Abstract
Here we show that inactivation of the ATR-related kinase ATL-1 results in a significant reduction in mitochondrial DNA (mtDNA) copy numbers in Caenorhabditis elegans. Although ribonucleotide reductase (RNR) expression and the ATP/dATP ratio remained unaltered in atl-1 deletion mutants, inhibition of RNR by RNAi or hydroxyurea treatment caused further reductions in mtDNA copy number. These results suggest that ATL-1 functions to maintain mtDNA independently of RNR.
PROTEIN kinases in the ataxia-telangiectasia mutated (ATM) family initiate a well-characterized response to DNA damage, resulting in cell-cycle arrest, DNA repair, or apoptosis (Abraham 2001; Shiloh 2003). This family is highly conserved among eukaryotes, comprising two related proteins: ATM and ATR (AT mutant and rad3+ related) in humans; Tel1 and Mec1/Esr1 in Saccharomyces cerevisiae; Tel1 and Rad3 in Schizosaccharomyces pombe; and ATM-1 and ATL-1 in Caenorhabditis elegans (Jimenez et al. 1992; Kato and Ogawa 1994; Weinert et al. 1994; Keith and Schreiber 1995; Lavin et al. 1995; Morrow et al. 1995; Savitsky et al. 1995; Keegan et al. 1996; Aoki et al. 2000; Boulton et al. 2002). These proteins exhibit distinct, but partially overlapping biological functions (Morrow et al. 1995; Cliby et al. 1998; Ritchie et al. 1999). Ataxia telangiectasia (AT) is a human autosomal recessive disease caused by mutations in ATM, which result in a wide variety of symptoms; its hallmarks include progressive neuronal degeneration, oculocutaneous telangiectasias, immune dysfunction, predisposition to cancer, incomplete sexual maturation, endocrine abnormalities, and premature aging of the skin and hair (Boder 1975; Shiloh 2001). An aberrant DNA damage response appears to cause immune dysfunction, cancer predisposition, and incomplete sexual maturation, but may not be sufficient to explain all the symptoms of the disease (Shiloh 2001).
In budding yeast, the ATR pathway, governed by Mec1 and Rad53, is essential for cell growth and the DNA damage checkpoint response (Zheng et al. 1993; Kato and Ogawa 1994; Weinert et al. 1994; Sun et al. 1996; Zhao et al. 1998). The lethality of mec1 or rad53 deletion mutants is suppressed by a mutation in sml1, which encodes an inhibitor of ribonucleotide reductase (RNR) (Zhao et al. 1998). RNR is a rate-limiting enzyme in de novo synthesis of deoxynucleoside triphosphates (dNTP), suggesting that mutation of sml1 allows mec1 cells to survive by resulting in increased RNR activity and dNTP levels. Sml1 overproduction frequently causes the formation of petit colonies, due to loss of mitochondria, indicating that decreases in dNTP levels preferentially affect mitochondrial DNA (mtDNA) replication in comparison to chromosomal DNA (chrDNA) replication, which is more mildly affected (Zhao et al. 1998). The Mec1 and Rad53 checkpoint pathways, therefore, regulate mtDNA copy number (Taylor et al. 2005). However, Sml1-like proteins have not been isolated from other organisms and the effect of ATR on dNTP pools or mtDNA copy number has not been examined in metazoans.
