Abstract
Familial adenomatous polyposis (FAP) is a human cancer syndrome characterized by the development of hundreds to thousands of colonic polyps and extracolonic lesions including desmoid fibromas, osteomas, epidermoid cysts, and congenital hypertrophy of the pigmented retinal epithelium. Afflicted individuals are heterozygous for mutations in the APC gene. Detailed investigations of mice heterozygous for mutations in the ortholog Apc have shown that other genetic factors strongly influence the phenotype. Here we report qualitative and quantitative modifications of the phenotype of Apc mutants as a function of three genetic variables: Apc allele, p53 allele, and genetic background. We have found major differences between the Apc alleles Min and 1638N in multiplicity and regionality of intestinal tumors, as well as in incidence of extracolonic lesions. By contrast, Min mice homozygous for either of two different knockout alleles of p53 show similar phenotypic effects. These studies illustrate the classic principle that functional genetics is enriched by assessing penetrance and expressivity with allelic series. The mouse permits study of an allelic gene series on multiple genetic backgrounds, thereby leading to a better understanding of gene action in a range of biological processes.
HIGHLY penetrant familial cancers, including 200 variants, represent 5–10% of the total cancer burden (Fearon 1997; Nagy et al. 2004; Garber and Offit 2005). Individuals in such families inherit a germline mutation that predisposes them to cancer of one or more tissues. Identification of the mutated gene is only the first step in understanding the molecular basis of a particular familial cancer. Patients carrying the same mutation can have quite different clinical outcomes because other genes affect both penetrance (the fraction of mutation carriers exhibiting one or another of the neoplastic phenotypes) and expressivity (tumor multiplicity and progression). Thus, a familial cancer is not a fixed consequence of an alteration in a single gene, but involves the effect of such an alteration in the context of the alleles of other genes.
Patients afflicted with familial adenomatous polyposis (FAP) carry mutations in the APC gene (Groden et al. 1991; Kinzler et al. 1991). They are predisposed to the development of colorectal tumors by the third decade of life (Croner et al. 2005), with tumor multiplicities from 10 to 2000. FAP is a rare disease, whereas sporadic colorectal cancer is common. The vast majority of tumors from these patients carry somatic mutations in APC (Nishisho et al. 1991)—a gene essential to homeostasis in the intestine. Consequently, a full characterization of the molecular basis of this familial colon cancer syndrome may facilitate the understanding and management of the more prevalent sporadic disease.
The APC gene is broadly expressed in mammals and has been shown to be necessary not only for growth of the early postimplantation embryo (Moser et al. 1995), but also for skin and thymus (Kuraguchi et al. 2006). This may explain the observation that FAP patients are predisposed to a number of extracolonic neoplasms. The relative risks vary dramatically by site: from 852 for desmoid fibromas to 4.46 for pancreatic carcinomas (Giardiello et al. 1993; Gurbuz et al. 1994). Multiplicity of colorectal polyps and incidence of extracolonic manifestations are at least partially affected by the particular APC germline mutation (Enomoto et al. 2000; Bertario et al. 2001, 2003): mutations in the 5′ end of the gene result in an attenuated form of FAP that is characterized by the development of few colonic polyps, while mutations in the 3′ end increase the tendency to develop desmoid fibromas. Apc has been linked to several different biological processes through its interactions with other proteins. The binding of APC to β-catenin and consequent degradation of the latter is essential for maintaining homeostasis in the mammalian intestine (reviewed in Matushansky et al. 2008).
Several mouse models of FAP have been generated over the past decade (Moser et al. 1990; Oshima et al. 1997; Shibata et al. 1997; Smits et al. 1998, 1999). The Min allele of Apc, induced by germline mutagenesis with ethylnitrosourea (Moser et al. 1990), is a T-to-A transversion that creates a translational stop at codon 850 (Su et al. 1992). C57BL/6J (B6) ApcMin/+ mice develop on average 100 adenomas along the entire length of the intestinal tract (Cormier and Dove 2000). Tumorigenesis in these mice is initiated when function of the wild-type allele of Apc is lost, commonly by somatic recombination (Haigis et al. 2004). Two alleles of Apc, 1638N and 1638T, were generated by inserting a selectable Neomycin resistance cassette at Apc codon 1638. B6 Apc1638N/+ mice develop on average one tumor near the duct of Vader in the proximal region of the small intestine (Smits et al. 1998), while B6 Apc1638T/+ mice are tumor free (Smits et al. 1999). These insertion alleles differ in the orientation of transcription from the insertion relative to transcription from the Apc promoter; they also differ in expression of a protein fragment from the 1638T but not the 1638N mutant allele (Smits et al. 1999). Smits et al. (1998) reported that only 17% of intestinal adenomas from Apc1638N/+ mice with a mixed genetic background exhibited maintenance of heterozygosity (MOH) at the Apc locus. By contrast, Haigis et al. (2004) found that 100% of the intestinal adenomas from Apc1638N/+ mice on a congenic B6 genetic background exhibited MOH at Apc. They interpreted this observation and the regionality of tumorigenesis in these mice to be consistent with a second hit involving intragenic mutation in the wild-type Apc allele. This interpretation has found support from the demonstration of intragenic Apc truncation mutations in 16 of 50 tumors from B6 Apc1638N mice (Kucherlapati et al. 2007).
The phenotype of a mouse model clearly depends on the particular mutant allele of Apc. The multiplicity of intestinal adenomas in ApcMin/+ is also profoundly affected by genetic background. AKR/J ApcMin/+ mice develop on average less than one adenoma per mouse (Shoemaker et al. 1998). One genetic factor contributing to this dramatic reduction in tumor multiplicity is Mom1 (Gould et al. 1996). This locus acts as a semidominant modifier; one copy of the resistance allele (Mom1R) reduces tumor multiplicity by a factor of two, whereas two copies reduce tumor multiplicity by a factor of four (Gould et al. 1996). Several other genetic modifiers and environmental factors affecting tumorigenesis in mouse models of FAP have been identified, using a wide variety of experimental approaches. The relevance of these genes and factors to human colorectal cancer continues to be examined.
The molecular basis of the Li–Fraumeni syndrome (LFS) is also beginning to be understood. A breakthrough occurred in early 1990 when Malkin and colleagues demonstrated that afflicted individuals carry a mutation in the p53 tumor suppressor gene (Malkin et al. 1990). LFS is characterized by the development at an unusually early age of breast carcinoma, soft-tissue sarcoma, osteosarcoma, leukemia, brain tumors, and adrenocortical carcinoma (Li and Fraumeni 1969). This diversity in the tumor spectrum probably reflects the involvement of p53 in numerous cellular processes, including cell cycle arrest, senescence, and apoptosis (reviewed in Harris and Levine 2005). For one specific example, p53 protein stimulates the transcription of p21, which encodes a protein that inhibits a complex of Cyclin E and Cdk2 and thereby blocks the cell cycle at G1. Mutations in p53 are among the most frequent genetic alterations found in sporadic forms of human cancer (Nigro et al. 1989). Changes in p53 activity may be a useful prognosticator of clinical outcome for patients with colorectal cancer (reviewed in Soussi and Beroud 2001).
