL2 and SLFL3. Both SLFL2 and SLFL3 are found in the vicinity of the S-locus (Entani et al. 2003; Ushijima et al. 2003), but the genomic location of SLFL4 and SLFL5 genes is unknown. Thus, it is not possible to determine whether the different genes originated through a series of regional duplications. Nevertheless, in P. spinosa, SLFL1 pseudogenes are commonly found between SLFL1 and the S-RNase gene. SLFL1 pseudogenes also have been found in P. mume (Entani et al. 2003). These pseudogene sequences do not form a monophyletic group (Figure 1). Thus, it is inferred that they have multiple origins. It is thus conceivable that SLFL genes were frequently duplicated during evolution.
In P. spinosa, the SLFL1 synonymous variability level is about four times higher than that found for the reference locus SLFL4. This suggests that variability patterns at SLFL1 are influenced by the neighboring S-locus. Nevertheless, the SLFL1 synonymous variability level is 2.3 times lower than those found for the S-RNase and SFB genes (Vieira et al. 2007, 2008a). Therefore, it is unlikely that the evolutionary histories of SLFL1 and the S-locus are completely correlated. Indeed, a nonsignificant correlation is obtained between synonymous divergence values at the SLFL1 and S-RNase genes.
Fewer than 10 recombinants per generation are expected for the SLFL1–S-locus intergenic region. In contrast, the SLFL1 gene is expected to experience ∼81 recombination events per generation, although, as noted (see results), this number may be an overestimate. Overall, the analyses performed here suggest that recombination levels increase near the SLFL1 coding region (see results). Under this scenario, the observed associations between SLFL1 alleles and S-locus specificities are expected, because to create an association between a given SLFL1 sequence and two different specificities, one of the recombination breakpoints must be located in the SLFL1–S-locus intergenic region and this is a rare event. Recombination events affecting the SLFL1 gene will uncouple the evolutionary histories of the two genes, as it is observed (see results).
Recombination seems to be severely repressed at the S-locus only. This region varies in size from 2.6 to ∼50 kb (Nunes et al. 2006; Tao et al. 2007). Evidence suggestive of rare recombination has been reported at the S-RNase (Ortega et al. 2006; Vieira et al. 2007) and SFB (Nunes et al. 2006; Vieira et al. 2008a) genes. Given the evidence for severely restricted recombination at the S-locus only, the accumulation of weak deleterious mutations in the S-locus region is unlikely. Therefore, in Prunus there should be no selection against closely related allele pairs, in contrast with what has been predicted by Uyenoyama (1997). Such an effect may be restricted to Solanaceae species showing gametophytic self-incompatibility. In these species, the S-locus has been shown to have a centromeric location (see review by Wang et al. 2003). Thus it is conceivable that in Solanaceae species recombination is severely repressed in a large region around the S-locus.
Acknowledgments
We thank the anonymous reviewers for the constructive criticisms of earlier versions of the manuscript. This work has been funded by Fundação para a Ciência e Tecnologia [research project Programa Operacional Ciência e Inovação (POCI)/BIA-BDE/59887/2004 funded by POCI 2010, cofunded by Fundo Europeu de Desenvolvimento Regional funds].
References
- Baiashvili, E. I., 1980. Karyological study of Prunus spinosa L. Bull. Georgian Acad. Sci. 100 645–647. [Google Scholar]
- Charlesworth, B., M. Nordborg and D. Charlesworth, 1997. The effects of local selection, balanced polymorphism and background selection on equilibrium patterns of genetic diversity in subdivided inbreeding and outcrossing populations. Genet. Res. 70 155–174. [DOI] [PubMed] [Google Scholar]
- Cunningham, C. W., 1997. Can three incongruence tests predict when data should be combined? Mol. Biol. Evol. 14 733–740. [DOI] [PubMed] [Google Scholar]
- de Nettancourt, D., 1997. Incompatibility in Angiosperms. Springer-Verlag, Berlin.
- Entani, T., M. Iwano, H. Shiba, F. S. Che, A. Isogai et al., 2003. Comparative analysis of the self-incompatibility (S-) locus region of Prunus mume: identification of a pollen-expressed F-box gene with allelic diversity. Genes Cells 8 203–213. [DOI] [PubMed] [Google Scholar]
- Halliday, G., and M. Beadle, 1983. Flora Europaea. Cambridge University Press, Cambridge, UK.