To investigate whether C. elegans checkpoint control-related genes participate in mtDNA maintenance, we compared mtDNA copy numbers in wild-type, atl-1(tm853, ATR homolog), atm-1(gk186, ATM homolog), and cep-1(w40, p53 homolog) adult hermaphrodites [3 days old from the laid egg (3d): young adult stage] using real-time PCR and normalizing against chrDNA copy number (Sugimoto et al. 2008). atl-1(tm853) and atm-1(gk186) delete a 720-bp region within the exon-7 and a 548-bp region within the parts of intron 1 and exon 2, respectively (http://www.wormbase.org). Both mutations result in frame shifts, which prematurely terminate translation prior to the catalytic center of the respective protein kinase. Thus, both mutations are likely to constitute null alleles (Garcia-Muse and Boulton 2005). The cep-1(w40) mutant strain contains an intact copy of cep-1 at the normal locus, and a 1832-bp deletion encoding a truncated protein lacking the DNA binding domain translocated to elsewhere in the genome (Derry et al. 2001). atl-1(tm853), cep-1(w40), and atm-1(gk186) homozygotes exhibit defects in DNA damage-induced germ cell apoptosis (Derry et al. 2001; Stergiou et al. 2007). For atl-1(tm853) and cep-1(w40), these defects are dosage sensitive: both atl-1(tm853)/+ and cep-1(w40)/+ heterozygotes exhibit reduced levels of germ cell apoptosis in response to DNA damage (Derry et al. 2001; Stergiou et al. 2007). The atm-1- and the cep-1-defective homozygotes were viable and fertile. Although atl-1(tm853) homozygotes appeared to develop into normal adults, their eggs did not hatch, dying during early embryogenesis (Garcia-Muse and Boulton 2005). Garcia-Muse and Boulton (2005) also show that atl-1(tm853) causes mitotic catastrophe and loses the S-phase checkpoint and the atm-1 cooperative checkpoint response to DNA double-strand breaks to induce cell-cycle arrest or apoptosis via the cep-1 pathway. In addition, atl-1(RNAi) affects asymmetric division at the two-cell stage of embryonic development; moreover, atl-1(RNAi) frequently produces male (XO) progeny due to nondisjunction of the X chromosome at meiosis I (the Him phenotype, for high incidence of males) (Aoki et al. 2000; Boulton et al. 2002; Brauchle et al. 2003).
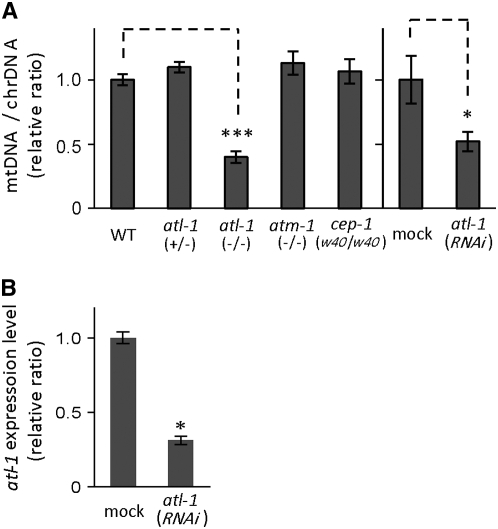
In atl-1(tm853) homozygous mutant hermaphrodites, we observed a substantial decrease in relative mtDNA copy number (to less than half of wild-type levels), whereas no reductions were detected in atl-1(tm853)/+ heterozygotes or mutants defective for atm-1 and cep-1 (Figure 1A). Similarly, mtDNA levels decreased significantly (by half) when RNAi feeding was used to silence 80% of atl-1 expression (Figure 1, A and B).
Figure 1.—
Relative ratios of mtDNA to chrDNA in adult hermaphrodites with defects in checkpoint-related genes. (A) The mtDNA copy numbers were quantified by normalizing amplifications of mtDNA and chrDNA. C. elegans N2 wild-type hermaphrodites, as well as the following strains: JR1279 cep-1(w40); VC381 atm-1(gk186); and HS1208 atl-1(tm853)/nT1 (HS1208), prepared from an atl-1(tm853)/+ (FX853) and unc-76/nT1 male were used. Worm lysates from young adult hermaphrodites (3d) were prepared as described previously (Tsang and Lemire 2002). Real-time quantitative PCR was performed using SYBER Premix Ex Taq (TaKaRa). The primer sets used to amplify mtDNA and chrDNA were described previously (Sugimoto et al. 2008). A slight modification was made to the RNAi feeding method (Kamath et al. 2001). The atl-1 RNAi feeding plate was changed twice a day during the parental generation, after which the F1 progeny were fed on RNAi-containing bacteria during the L4 larval stage, in order to maintain continuous feeding across generations (Sugimoto et al. 2008). Escherichia coli HT115 (DE3) (Kamath et al. 2001) expressing ∼930 bp atl-1 dsRNA (68-1001) cloned with LITMUS28 plasmid vector (New England Biolabs) and E. coli HT115 (DE3) harboring the LITMUS28 plasmid (mock control) were used. Experiments were performed on F2 generations at the young adult stage (24 hr from the L4 larval stage). (B) The expression levels of the atl-1 gene were monitored by real-time quantitative RT–PCR as compared with the expression level of an elongation factor eft-2. Total RNAs were isolated from the young adult stage in the atl-1 RNAi and mock RNAi. A mock feeding control was performed using E. coli HT115 (DE3) harboring the LITMUS28 plasmid. The following primer sets were used to amplify atl-1: (forward) 5′-CAG TTT GGC TTC GAT TGC TC and (reverse) 5′-TGA AGC TGG TCC TCT GTC TG, and eft-2: (forward) 5′-GAC GCT ATC CAC AGA GGA GG and (reverse) 5′-TTC CTG TGA CCT GAG ACT CC. Real-time PCR experiments were performed in triplicate for each sample. Vertical bars indicate standard error. * and *** indicate statistical significance at P < 0.05 and < 0.001, respectively (Student's t-test).