Mice have been generated that either express a dominant-negative mutant allele of p53 or lack p53 activity (Donehower et al. 1992; Jacks et al. 1994; De Vries et al. 2002). In particular, Donehower and his colleagues have generated a p53 knockout allele (p53BCM) in which intron 4 and exon 5 are replaced with a selectable marker cassette, and Jacks and his colleagues have generated a p53 knockout allele (p53tm1TyjJ) in which exons 2–6 are replaced with a selectable marker cassette. Mice that are homozygous for either of these knockout alleles are viable but develop tumors that are similar to those commonly seen in LFS patients (Donehower et al. 1992; Jacks et al. 1994; De Vries et al. 2002). These mouse models have been treated with carcinogens and bred to other mice each predisposed to a particular cancer to understand more fully the role of p53 in suppressing tumorigenesis in particular tissues (Kemp et al. 1993; Clarke et al. 1995; Nacht et al. 1996; Xu et al. 1998; Reilly et al. 2000; Yamamoto et al. 2000; Halberg et al. 2000; Shirai et al. 2002; Sakai et al. 2003; Hirata et al. 2005).
This study extends earlier studies by exploring the phenotype of mice carrying Apc mutations as a function of several genetic variables: the Apc allele, the p53 allele and its target p21, and the genetic background—C57BL/6J (B6) vs. 129S6/SvEvTac (129) vs. (B6 × 129)F1 (F1).
MATERIALS AND METHODS
Mice:
All mice were bred, housed, and investigated in the McArdle Laboratory for Cancer Research, under approved guidelines set forth by the Institutional Animal Care and Use Committee of the American Association for the Assessment and Accreditation of Laboratory Animal Care at the University of Wisconsin. We bred several C57BL/6J (B6) congenic strains to test different combinations of two mutant alleles of Apc with two knockout alleles of p53. B6 mice carrying the 1638N allele of Apc were obtained through the Mouse Models of Human Cancer Consortium and crossed to our B6 stock carrying p53BCM (Donehower et al. 1992; Yang et al. 1997). The resulting progeny were intercrossed to generate Apc1638N/+ p53+/+, Apc1638N/+ p53BCM/BCM, and Apc+/+ p53BCM/BCM mice on the B6 genetic background. In addition, B6 mice carrying p53tm1TyjJ were obtained from The Jackson Laboratory in Bar Harbor, Maine (Jacks et al. 1994). Females were crossed to B6 ApcMin/+ males from our laboratory stock (Moser et al. 1990). The resulting p53-heterozygous progeny were intercrossed to generate ApcMin/+ p53+/+, ApcMin/+ p53tm1TyjJ/+, ApcMin/+ p53tm1TyjJ/tm1TyjJ, and Apc+/+ p53tm1TyjJ/tm1TyjJ mice on the B6 genetic background. To test whether the effect of p53 on tumorigenesis in ApcMin/+ mice was also affected by genetic background, the ApcMin and p53BCM alleles present in our B6 stock were moved onto the129S6/SvEvTac (129) genetic background by backcrossing for 10 generations. Populations of ApcMin/+ p53+/+, ApcMin/+ p53+/BCM, ApcMin/+ p53BCM/BCM, and Apc+/+ p53BCM/BCM mice on the 129 genetic background were generated by intercrossing p53-heterozygous mice from the N10 through N14 generations. In parallel, we generated test mice of all three p53 genotypes on the (B6 × 129)F1 hybrid background by crossing B6 Apc+/+ Mom1R/R p53+/BCM females to 129 ApcMin/+ Mom1S/S p53+/BCM males.
To assess the role of p21, (129 × B6)F2 mice carrying a knockout allele of p21 (p21tm1Tyj) were obtained from The Jackson Laboratory. Females were bred to congenic 129 ApcMin/+ mice from our laboratory stock. The resulting p21-heterozygous progeny were intercrossed to generate ApcMin/+ p21+/+, ApcMin/+ p21tm1Tyj/+, ApcMin/+ p21tm1Tyj/tm1Tyj, and Apc+/+ p21tm1Tyj/tm1Tyj mice. Since these mice had a heterogeneous genetic background composed of 129 and B6 alleles, we moved the p21tm1Tyj allele onto the 129 genetic background by backcrossing for 10 generations. This congenic p21-deficient line was then bred to the congenic 129 ApcMin/+ stock described above. The resulting p21-heterozygous progeny were intercrossed to generate ApcMin/+ p21+/+, ApcMin/+ p21+/tm1Tyj, ApcMin/+ p21tm1Tyj/tm1Tyj, and Apc+/+ p21tm1Tyj/tm1Tyj mice. The test mice in this study were genotyped at Apc, Mom1, p21, and p53 by analyzing DNA prepared from toe clips using PCR assays as described previously (Jacks et al. 1994; Halberg et al. 2000; Yang et al. 2001).
Tumor counts:
Mice were killed between 90 and 100 days of age. The number of desmoid fibromas was scored on the abdominal wall and the intestinal tract was removed, opened longitudinally, and laid out as described previously (Moser et al. 1990). Samples were fixed in 10% buffered formalin for 16 hr and then placed in 70% ethanol for long-term storage. The number of intestinal tumors was scored under a dissecting microscope. All scoring was performed by a single observer (R. B. Halberg) blind to the genotype of the mice. Differences in tumor multiplicities between groups of mice were tested for statistical significance, using the two-sided Wilcoxon rank sum test. In addition, the pancreas and testes were removed from mice chosen at random. The tissue was fixed in 10% buffered formalin for 16 hr and then placed in 70% ethanol for long-term storage.
Histological analysis:
Fixed desmoid fibromas and pancreatic, testicular, and intestinal tumors were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Sections were analyzed by two pathologists, H. C. Pitot and R. Sullivan, blind to the genotype of the mice.