- Hauck, N. R., H. Yamane, R. Tao and A. F. Iezzoni, 2006. Accumulation of nonfunctional S-haplotypes results in the breakdown of gametophytic self-incompatibility in tetraploid Prunus. Genetics 172 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, R. R., and N. L. Kaplan, 1985. Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics 111 147–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson, D. H., and D. Bryant, 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23 254–267. [DOI] [PubMed] [Google Scholar]
- Ingram, G. C., S. Doyle, R. Carpenter, E. A. Schultz, R. Simon et al., 1997. Dual role for fimbriata in regulating floral homeotic genes and cell division in Antirrhinum. EMBO J. 16 6521–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innan, H., and M. Nordborg, 2003. The extent of linkage disequilibrium and haplotype sharing around a polymorphic site. Genetics 165 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, J. K., 1997. A test of neutrality based on interlocus associations. Genetics 146 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheyr-Pour, A., S. C. Bintrim, T. R. Ioerger, R. Remy, S. A. Hammond et al., 1990. Sequence diversity of pistil S-proteins associated with gametophytic self-incompatibility in Nicotiana alata. Sex. Plant Reprod. 3 88–97. [Google Scholar]
- Kamau, E., and D. Charlesworth, 2005. Balancing selection and low recombination affects diversity near the self-incompatibility loci of the plant Arabidopsis lyrata. Curr. Biol. 15 1773–1778. [DOI] [PubMed] [Google Scholar]
- Kumar, S., K. Tamura and M. Nei, 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinformatics 5 150–163. [DOI] [PubMed] [Google Scholar]
- Martin, D. P., C. Williamson and D. Posada, 2005. RDP2: recombination detection and analysis from sequence alignments. Bioinformatics 21 260–262. [DOI] [PubMed] [Google Scholar]
- Matsumoto, D., H. Yamane and R. Tao, 2008. Characterization of SLFL1, a pollen-expressed F-box gene located in the Prunus S locus. Sex. Plant Reprod. 21 113–121. [Google Scholar]
- Nei, M., 1987. Molecular Evolutionary Genetics. Columbia University Press, New York.
- Nordborg, M., B. Charlesworth and D. Charlesworth, 1996. Increased levels of polymorphism surrounding selectively maintained sites in highly selfing species. Proc. R. Soc. Lond. B Biol. Sci. 163 1033–1039. [Google Scholar]
- Nunes, M. D. S., R. A. M. Santos, S. M. Ferreira, J. Vieira and C. P. Vieira, 2006. Variability patterns and positively selected sites at the gametophytic self-incompatibility pollen SFB gene in a wild self-incompatible Prunus spinosa (Rosaceae) population. New Phytol. 172 577–587. [DOI] [PubMed] [Google Scholar]
- Ortega, E., R. I. Boskovic, D. J. Sargent and K. T. Tobutt, 2006. Analysis of S-RNase alleles of almond (Prunus dulcis): characterization of new sequences, resolution of synonyms and evidence of intragenic recombination. Mol. Genet. Genomics 276 413–426. [DOI] [PubMed] [Google Scholar]
- Posada, D., and K. A. Crandall, 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14 817–818. [DOI] [PubMed] [Google Scholar]
- Rozas, J., J. C. Sanchez-DelBarrio, X. Messeguer and R. Rozas, 2003. DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19 2496–2497. [DOI] [PubMed] [Google Scholar]
- Salesses, G., 1973. Études cytologiques chez les Prunus II. Hybrides interspécifiques impliquant P. cerasifera, P. spinosa, P. domestica et P. insititia. Ann. Améloriement Plantes 23 145–161. [Google Scholar]
- Schierup, M. H., X. Vekemans and F. B. Christiansen, 1998. Allelic genealogies in sporophytic self-incompatibility systems in plants. Genetics 150 1187–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierup, M. H., X. Vekemans and D. Charlesworth, 2000. The effect of subdivision on variation at multi-allelic loci under balancing selection. Genet. Res. 76 51–62. [DOI] [PubMed] [Google Scholar]
- Stoeckel, S., V. Castric, S. Mariette and V. Vekemans, 2008. Unequal allelic frequencies at the self-incompatibility locus within local populations of Prunus avium L.: An effect of population structure? J. Evol. Biol. 21 889–899. [DOI] [PubMed] [Google Scholar]
- Swofford, D. L., 2002. PAUP*: Phylogenetic Analysis Using Parsimony (and Other Methods), Version 4.0b10. Sinauer, Sunderland, MA.