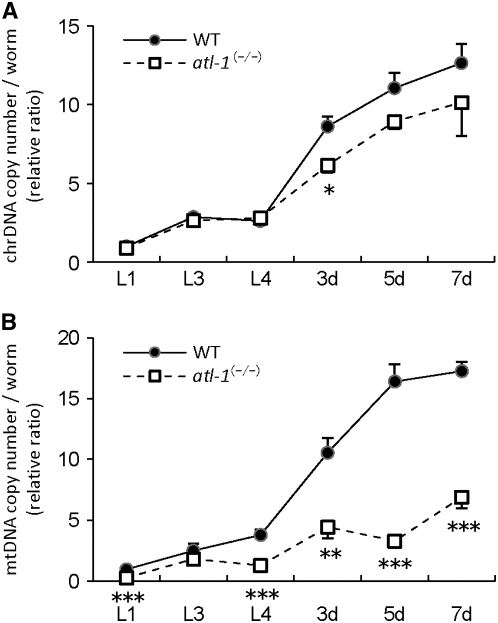
We examined the copy number of mtDNA and chrDNA in atl-1(tm853) and wild-type hermaphrodites at sequential developmental stages (Figure 2). Both mtDNA and chrDNA copy numbers increased from the L4 to the adult stage in wild-type hermaphrodites (Figure 2). In C. elegans, somatic cell proliferation is nearly completed prior to hatching (550 somatic cells at hatching vs. 959 in adults), and germline proliferation most robustly occurs in the L4 and adult stages (Schedl 1997). Maternally derived mtDNA remains unchanged before the early L3 larval stage and thereafter increases significantly in association with germline proliferation (Tsang and Lemire 2002). atl-1(tm853) homozygotes exhibited a reduced rate of mtDNA accumulation compared to the wild type (Figure 2B). By contrast, chrDNA accumulation was unaffected (Figure 2A). These results suggest that ATL-1 is involved in effective mtDNA replication during germline proliferation.
Figure 2.—
atl-1 is required for effective mtDNA replication during germline proliferation. The copy numbers of chrDNA (A) or mtDNA (B) in wild-type hermaphrodites and atl-1(tm853) homozygous mutants were measured using real-time PCR; data were normalized relative to wild-type L1 stage larvae. Total DNA was isolated from L1, L3, and L4 larvae, as well as from 3-, 5-, and 7-day-old hermaphrodites. Values represent means obtained from five synchronized but independent worms. Vertical bars indicate standard error. *, **, and *** show statistical significance at P < 0.05, <0.01, and < 0.001, respectively (Student's t-test).
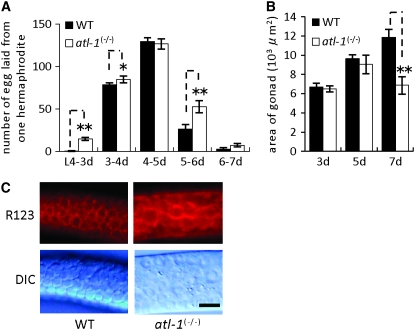
Following self-fertilization, a single hermaphrodite will lay ∼250–300 eggs. After hatching, these individuals grow to adulthood in ∼3 days at 20°, passing through four larval stages (L1–L4). The timing and rate of egg production were the same or slightly faster in the atl-1(tm853) homozygotes than in the wild-type individuals (Figure 3A), and we observed no significant difference in their gonadal development (Figure 3B). Rhodamine-123 fluorescence staining indicated very similar mitochondrial membrane potentials in the gonads of the atl-1(tm853) homozygote and wild-type worms (Figure 3C), despite the former's marked reduction in mtDNA (Figure 2B). C. elegans produces more mtDNA than it requires; ∼25% mtDNA is sufficient for gonadal development, whereas 10% mtDNA is not (Sugimoto et al. 2008). Interestingly, atl-1(tm853) germ cells were irregular in both shape and size (Figure 3C), presumably as a result of defects in the cell cycle and in chromosome segregation (Aoki et al. 2000; Garcia-Muse and Boulton 2005). In the future, it will be important to analyze mitochondrial numbers and morphology in atl-1(tm853) mutants.