Apc1638N pyrosequencing:
Pyrosequencing primers were designed for the single-nucleotide polymorphism rs29821669 that was confirmed to be polymorphic between B6 and the ancestral 129/S6 allele of Apc that was targeted and gave rise to the 1638N transgenic line. The final PCR mix concentrations were as follows: 1× GoTaq clear buffer, 1.2 mm MgCl2, 0.2 mm dNTPs, 0.5 μm of each primer (forward, F; reverse, R), 0.6 unit of GoTaq Flexi (Promega, Madison, WI), 8 μl of DNA, and ddH2O to 50 μl. The PCR cycling profile was 94° for 3 min, followed by 50 cycles of 94° for 15 sec, 57° for 1 min 30 sec, and 72° for 2 min, with a final elongation step of 72° for 10 min. Pyrosequencing was performed according to the manufacturer's protocols using Pyro Gold Reagents on a PSQ96MA machine and PSQ 96MA v2.1 software (Biotage, Uppsala, Sweden). A total of 40 μl of PCR reaction were used per well and only sequence reads with single-base peak heights of >120 units were included. Primer sequences were as follows: forward biotin, CCTTCCATTCTTCCGTCAAA; reverse, GGGGCTTCAATGTTCTCAAA; and pyrosequencing primer, TGTCTTGGTTTTGTACTG.
RESULTS
The p53 effect is independent of the specific Apc and p53 alleles:
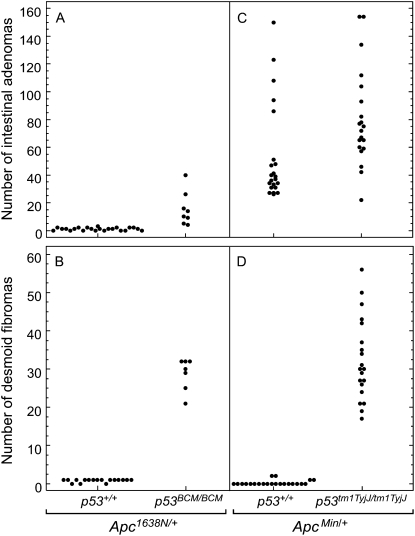
The ability of p53 to modulate tumorigenesis in mice carrying a mutant allele of Apc is evident with different mutant alleles of Apc and of p53. First, we tested whether a lack of p53 activity affected the development of intestinal tumors and desmoid fibromas in B6 mice carrying the 1638N allele of Apc. B6 Apc1638N/+ mice were crossed to B6 mice carrying the p53BCM knockout allele and the progeny were intercrossed to generate Apc1638N/+ mice carrying p53+/+ and p53BCM/BCM. B6 Apc1638N/+ p53BCM/BCM mice developed on average 15 intestinal tumors and 28 desmoid fibromas, compared to B6 Apc1638N/+ p53+/+ with an average of 1 intestinal tumor and 1 desmoid fibroma (Figure 1, A and B; P = 2.2 × 10−5 and P = 2.4 × 10−5, respectively). Previous reports have shown that a lack of p53 affects intestinal neoplasia: Smits et al. (1998), using Apc1638N/+ on a hybrid genetic background, and Halberg et al. (2000), using ApcMin/+ on the inbred B6 background. The majority of tumors (8/10) from B6 Apc1638N/+ mice maintain the wild-type allele of Apc during tumorigenesis in the intestine as determined by assessing the allelic ratio of a 129/B6 single-nucleotide polymorphism linked to the Apc gene by pyrosequencing. This observation is consistent with an earlier study (Haigis et al. 2004).
Figure 1.—
The p53 effect on tumorigenesis in Apc mutants was observed with different Apc and p53 alleles. Crosses between B6 congenic strains were performed to test the interaction between Apc1638N and p53BCM (A and B: P = 2.2 × 10−5 and P = 2.4 × 10−5, respectively) or to test the interaction between ApcMin and p53tm1TyjJ (C and D: P = 0.003 and P = 7.8 × 10−4, respectively). Progeny homozygous for the mutant p53 allele were sacrificed at 100 days of age. The total numbers of intestinal tumors (A and C) and desmoid fibromas (B and D) were scored for each mouse.
Next, we tested whether the effect of p53 on tumorigenesis depended on a specific knockout allele of this gene. B6 mice carrying the p53tm1TyjJ knockout allele were bred to B6 ApcMin/+ mice and the progeny were intercrossed to generate ApcMin/+ mice carrying p53+/+, p53tm1TyjJ/+, and p53tm1TyjJ/tm1TyjJ. B6 ApcMin/+ p53tm1TyjJ/tm1TyjJ mice developed significantly more intestinal tumors and desmoid fibromas than B6 ApcMin/+ p53+/+ controls (Figure 1, C and D; P = 0.003 and P = 7.8 × 10−4, respectively). Interestingly, a heterozygous effect of p53 deficiency was also observed. B6 ApcMin/+ p53tm1TyjJ/+ mice developed significantly more intestinal tumors than B6 ApcMin/+ p53+/+ controls (72 ± 49 vs. 53 ± 35, P = 0.01). These observations are consistent with results of our previous study utilizing ApcMin and p53BCM (Halberg et al. 2000).
In summary (see Figure 1 and Table 1), the effect of p53 deficiency on Apc-dependent intestinal and desmoid neoplasia is evident on the congenic B6 background for each pairwise combination involving heterozygosity for either of two mutant alleles of Apc and homozygosity for either of two knockout alleles of p53. Notably, we also consistently detected a subtle but significant change in the multiplicity of intestinal tumors in ApcMin/+ caused by heterozygosity for p53 deficiency. The heterozygous effect of p53 deficiency was observed on the F1 background, consistent with the previous report of Halberg et al. (2000) on the B6 genetic background: ApcMin/+ p53+/BCM mice developed more intestinal tumors than controls but fewer than ApcMin/+ p53BCM/BCM mice (Table 1; P = 0.001 and 0.03, respectively).
TABLE 1.
Effect of p53 and genetic background on the development of neoplasms in ApcMin/+ mice
| Extracolonic tumors
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Intestinal tumor counta, mean ± SD (no. of mice)
|
Desmoid fibroma count, mean ± SD (no. of mice)
|
Pancreatic tumors, tumor bearing/totalb
|
|||||||
| B6 | 129 | F1 | B6 | 129 | F1 | B6 | 129 | F1 | |
| Genotype | Mom1R/Rc | Mom1S/S | Mom1R/S | Mom1R/R | Mom1S/S | Mom1R/S | Mom1S/S | Mom1S/S | Mom1R/S |
| ApcMin/+p53+/+ | 27 ± 13 (13) | 50 ± 50 (36) | 62 ± 30 (27) | 0 (8) | 1 ± 2 (29) | 3 ± 6 (19) | 0/6 | 0/8 | 1/14 |
| ApcMin/+p53+/BCM | 40 ± 19 (28) | 47 ± 26 (31) | 84 ± 36 (77) | 1 ± 2 (20) | 2 ± 4 (24) | 6 ± 5 (67) | 0/13 | 0/2 | 0/49 |
| ApcMin/+p53BCM/BCM | 75 ± 25 (22) | 46 ± 29 (10) | 103 ± 36 (20) | 33 ± 8 (22) | 22 ± 4 (10) | 28 ± 9 (17) | 0/8c | 9/9 | 8/9 |
| Apc+/+p53BCM/BCM | ND | 0 (5) | 0 (11) | 0 (14) | 0 (5) | 0 (11) | 0/6 | 0/3 | 0/11 |
Total tract.