- Tao, R., A. Watari, T. Hanada, T. Habu, H. Yaegaki et al., 2007. Self-compatible peach (Prunus persica) has mutant versions of the S haplotypes found in self-incompatible Prunus species. Plant Mol. Biol. 63 109–123. [DOI] [PubMed] [Google Scholar]
- Thompson, J., T. J. Gibson, F. Plewniak, F. Jeanmougin and D. G. Higgins, 1997. The ClustalX window interface: flexible stategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima, K., H. Sassa, M. Tamura, M. Kusaba, R. Tao et al., 2001. Characterization of the S-locus region of almond (Prunus dulcis): analysis of a somaclonal mutant and a cosmid contig for an S haplotype. Genetics 158 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima, K., H. Sassa, A. M. Dandekar, T. M. Gradziel, R. Tao et al., 2003. Structural and transcriptional analysis of the self-incompatibility locus of almond: identification of a pollen-expressed F-box gene with haplotype-specific polymorphism. Plant Cell 15 771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyenoyama, M. K., 1997. Genealogical structure among alleles regulating self-incompatibility in natural populations of flowering plants. Genetics 147 1389–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyenoyama, M. K., 2000. Mutational origin of new mating type specificities in flowering plants. Genes Genet. Syst. 75 305–311. [DOI] [PubMed] [Google Scholar]
- Uyenoyama, M. K., 2005. Evolution under tight linkage to mating type. New Phytol. 165 63–70. [DOI] [PubMed] [Google Scholar]
- Uyenoyama, M. K., Y. Zhang and E. Newbigin, 2001. On the origin of self-incompatibility haplotypes: transition through self-compatible intermediates. Genetics 157 1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekemans, X., and M. Slatkin, 1994. Gene and allelic genealogies at a gametophytic self-incompatibility locus. Genetics 137 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, C. P., D. Charlesworth and J. Vieira, 2003. Evidence for rare recombination at the gametophytic self-incompatibility locus. Heredity 91 262–267. [DOI] [PubMed] [Google Scholar]
- Vieira, J., R. Morales-Hojas, R. A. M. Santos and C. P. Vieira, 2007. Different positively selected sites at the gametophytic self-incompatibility pistil S-RNase gene in the Solanaceae and Rosaceae (Prunus, Pyrus and Malus). J. Mol. Evol. 65 175–185. [DOI] [PubMed] [Google Scholar]
- Vieira, J., R. A. M. Santos, S. M. Ferreira and C. P. Vieira, 2008. a Molecular evolution at the Prunus spinosa SFB: allele diversity, population structure and amino acid sites under positive selection. Heredity (in press).
- Vieira, J., N. A. Fonseca, R. A. M. Santos, T. Habu, R. Tao et al., 2008. b The number, age, sharing and relatedness of S-locus specificities in Prunus. Genet. Res. 90 17–26. [DOI] [PubMed] [Google Scholar]
- Wang, Y., X. Wang, A. L. Skirpan and T. H. Kao, 2003. S-RNase-mediated self-incompatibility. J. Exp. Bot. 54 115–122. [DOI] [PubMed] [Google Scholar]
- Wilson, D. J., and G. McVean, 2006. Estimating diversifying selection and functional constraint in the presence of recombination. Genetics 172 1411–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiuf, C., K. Zhao, H. Innan and M. Nordborg, 2004. The probability and chromosomal extent of trans-specific polymorphism. Genetics 168 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, S., 1939. The distribution of self-sterility alleles in populations. Genetics 24 538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z., 1997. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput. Appl. Biosci. 13 555–556. [DOI] [PubMed] [Google Scholar]