Figure 3.—
atl-1 is not required for gonadal development or mitochondrial function. Numbers of eggs laid by a single hermaphrodite (A) and gonadal size from the tip to hinge site (B) in adult hermaphrodites (3, 5, and 7 days old) of wild-type and atl-1 homozygotes. Vertical bars indicate standard error. * and ** show statistical significance at P < 0.05 and < 0.01, respectively (Student's t-test). (C) Mitochondrial membrane potential in germ cells from the meiotic pachytene region of wild-type and atl-1 homozygotes (3-day-old adult hermaphrodites), stained with rhodamine 123 fluorescence, as described previously (Sugimoto et al. 2008). Bar represents 10 μm.
Depletion or inhibition of RNR activity strongly suppresses mtDNA replication; in contrast, chrDNA replication is less severely affected under normal growth conditions in mammalian cells (Eaton et al. 2007). RNR contains a large (R1) and small [R2 or p53-inducible R2 (p53R2)] subunit, both of which are essential for maintenance of mtDNA copy number (Bourdon et al. 2007; Eaton et al. 2007). Eaton et al. (2007) also reported that mammalian ATM regulates RNR expression and human atm primary fibroblasts, which were derived from atm patients, exhibit lower steady-state levels of R1 and higher levels of p53R2. This decrease in RNR levels in atm mutants appears to cause reduced mtDNA copy numbers in actively dividing cells but not in quiescent cells. Similarly, lower R1 levels were found in all tissues of atm-deficient knockout mice, but reduced mtDNA levels were only observed in some of these tissues (Eaton et al. 2007). Therefore, it is difficult to explain fully the control of mtDNA copy number in the mammalian system.
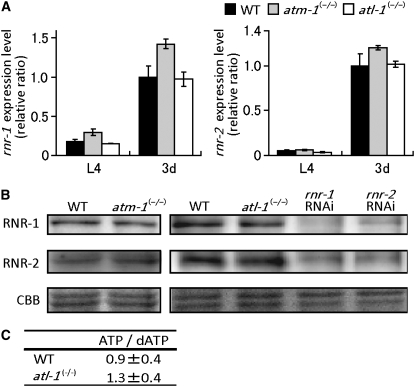
C. elegans RNR comprises two subunits, RNR-1 (large subunit) and RNR-2 (small subunit) (Hong et al. 1998; van den Heuvel 2005). In addition, its genome contains the gene F19G12.2, which encodes a protein with greater similarity to mammalian p53R2 than R2. Although F19G12.2 expression is not induced by the DNA damage response, it is strongly expressed in males and L1 hermaphrodite larvae, but found rarely in adult hermaphrodites (Jiang et al. 2001; Roy et al. 2002). Therefore, it appears that p53R2 may be a vertebrate-specific protein. To determine whether the C. elegans AT family proteins regulate expression of either rnr-1 or rnr-2, we performed quantitative analyses of gene expression using real-time RT–PCR and Western blots. We observed significant upregulation of rnr-1 and rnr-2 transcription during germline proliferation in wild-type hermaphrodites between the L4 larval and young adult stages (Figure 4A). Transcriptional upregulation occurred normally in atm-1(gk186) and atl-1(tm853) mutant homozygotes (Figure 4A) and Western blot analyses did not reveal reductions in RNR-1 or RNR-2 protein levels in atm-1(gk186) or atl-1(tm853) mutants (Figure 4B). We also measured ATP/dATP ratios in atl-1(tm853) mutants using LC-MS/MS on an API-400 instrument (Applied Biosystems). We observed no significant differences between the ATP/dATP ratios of wild-type and atl-1(tm853) homozygotes (P-value = 0.54, Student's t-test, Figure 4C). In contrast, in S. cerevisiae, dNTP levels increase twofold in sml1 null mutants (Zhao et al. 1998). Taken together, these results suggest that C. elegans ATL-1 affects mtDNA copy number without affecting the steady state RNR levels.