Mice were scored as tumor bearing if a tumor was detected during necropsy at 90–100 days of age. Microscopic adenomas in the pancreas of some B6 ApcMin/+ p53tm1TyjJ/tm1TyjJ mice were detected only when the pancreas was sectioned and stained with hematoxylin and eosin.
Mom1 is a semidominant modifier of intestinal tumorigenesis that acts independently of p53 (Halberg et al. 2000). Testicular tumors were also observed in mice homozygous for p53BCM/BCM and heterozygous or homozygous for the 129 background, but not depending on Apc deficiency.
Suppression of tumorigenesis in ApcMin/+ mice by p53 is affected by polymorphic modifiers:
Does the 129 genetic background differ from B6 by carrying polymorphic modifier alleles that affect the role of Apc and p53 in intestinal or extracolonic neoplasia? A 129 congenic strain was bred, carrying ApcMin and p53BCM. 129 ApcMin/+ p53BCM/BCM mice developed 46 ± 29 intestinal tumors, whereas 129 ApcMin/+ p53+/+ controls developed 50 ± 50 (Table 1; P = 0.58). The statistical power to detect an effect of p53 deficiency on the multiplicity of intestinal tumors in this experiment was 0.8, based on the number of mice in each group and the variance in tumor counts among controls. Thus, the 129 genetic background carries at least one modifier allele that supplants the role of p53 in suppressing tumorigenesis in the intestine. The 129 modifier system is formally recessive to B6 since the enhancing effect of homozygosity for p53 deficiency is evident both on B6 and on the F1 hybrid genetic background. (B6 × 129)F1 ApcMin/+ p53BCM/BCM mice developed significantly more intestinal tumors than controls (Table 1; P = 0.0002).
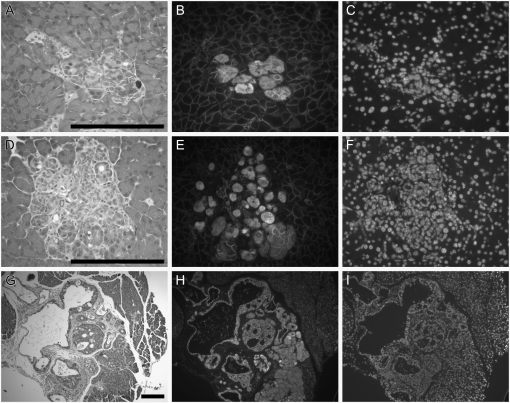
Modifiers polymorphic between 129 and B6 also affect tumorigenesis in the pancreas. 129 ApcMin/+ p53BCM/BCM mice and (B6 × 129)F1 ApcMin/+ p53BCM/BCM hybrids develop large pancreatic tumors that are evident during necropsy (Table 1); multiple adenomas and adenocarcinomas, primarily derived from acinar cells, were present throughout the pancreas. Apc activity appeared to be reduced or lost in both types of pancreatic lesions since β-catenin was present throughout the nucleus (Figure 2). By contrast, B6 ApcMin/+ p53tm1Tyj/tm1Tyj mice developed only microscopic adenomas in the pancreas (Figure 2). Thus, pancreatic neoplasms arise when both Apc and p53 are deficient and a dominant 129 susceptibility modifier system is present. This observation extends the report by Clarke et al. (1995).
Figure 2.—
β-Catenin is detectible in the nucleus of neoplastic cells within the pancreas. The pancreas was removed from a B6 ApcMin/+ p53tm1TyjJ/tm1TyjJ mouse (A–C) or a 129 ApcMin/+ p53BCM/BCM mouse (D–F and G–I) at 100 days of age, embedded in paraffin, and sectioned. Every 10th slide was stained with hematoxylin and eosin (A, D, and G). Adenomas (A and D) and adenocarcinomas (G) were apparent. The levels of β-catenin antigens (B, E, and H) within cells were determined by immunofluorescence microscopy. Nuclei were counterstained with DAPI (C, F, and I). Bars, 200 μm.
The polymorphic modifier(s) affecting the development of tumors in the intestine and pancreas of B6 and 129 mice do not affect the development of desmoid fibromas on the abdominal body wall. 129 ApcMin/+ p53BCM/BCM mice developed significantly more desmoid fibromas than 129 ApcMin/+ p53+/+ controls (Table 1; P = 5.5 × 10−8). A similar result was observed in (B6 × 129)F1 hybrids (Table 1; P = 1.4 × 10−6). Thus, the activity of polymorphic modifiers can be tissue specific. Note that we demonstrated in our earlier study that the activity of the modifier locus Mom1 is also tissue specific (Halberg et al. 2000).
The p53 effect is partially mediated by p21:
Yang and her colleagues reported that Apc1638N/+ mice homozygous for a knockout allele of p21 with a mixed genetic background of B6 and 129 on a high-fat “Western diet” developed on average 7.5 ± 4.9 tumors in the proximal small intestine, significantly more than the average of 4.3 ± 1.9 developed by controls homozygous for the wild-type allele of the negative regulator p21 (Yang et al. 2001). One of the targets activated by the p53 transcription factor is p21. Thus, the suppressive effect of p53 function on tumorigenesis in ApcMin/+ mice might be mediated by p21 function. To test this possibility, (129 × B6)F2 mice carrying a knockout allele of p21 (p21tm1Tyj) were bred to congenic 129 ApcMin/+ mice from our laboratory stock. The resulting heterozygous progeny were intercrossed to generate ApcMin/+ mice carrying p21+/+, p21tm1Tyj/+, and p21tm1Tyj/tm1Tyj. The multiplicity of intestinal tumors in ApcMin/+ p21tm1TyjJ/tm1TyjJ mice ranged from 35 to 426 with an average of 178 ± 136 whereas the multiplicity of intestinal tumors in ApcMin/+ p21+/+ mice ranged from 29 to 107 with an average of 59 ± 30 (Table 2). Here again, the p21 deficiency displayed a heterozygous effect on intestinal tumors, but not on desmoid fibromas or pancreatic tumors.
TABLE 2.
Effect of p21 and genetic background on the development of neoplasms in ApcMin/+ mice
| Intestinal tumor count,a mean ± SD (no. of mice)
|
Desmoid fibroma count, mean ± SD (no. of mice)
|
Pancreatic tumors, tumor bearing/totalb
|
||||
|---|---|---|---|---|---|---|
| Genotype | Mixed backgroundc | 129 | Mixed backgroundc | 129 | Mixed backgroundc | 129 |
| ApcMin/+p21+/+ | 59 ± 30 (7) | 54 ± 34 (11) | 0 ± 0 (4) | 2 ± 3 (10) | 0/7 | 0/11 |
| ApcMin/+p21+/− | 120 ± 76 (20) | 97 ± 51 (26) | 2 ± 3 (9) | 3 ± 3 (25) | 0/20 | 0/26 |
| ApcMin/+p21−/− | 178 ± 136 (16) | 101 ± 39 (12) | 1 ± 2 (9) | 4 ± 4 (12) | 0/16 | 0/12 |
| Apc+/+p21−/− | 0 ± 0 (10) | 0 ± 0 (8) | 0 ± 0 (7) | 0 ± 0 (8) | 0/10 | 0/8 |
Total tract.
Mice were scored as tumor bearing if a tumor was detected during necropsy at 90–100 days of age.
Heterozygous p21+/− progeny of the cross (B6 × 129)F2 p21+/− × 129 ApcMin/+ were intercrossed to generate these mice.
The p21 knockout allele was then bred onto the 129 genetic background to determine whether our initial analysis of the role of p21 is confirmed under congenic conditions on the background that obscures the p53 deficiency on Apc-dependent intestinal neoplasia. The multiplicity of intestinal tumors in 129 ApcMin/+ p21tm1TyjJ/tm1TyjJ and ApcMin/+ p21tm1TyjJ/+ mice was again significantly higher than that in 129 ApcMin/+ p21+/+ mice (Table 2). Thus, the dominant 129 modifier system that supplants the p53 effect on intestinal tumorigenesis in ApcMin/+ mice does not supplant the p21 effect. Here too, a lack of p21 activity did not lead to the development of desmoid fibromas or pancreatic tumors in 129 ApcMin/+ mice (Table 2). Taken together, these results indicate that p21 mediates the p53 effect only on intestinal tumorigenesis, and in that case only in part.
DISCUSSION
FAP patients carrying the same Apc mutation often have different clinical presentations, varying in both multiplicity of colonic tumors and incidence of extracolonic lesions. Perhaps these two modes of variation reflect polymorphic genetic modifiers. In the Min mouse model, such modifiers have been associated with the alterations in tumor multiplicity (Mom1 on chromosome 4 and Mom2, -3, and -7 on chromosome 18—linked to Apc). In this study, we have investigated whether the distinct tissues in which ApcMin/+ mice develop neoplasms involve a contribution by the negative regulators p53 and p21. Further, we have used controlled variation in the genetic background to ascertain whether polymorphic genetic modifiers act in a tissue-specific manner to influence further the qualitative Apc-dependent neoplastic phenotype.
The difference in intestinal tumor multiplicity between two Apc alleles, the nonsense allele Min and the insertion allele 1638N, can be explained either by a dominant-negative activity of the Min allele or, as for Mom2 (Baran et al. 2007), by a position effect in which the 1638N insertion affects expression of a neighboring cell-vital gene (Haigis et al. 2004). In contrast to the vast preponderance of loss-of-heterozygosity (LOH) seen in Min tumors on the B6 background (Luongo et al. 1994; Haigis et al. 2002; Haigis and Dove 2003), the majority of tumors in B6 Apc1638N/+ mice do not show LOH. That these tumors can be cleanly dissected away from normal tissue is shown by finding that a minority of tumors from B6 Apc1638N/+ mice do show LOH. Instead, as inferred by the regional distribution of these tumors (Haigis et al. 2004) and as demonstrated by Kucherlapati et al. (2007), the major pathway for tumorigenesis in Apc1638N/+ mice involves intragenic mutations in the wild-type Apc allele. Thus, the nature of the predisposing Apc allele can influence the pathway whereby the function of the wild-type allele is lost. The scenarios described here (LOH vs. intragenic Apc mutation) are not the only factors to consider in understanding allelic specificity of the intestinal phenotype. Dominant negative alleles, haploinsufficiency, alternative splicing, and variable levels of Wnt signaling each have been raised as possible contributing factors to allele-specific effects on intestinal multiplicity (Solomon et al. 1987; Thliveris et al. 1994; Samowitz et al. 1995; Rowan et al. 2000; Albuquerque et al. 2002; Sieber et al. 2002).
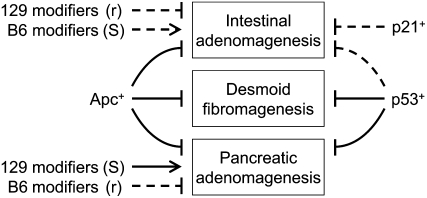
Inspection of Table 1 and Figure 3 shows several distinct patterns of interaction between the two tumor suppressor genes Apc and p53. First, for intestinal tumorigenesis, the dependence on Apc deficiency is complete within the range of variables tested in this study. However, genetic background affects the influence of p53 deficiency. Second, for desmoid fibromas, either Apc or p53 function can suppress development but no significant effects of genetic background have been observed. Finally, for acinar pancreatic neoplasms, either Apc or p53 function can suppress the pathway, but here there is a strong effect of genetic background. The instances of desmoid fibromagenesis and acinar pancreatic adenocarcinomagenesis, in which either Apc or p53 activity is sufficient to suppress a neoplastic pathway, provide formal examples of functional redundancy, as elaborated in the notion of robustness (Halberg et al. 2000; Hartwell 2004).
Figure 3.—
The development of three distinct neoplasms in the laboratory mouse is affected by Apc, p21, p53, and modifiers that are polymorphic between 129 and B6. The tumor suppressor genes and polymorphic modifiers can have a partial (dashed line) or complete (solid line) effect. Whether modifiers act in a recessive fashion to resist (r) or a dominant fashion to enhance susceptibility (S) was ascertained by analyzing two parental strains and their F1 hybrid.
One interpretation of the data in Table 2 is that p21 alone can mediate a portion of the p53 effect on intestinal tumorigenesis. But note that p21 does not regulate desmoid fibromagenesis or pancreatic neoplasia. An interesting possibility is that p21 function is not important for specific neoplastic pathways in the intestine—the microsatellite instability (MSI) and CpG island methylator (CIMP) pathways (see Ogino et al. 2006). By extrapolation of this hypothesis, desmoid fibromagenesis and acinar pancreatic neoplasia would share features with the MSI and CIMP intestinal pathways and not with the LOH pathway. Evaluation of the silencing pathway in these several Apc-dependent neoplastic processes may shed light on this possibility.