Figure 4.—
atm-1 and atl-1 are not required for RNR expression. (A) The relative transcriptional levels of rnr-1 and rnr-2 were normalized using eft-2 expression. Total RNAs were isolated from L4 and 3-day-old hermaphrodites of wild-type, atm-1, and atl-1 homozygotes. Real-time PCR experiments were performed in triplicate for each sample. The following primer sets were used to amplify rnr-1: (forward) 5′-GCG AGT CGA GAA GGA TCA AG and (reverse) 5′-GGC TTC GTA TTT GGC GTA AA, rnr-2: (forward) 5′-ATC AGT GCC GAA ACA CTC ATC and (reverse) 5′-CAG CTC ATT CAC CTC CGA CT, and eft-2 as described in the legend of Figure 1. Vertical bars indicate standard error. (B) Western blot analysis of RNR-1 and RNR-2 protein levels. Wild-type, atm-1 and atl-1 homozygous mutant hermaphrodites (3-day-old) and rnr-1 and rnr-2 RNAi hermaphrodites were boiled for 5 min in 2× SDS-loading buffer (63 mm Tris-HCl [pH 6.8], 4% SDS, 5% β-mercaptoethanol, 20% glycerol) and then homogenized with a sonicator for 30 sec. The RNAi samples were obtained from the young adults (3 days from L1) that had been fed from the L1 larval stage on E. coli HT115 (DE3) expressing rnr-1 dsRNA (541-1559) or rnr-2 dsRNA (254-1397) (Kamath et al. 2001). Total protein from 25 adult hermaphrodites was separated by discontinuous 8 or 12% SDS–PAGE and then electrotransferred to Hybond ECL nitrocellulose membranes (GE Healthcare). Immunochemical hybridization was performed using anti-RNR-1 (Santa Cruz Biotechnology) and anti-RNR-2 (Calbiochem) polyclonal antibodies, with horseradish peroxidase-coupled secondary antibodies of donkey anti-goat IgG (AP-180P, Chemicon) and goat anti-rabbit IgG (GE Healthcare), respectively. Signals were visualized using the ECL Plus Western blotting detection system (GE Healthcare) and quantified with a Fuji LAS 1000 digital image analyzer (Fuji film). Coomassie Brilliant Blue (CBB) staining of major proteins was used as an internal standard. (C) The ATP/dATP ratios of wild-type hermaphrodites and atl-1(tm853) homozygous mutants were measured using LC-MS/MS. Approximately 1000 wild-type hermaphrodites and atl-1(tm853) homozygous mutants (3 days old) were suspended in PBS containing 2% SDS and boiled for 5 min, followed by homogenization with a sonicator for 30 sec. The extract was deproteinized with TCA (final concentration of 5%), vortexed, placed in an ice bath to incubate for 10 min, and then revortexed. The supernatant was collected, neutralized with potassium carbonate, and filtered through a 0.45 μm membrane filter. Finally, the extract was purified by centrifugal ultrafiltration using a 30,000 MW cutoff membrane (Amicon YM-3, Millipore).