Alternatively, p21 might act independently of p53 to affect intestinal tumorigenesis in Apc mutants. Chen et al. (2004) demonstrated that clusterin-mediated apoptosis is independent of p53 but dependent on p21. Clusterin expression and apoptosis were induced in HCT116 cells and HCT116 cells lacking p53 activity following treatment with 5-fluorouracil. By contrast, clusterin was not induced and apoptosis was inhibited in HCT116 cells lacking p21 activity. Action of p21 independent of p53 could explain why the dominant 129 modifier system supplants the p53 effect on intestinal tumorigenesis in ApcMin/+ mice but not the p21 effect.
We are interested in the consistent observation of a subtle but significant change in multiplicity of intestinal tumors in ApcMin/+ observed in both homozygous and heterozygous p53 deficiency. These effects can be detected only by analyzing a cohort of mice with a homogenous genetic background, where the random segregation of polymorphic modifiers has been eliminated. These observations point to an important basic issue in the genetics of “tumor suppressor” genes: Do they commonly have a heterozygous mutant phenotype, even if finally the emergent tumor is homozygous for the mutant allele or doubly heterozygous for two mutant alleles?
Normal regulation in the intestine and pancreas of p53-deficient ApcMin/+ mice is partially restored by modifiers polymorphic between 129 and B6. ApcMin/+ p53−/− mice develop significantly more intestinal tumors than ApcMin/+ p53+/+ controls on B6 and (B6 × 129)F1, but not on the 129 genetic background (Table 1 and Figure 3). Thus, homozygosity for a recessive 129 allele or alleles blunts the enhancement of intestinal adenomagenesis observed in p53-deficient backgrounds. An analogous situation exists in the pancreas. 129 and (B6 × 129)F1 ApcMin/+ p53−/− mice develop massive adenocarcinomas in the pancreas, whereas B6 ApcMin/+ p53−/− mice develop only microscopic adenomas (Table 1 and Figure 3). In this case, homozygosity for a recessive B6 allele attenuates the enhancement of acinar pancreatic tumorigenesis when both Apc and p53 are mutated. These polymorphic differences between B6 and 129 apparently cannot be explained by differences in the coding region of either p21 or p53. The 129S6/SvEvTac strain has not been resequenced, but the 129S1 and 129X1 strains do not show any coding-region differences from B6 in either p21 or p53 (dbSNP database build 129, http://www.ncbi.nlm.nih.gov/SNP/).
Recessive resistance may reflect a polymorphism that reduces or eliminates the activity of a tumor-promoting factor. For example, a single-nucleotide polymorphism in the promoter of the human ornithine decarboxylase (ODC) gene that reduces the level of ODC expression also reduces the risk of adenoma recurrence in patients using aspirin for chemoprevention of colonic neoplasia (Erdman et al. 1999). Consistent with this observation, treatment of ApcMin/+ mice with dimethylfluoroornithine, a suicide inhibitor of ODC activity, reduces tumor multiplicity in the small intestine by a factor of two (Martinez et al. 2003). High-resolution mapping and positional cloning are required to identify the proposed modifiers polymorphic between B6 and 129 affecting tumorigenesis in ApcMin/+ p53−/− mice. These modifiers may be novel targets for chemotherapeutics because some human pancreatic tumors lack Apc and p53 activity (Abraham et al. 2001, 2002; Dong et al. 2005). Thus, polymorphic modifiers can profoundly affect tumorigenesis in a specific tissue pattern under certain genetic conditions that predispose mice to cancer, emphasizing further the importance of characterizing mutant alleles on a series of homogenous genetic backgrounds—for example, as reported here, on two distinct inbred backgrounds and the F1 between them.
Acknowledgments
We thank Harlene Edwards, Jen Triemstra, and Jane Weeks for highly capable technical assistance and Jeff Bacher, Norman Drinkwater, Paul Lambert, and Alexandra Shedlovsky for critical review of this manuscript. This is publication no. 3640 from the Laboratory of Genetics. This work was supported by grants R37 CA63677 and U01 CA-58085 (The Mouse Models of Human Cancer Consortium) from the National Institutes of Health.
References
- Abraham, S. C., T. T. Wu, D. S. Klimstra, L. S. Finn, J. H. Lee et al., 2001. Distinctive molecular genetic alterations in sporadic and familial adenomatous polyposis-associated pancreatoblastomas: frequent alterations in the APC/beta-catenin pathway and chromosome 11p. Am. J. Pathol. 159 1619–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham, S. C., T. T. Wu, R. H. Hruban, J. H. Lee, C. J. Yeo et al., 2002. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am. J. Pathol. 160 953–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque, C., C. Breukel, L. R. van der Luijt, P. Fidalgo, P. Lage et al., 2002. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade. Hum. Mol. Genet. 11 1549–1560. [DOI] [PubMed] [Google Scholar]
- Baran, A. A., K. A. Silverman, J. Zeskand, R. Koratkar, A. Palmer et al., 2007. The modifier of Min 2 (Mom2) locus: embryonic lethality of a mutation in the Atp5a1 gene suggests a novel mechanism of polyp suppression. Genome Res. 17 566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertario, L., A. Russo, P. Sala, M. Eboli, M. Giarola et al., 2001. Genotype and phenotype factors as determinants of desmoid tumors in patients with familial adenomatous polyposis. Int. J. Cancer 95 102–107. [DOI] [PubMed] [Google Scholar]
- Bertario, L., A. Russo, P. Sala, L. Varesco, M. Giarola et al., 2003. Multiple approach to the exploration of genotype-phenotype correlations in familial adenomatous polyposis. J. Clin. Oncol. 21 1698–1707. [DOI] [PubMed] [Google Scholar]
- Chen, T., J. Turner, S. McCarthy, M. Scaltriti, S. Bettuzzi et al., 2004. Clusterin-mediated apoptosis is regulated by adenomatous polyposis coli and is p21 dependent but p53 independent. Cancer Res. 64 7412–7419. [DOI] [PubMed] [Google Scholar]
- Clarke, A. R., M. C. Cummings and D. J. Harrison, 1995. Interaction between murine germline mutations in p53 and APC predisposes to pancreatic neoplasia but not to increased intestinal malignancy. Oncogene 11 1913–1920. [PubMed] [Google Scholar]
- Cormier, R. T., and W. F. Dove, 2000. Dnmt1N/+ reduces the net growth rate and multiplicity of intestinal adenomas in C57BL/6-multiple intestinal neoplasia (Min)/+ mice independently of p53 but demonstrates strong synergy with the modifier of Min 1(AKR) resistance allele. Cancer Res. 60 3965–3970. [PubMed] [Google Scholar]
- Croner, R. S., W. M. Brueckl, B. Reingruber, W. Hohenberger and K. Guenther, 2005. Age and manifestation related symptoms in familial adenomatous polyposis. BMC Cancer 5 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries, A., E. R. Flores, B. Miranda, H. M. Hsieh, C. T. van Oostrom et al., 2002. Targeted point mutations of p53 lead to dominant-negative inhibition of wild-type p53 function. Proc. Natl. Acad. Sci. USA 99 2948–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower, L. A., M. Harvey, B. L. Slagle, M. J. McArthur, C. A. Montgomery, Jr. et al., 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356 215–221. [DOI] [PubMed] [Google Scholar]
- Dong, M., G. Ma, W. Tu, K. J. Guo, Y. L. Tian et al., 2005. Clinicopathological significance of p53 and mdm2 protein expression in human pancreatic cancer. World J. Gastroenterol. 11 2162–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto, M., M. Konishi, T. Iwama, J. Utsunomiya, K. I. Sugihara et al., 2000. The relationship between frequencies of extracolonic manifestations and the position of APC germline mutation in patients with familial adenomatous polyposis. Jpn. J. Clin. Oncol. 30 82–88. [DOI] [PubMed] [Google Scholar]
- Erdman, S. H., N. A. Ignatenko, M. B. Powell, K. A. Blohm-Mangone, H. Holubec et al., 1999. APC-dependent changes in expression of genes influencing polyamine metabolism, and consequences for gastrointestinal carcinogenesis, in the Min mouse. Carcinogenesis 20 1709–1713. [DOI] [PubMed] [Google Scholar]
- Fearon, E. R., 1997. Human cancer syndromes: clues to the origin and nature of cancer. Science 278 1043–1050. [DOI] [PubMed] [Google Scholar]
- Garber, J. E., and K. Offit, 2005. Hereditary cancer predisposition syndromes. J. Clin. Oncol. 23 276–292. [DOI] [PubMed] [Google Scholar]
- Giardiello, F. M., G. J. Offerhaus, D. H. Lee, A. J. Krush, A. C. Tersmette et al., 1993. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut 34 1394–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, K. A., W. F. Dietrich, N. Borenstein, E. S. Lander and W. F. Dove, 1996. Mom1 is a semi-dominant modifier of intestinal adenoma size and multiplicity in Min/+ mice. Genetics 144 1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groden, J., A. Thliveris, W. Samowitz, M. Carlson, L. Gelbert et al., 1991. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 66 589–600. [DOI] [PubMed] [Google Scholar]
- Gurbuz, A. K., F. M. Giardiello, G. M. Petersen, A. J. Krush, G. J. Offerhaus et al., 1994. Desmoid tumours in familial adenomatous polyposis. Gut 35 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis, K. M., and W. F. Dove, 2003. A Robertsonian translocation suppresses a somatic recombination pathway to loss of heterozygosity. Nat. Genet. 33 33–39. [DOI] [PubMed] [Google Scholar]
- Haigis, K. M., J. G. Caya, M. Reichelderfer and W. F. Dove, 2002. Intestinal adenomas can develop with a stable karyotype and stable microsatellites. Proc. Natl. Acad. Sci. USA 99 8927–8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis, K. M., P. D. Hoff, A. White, A. R. Shoemaker, R. B. Halberg et al., 2004. Tumor regionality in the mouse intestine reflects the mechanism of loss of Apc function. Proc. Natl. Acad. Sci. USA 101 9769–9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg, R. B., D. S. Katzung, P. D. Hoff, A. R. Moser, C. E. Cole et al., 2000. Tumorigenesis in the multiple intestinal neoplasia mouse: redundancy of negative regulators and specificity of modifiers. Proc. Natl. Acad. Sci. USA 97 3461–3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S. L., and A. J. Levine, 2005. The p53 pathway: positive and negative feedback loops. Oncogene 24 2899–2908. [DOI] [PubMed] [Google Scholar]
- Hartwell, L., 2004. Genetics. Robust interactions. Science 303 774–775. [DOI] [PubMed] [Google Scholar]
- Hirata, A., T. Tsukamoto, M. Yamamoto, H. Sakai, T. Yanai et al., 2005. Organ-specific susceptibility of p53 knockout mice to N-bis(2-hydroxypropyl)nitrosamine carcinogenesis. Cancer Lett. 238 271–283. [DOI] [PubMed]
- Jacks, T., L. Remington, B. O. Williams, E. M. Schmitt, S. Halachmi et al., 1994. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 4 1–7. [DOI] [PubMed] [Google Scholar]
- Kemp, C. J., L. A. Donehower, A. Bradley and A. Balmain, 1993. Reduction of p53 gene dosage does not increase initiation or promotion but enhances malignant progression of chemically induced skin tumors. Cell 74 813–822. [DOI] [PubMed] [Google Scholar]
- Kinzler, K. W., M. C. Nilbert, L.-K. Su, B. Vogelstein, T. M. Bryan et al., 1991. Identification of FAP locus genes from chromosome 5q21. Science 253 661–665. [DOI] [PubMed] [Google Scholar]
- Kucherlapati, M., A. Nguyen, M. Kuraguchi, K. Yang, K. Fan et al., 2007. Tumor progression in Apc(1638N) mice with Exo1 and Fen1 deficiencies. Oncogene 26 6297–6306. [DOI] [PubMed] [Google Scholar]
- Kuraguchi, M., X. P. Wang, R. T. Bronson, R. Rothenberg, N. Y. Ohene-Baah et al., 2006. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2 1362–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F. P., and J. F. Fraumeni, Jr., 1969. Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann. Intern. Med. 71 747–752. [DOI] [PubMed] [Google Scholar]
- Luongo, C., A. R. Moser, S. Gledhill and W. F. Dove, 1994. Loss of Apc+ in intestinal adenomas from Min mice. Cancer Res. 54 5947–5952. [PubMed] [Google Scholar]
- Malkin, D., F. P. Li, L. C. Strong, J. F. Fraumeni, Jr., C. E. Nelson et al., 1990. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 250 1233–1238. [DOI] [PubMed] [Google Scholar]