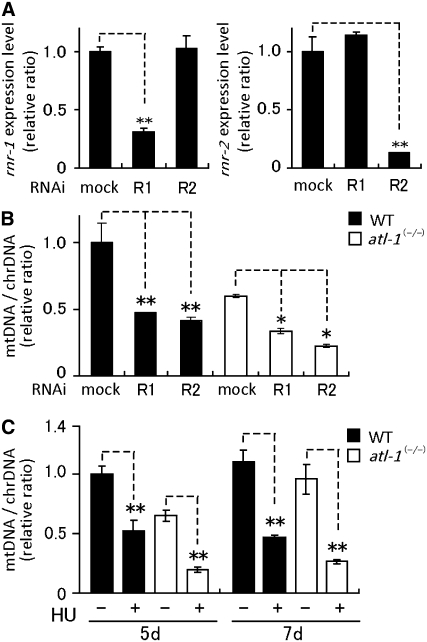
To determine if C. elegans RNR activity affects mtDNA copy numbers, we performed knockdown of rnr-1 or rnr-2 expression by feeding animals with gene-specific RNAi and treatment of an RNR inhibitor, hydroxyurea (HU). Measurement of mRNA levels for rnr-1 or rnr-2 showed that the RNAi treatment was efficacious, reducing rnr-1 mRNA levels by 70% and rnr-2 mRNA levels by 90% (Figure 5A). Both rnr-1(RNAi) and rnr-2(RNAi) resulted in reductions in the mtDNA copy number (Figure 5B). Both rnr-1(RNAi) and rnr-2(RNAi) hermaphrodites also exhibited a protruding vulva and sterility (data not shown). At the protein level, RNR-1 and RNR-2 subunits appear to be coordinately regulated, as rnr-1(RNAi) reduced RNR-2 levels and rnr-2(RNAi) reduced RNR-1 levels (Figure 4B). Since rnr-1(RNAi) does not affect rnr-2 mRNA levels, and vice versa (Figure 5A), complex formation may be important for the accumulation of both RNR subunits. The treatment of wild-type hermaphrodites with 30 mm HU resulted in a reduction of mtDNA at each developmental stage (Figure 5C). Following RNR inhibition by HU treatment or rnr-1(RNAi) or rnr-2(RNAi), atl-1(tm853) homozygotes exhibited further reductions in mtDNA levels (Figure 5, B and C). Taken together, our results indicate that C. elegans RNR is necessary for mtDNA replication during germline proliferation, but that RNR levels are not dependent on ATL-1 function. Thus, ATL-1 likely affects mtDNA levels by a different mechanism, the nature of which is unclear.
Figure 5.—
Depletion or inhibition of RNR causes a reduction in mtDNA copy number. (A) The expression levels of the rnr-1 and rnr-2 genes in either rnr-1(RNAi) (R1) or rnr-2(RNAi) (R2) were monitored by real-time quantitative RT–PCR as compared with the expression level of an elongation factor eft-2. The primer sets were used as described in the legend of Figure 4. (B) Relative ratios of mtDNA to chrDNA copy numbers in wild-type and atl-1(tm853) homozygotes with RNAi-depleted rnr-1 or rnr-2. (C) Effect of HU treatment on mtDNA copy number in wild-type and atl-1(tm853) hermaphrodites. Three-day-old young adult hermaphrodites were cultured for 2–4 days on NGM plates containing 30 mm HU, and then mtDNA and chrDNA were isolated from 5- and 7-day-old hermaphrodites. Real-time PCR amplifications were performed in triplicate for each sample. Vertical bars indicate standard error. * and ** show statistical significance at P < 0.05 and < 0.01, respectively (Student's t-test).
In addition, we used quantitative real-time RT–PCR to investigate whether or not ATL-1 regulates the transcriptional levels of other proteins involved in mtDNA replication, including DNA polymerase gamma (Y57A10A.15 gene), mitochondrial single-stranded DNA-binding protein (mtss-1), and the predicted mitochondrial transcription factor A (hmg-5). However, we found no evidence to indicate that ATL-1 regulates their transcription (P-values were 0.94, 0.68, and 0.88, respectively).
Mammalian ATM regulates mtDNA copy number through RNR expression levels, and p53R2 is essential for maintenance of mtDNA (Bourdon et al. 2007; Eaton et al. 2007). In addition, Fu et al. (2008) recently reported that phosphorylation of AMP-activated protein kinase by ATM controls mitochondrial biogenesis in response to DNA damage. In multicellular organisms, there is growing evidence of mtDNA maintenance controlled by checkpoint related proteins but not ATR. Our study suggests that the C. elegans checkpoint protein ATL-1 participates in mtDNA replication by a mechanism that is separate from the control of dNTP pools or RNR protein levels.