- Martinez, M. E., T. G. O'Brien, K. E. Fultz, N. Babbar, H. Yerushalmi et al., 2003. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc. Natl. Acad. Sci. USA 100 7859–7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matushansky, I., R. G. Maki and C. C. Cardo, 2008. A context dependent role for Wnt signaling in tumorigenesis and stem cells. Cell Cycle 7 720–724. [DOI] [PubMed] [Google Scholar]
- Moser, A. R., H. C. Pitot and W. F. Dove, 1990. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247 322–324. [DOI] [PubMed] [Google Scholar]
- Moser, A. R., A. R. Shoemaker, C. S. Connelly, L. Clipson, K. A. Gould et al., 1995. Homozygosity for the Min allele of Apc results in disruption of mouse development prior to gastrulation. Dev. Dyn. 203 422–433. [DOI] [PubMed] [Google Scholar]
- Nacht, M., A. Strasser, Y. R. Chan, A. W. Harris, M. Schlissel et al., 1996. Mutations in the p53 and SCID genes cooperate in tumorigenesis. Genes Dev. 10 2055–2066. [DOI] [PubMed] [Google Scholar]
- Nagy, R., K. Sweet and C. Eng, 2004. Highly penetrant hereditary cancer syndromes. Oncogene 23 6445–6470. [DOI] [PubMed] [Google Scholar]
- Nigro, J. M., S. J. Baker, A. C. Preisinger, J. M. Jessup, R. Hostetter et al., 1989. Mutations in the p53 gene occur in diverse human tumour types. Nature 342 705–708. [DOI] [PubMed] [Google Scholar]
- Nishisho, I., Y. Nakamura, Y. Miyoshi, Y. Miki, H. Ando et al., 1991. Mutation of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 253 665–668. [DOI] [PubMed] [Google Scholar]
- Ogino, S., M. Brahmandam, T. Kawasaki, G. J. Kirkner, M. Loda et al., 2006. Epigenetic profiling of synchronous colorectal neoplasias by quantitative DNA methylation analysis. Mod. Pathol. 19 1083–1090. [DOI] [PubMed] [Google Scholar]
- Oshima, H., M. Oshima, M. Kobayashi, M. Tsutsumi and M. M. Taketo, 1997. Morphological and molecular processes of polyp formation in ApcΔ716 knockout mice. Cancer Res. 57 1644–1649. [PubMed] [Google Scholar]
- Reilly, K. M., D. A. Loisel, R. T. Bronson, M. E. McLaughlin and T. Jacks, 2000. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat. Genet. 26 109–113. [DOI] [PubMed] [Google Scholar]
- Rowan, A. J., H. Lamlum, M. Ilyas, J. Wheeler, J. Straub et al., 2000. APC mutations in sporadic colorectal tumors: a mutational “hotspot” and interdependence of the “two hits.” Proc. Natl. Acad. Sci. USA 97 3352–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, H., T. Tsukamoto, M. Yamamoto, N. Shirai, T. Iidaka et al., 2003. High susceptibility of nullizygous p53 knockout mice to colorectal tumor induction by 1,2-dimethylhydrazine. J. Cancer Res. Clin. Oncol. 129 335–340. [DOI] [PubMed] [Google Scholar]
- Samowitz, W. S., A. Thliveris, L. N. Spirio and R. White, 1995. Alternatively spliced adenomatous polyposis coli (APC) gene transcripts that delete exons mutated in attenuated APC. Cancer Res. 55 3732–3734. [PubMed] [Google Scholar]
- Shibata, H., K. Toyama, H. Shioya, M. Ito, M. Hirota et al., 1997. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science 278 120–123. [DOI] [PubMed] [Google Scholar]
- Shirai, N., T. Tsukamoto, M. Yamamoto, T. Iidaka, H. Sakai et al., 2002. Elevated susceptibility of the p53 knockout mouse esophagus to methyl-N-amylnitrosamine carcinogenesis. Carcinogenesis 23 1541–1547. [DOI] [PubMed] [Google Scholar]
- Shoemaker, A. R., A. R. Moser, C. A. Midgley, L. Clipson, M. A. Newton et al., 1998. A resistant genetic background leading to incomplete penetrance of intestinal neoplasia and reduced loss of heterozygosity in ApcMin/+ mice. Proc. Natl. Acad. Sci. USA 95 10826–10831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber, O. M., H. Lamlum, M. D. Crabtree, A. J. Rowan, E. Barclay et al., 2002. Whole-gene APC deletions cause classical familial adenomatous polyposis, but not attenuated polyposis or “multiple” colorectal adenomas. Proc. Natl. Acad. Sci. USA 99 2954–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits, R., W. Van Oordt, A. Luz, C. Zurcher, S. Jagmohan-Changur et al., 1998. Apc1638N: a mouse model for familial adenomatous polyposis-associated desmoid tumors and cutaneous cysts. Gastroenterology 114 275–283. [DOI] [PubMed] [Google Scholar]
- Smits, R., M. F. Kielman, C. Breukel, C. Zurcher, K. Neufeld et al., 1999. Apc1638T: a mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev. 13 1309–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon, E., R. Voss, V. Hall, W. F. Bodmer, J. R. Jass et al., 1987. Chromosome 5 allele loss in human colorectal carcinomas. Nature 328 616–619. [DOI] [PubMed] [Google Scholar]
- Soussi, T., and C. Beroud, 2001. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat. Rev. Cancer 1 233–240. [DOI] [PubMed] [Google Scholar]
- Su, L. K., K. W. Kinzler, B. Vogelstein, A. C. Preisinger, A. R. Moser et al., 1992. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science 256 668–670. [DOI] [PubMed] [Google Scholar]
- Thliveris, A., W. Samowitz, N. Matsunami, J. Groden and R. White, 1994. Demonstration of promoter activity and alternative splicing in the region 5′ to exon 1 of the APC gene. Cancer Res. 54 2991–2995. [PubMed] [Google Scholar]
- Xu, Y., E. M. Yang, J. Brugarolas, T. Jacks and D. Baltimore, 1998. Involvement of p53 and p21 in cellular defects and tumorigenesis in Atm−/− mice. Mol. Cell. Biol. 18 4385–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto, M., T. Tsukamoto, H. Sakai, N. Shirai, H. Ohgaki et al., 2000. p53 knockout mice (−/−) are more susceptible than (+/−) or (+/+) mice to N-methyl-N-nitrosourea stomach carcinogenesis. Carcinogenesis 21 1891–1897. [DOI] [PubMed] [Google Scholar]
- Yang, K., W. Edelmann, K. Fan, K. Lau, V. R. Kolli et al., 1997. A mouse model of human familial adenomatous polyposis. J. Exp. Zool. 277 245–254. [PubMed] [Google Scholar]
- Yang, W. C., J. Mathew, A. Velcich, W. Edelmann, R. Kucherlapati et al., 2001. Targeted inactivation of the p21WAF1/cip1 gene enhances Apc-initiated tumor formation and the tumor-promoting activity of a Western-style high-risk diet by altering cell maturation in the intestinal mucosa. Cancer Res. 61 565–569. [PubMed] [Google Scholar]