ATR-type proteins are checkpoint factors that control the nuclear DNA replication fork by phosphorylation of proteins such as RPA2, which binds to nuclear single-stranded DNA (Olson et al. 2006). This work provides some insight into the roles played by ATR in maintaining mtDNA copy numbers in higher eukaryotes. It is possible that ATL-1 regulates the efficiency of mtDNA replication by phosphorylating protein(s) involved in the replication process, such as DNA polymerase γ and mitochondrial single-stranded DNA binding protein. Mitochondria contain their own DNA, which encodes proteins that are essential for the respiratory chain machinery. Thus, mitochondria must undergo DNA replication prior to cell proliferation. Since the copy number of mtDNA increases during late G0/G1 and early S phase (Trinei et al. 2006), specific signals must stimulate mtDNA replication to enable synchronization with the cell cycle. Our future experiments will focus on the molecular mechanism(s) underlying the coordination of mtDNA and chrDNA replication by ATR.
Acknowledgments
We are grateful to Tomoko Sugimoto and Nahoko Higashitani for helpful suggestions and discussions. We also thank Yukinobu Arata and Hitoshi Sawa of RIKEN, Center for Developmental Biology, Shohei Mitani from the National BioResource Project for kindly supplying the HS1208 atl-1(tm853)/nT1 (HS1208), and the Caenorhabditis elegans Genetic Center for the kind supply of mutant strains. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and from the Japan Society for the Promotion of Science (JSPS). This study was also performed as part of the “Ground-Based Research Announcement for Space Utilization,” promoted by the Japan Space Forum. In addition, C.M. was supported by the International Advanced Research and Education Organization in Tohoku University.
References
- Abraham, R. T., 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15 2177–2196. [DOI] [PubMed] [Google Scholar]
- Aoki, H., S. Sato, T. Takanami, T. Ishihara, I. Katsura et al., 2000. Characterization of Ce-atl-1, an ATM-like gene from Caenorhabditis elegans. Mol. Gen. Genet. 264 119–126. [DOI] [PubMed] [Google Scholar]
- Boder, E., 1975. Ataxia-telangiectasia: some historic, clinical and pathologic observations. Birth Defects Orig. Artic. Ser. 11 255–270. [PubMed] [Google Scholar]
- Boulton, S. J., A. Gartner, J. Reboul, P. Vaglio, N. Dyson et al., 2002. Combined functional genomic maps of the C. elegans DNA damage response. Science 295 127–131. [DOI] [PubMed] [Google Scholar]
- Bourdon, A., L. Minai, V. Serre, J. P. Jais, E. Sarzi et al., 2007. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat. Genet. 39 776–780. [DOI] [PubMed] [Google Scholar]
- Brauchle, M., K. Baumer and P. Gonczy, 2003. Differential activation of the DNA replication checkpoint contributes to asynchrony of cell division in C. elegans embryos. Curr. Biol. 13 819–827. [DOI] [PubMed] [Google Scholar]
- Cliby, W. A., C. J. Roberts, K. A. Cimprich, C. M. Stringer, J. R. Lamb et al., 1998. Overexpression of a kinase-inactive ATR protein causes sensitivity to DNA-damaging agents and defects in cell cycle checkpoints. EMBO J. 17 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry, W. B., A. P. Putzke and J. H. Rothman, 2001. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294 591–595. [DOI] [PubMed] [Google Scholar]
- Eaton, J. S., Z. P. Lin, A. C. Sartorelli, N. D. Bonawitz and G. S. Shadel, 2007. Ataxia-telangiectasia mutated kinase regulates ribonucleotide reductase and mitochondrial homeostasis. J. Clin. Invest. 117 2723–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, X., S. Wan, Y. L. Lyu, L. F. Liu and H. Qi, 2008. Etoposide induces ATM-dependent mitochondrial biogenesis through AMPK activation. PLoS ONE 3 e2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Muse, T., and S. J. Boulton, 2005. Distinct modes of ATR activation after replication stress and DNA double-strand breaks in Caenorhabditis elegans. EMBO J. 24 4345–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, Y., R. Roy and V. Ambros, 1998. Developmental regulation of a cyclin-dependent kinase inhibitor controls postembryonic cell cycle progression in Caenorhabditis elegans. Development 125 3585–3597. [DOI] [PubMed] [Google Scholar]
- Jiang, M., J. Ryu, M. Kiraly, K. Duke, V. Reinke et al., 2001. Genome-wide analysis of developmental and sex-regulated gene expression profiles in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 98 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez, G., J. Yucel, R. Rowley and S. Subramani, 1992. The rad3+ gene of Schizosaccharomyces pombe is involved in multiple checkpoint functions and in DNA repair. Proc. Natl. Acad. Sci. USA 89 4952–4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath, R. S., M. Martinez-Campos, P. Zipperlen, A. G. Fraser and J. Ahringer, 2001. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2 RESEARCH0002. [DOI] [PMC free article] [PubMed]
- Kato, R., and H. Ogawa, 1994. An essential gene, ESR1, is required for mitotic cell growth, DNA repair and meiotic recombination in Saccharomyces cerevisiae. Nucleic Acids Res. 22 3104–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan, K. S., D. A. Holtzman, A. W. Plug, E. R. Christenson, E. E. Brainerd et al., 1996. The Atr and Atm protein kinases associate with different sites along meiotically pairing chromosomes. Genes Dev. 10 2423–2437. [DOI] [PubMed] [Google Scholar]
- Keith, C. T., and S. L. Schreiber, 1995. PIK-related kinases: DNA repair, recombination, and cell cycle checkpoints. Science 270 50–51. [DOI] [PubMed] [Google Scholar]
- Lavin, M. F., K. K. Khanna, H. Beamish, K. Spring, D. Watters et al., 1995. Relationship of the ataxia-telangiectasia protein ATM to phosphoinositide 3-kinase. Trends Biochem. Sci. 20 382–383. [DOI] [PubMed] [Google Scholar]
- Morrow, D. M., D. A. Tagle, Y. Shiloh, F. S. Collins and P. Hieter, 1995. TEL1, an S. cerevisiae homolog of the human gene mutated in ataxia telangiectasia, is functionally related to the yeast checkpoint gene MEC1. Cell 82 831–840. [DOI] [PubMed] [Google Scholar]
- Olson, E., C. J. Nievera, V. Klimovich, E. Fanning and X. Wu, 2006. RPA2 is a direct downstream target for ATR to regulate the S-phase checkpoint. J. Biol. Chem. 281 39517–39533. [DOI] [PubMed] [Google Scholar]
- Ritchie, K. B., J. C. Mallory and T. D. Petes, 1999. Interactions of TLC1 (which encodes the RNA subunit of telomerase), TEL1, and MEC1 in regulating telomere length in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 19 6065–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, P. J., J. M. Stuart, J. Lund and S. K. Kim, 2002. Chromosomal clustering of muscle-expressed genes in Caenorhabditis elegans. Nature 418 975–979. [DOI] [PubMed] [Google Scholar]
- Savitsky, K., A. Bar-Shira, S. Gilad, G. Rotman, Y. Ziv et al., 1995. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268 1749–1753. [DOI] [PubMed] [Google Scholar]
- Schedl, T., 1997. Development genetics of the germ line, pp. 241–269 in C. elegans II, edited by D. L. Riddle, T. Blumenthal, B. J. Meyer, and J. R. Priess. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Shiloh, Y., 2001. ATM (ataxia telangiectasia mutated): expanding roles in the DNA damage response and cellular homeostasis. Biochem. Soc. Trans. 29 661–666. [DOI] [PubMed] [Google Scholar]
- Shiloh, Y., 2003. ATM and related protein kinases: safeguarding genome integrity. Nat. Rev. Cancer 3 155–168. [DOI] [PubMed] [Google Scholar]
- Stergiou, L., K. Doukoumetzidis, A. Sendoel and M. O. Hengartner, 2007. The nucleotide excision repair pathway is required for UV-C-induced apoptosis in Caenorhabditis elegans. Cell Death Differ. 14 1129–1138. [DOI] [PubMed] [Google Scholar]
- Sugimoto, T., C. Mori, T. Takanami, Y. Sasagawa, R. Saito et al., 2008. Caenorhabditis elegans par2.1/mtssb-1 is essential for mitochondrial DNA replication and its defect causes comprehensive transcriptional alterations including a hypoxia response. Exp. Cell Res. 314 103–114. [DOI] [PubMed] [Google Scholar]
- Sun, Z., D. S. Fay, F. Marini, M. Foiani and D. F. Stern, 1996. Spk1/Rad53 is regulated by Mec1-dependent protein phosphorylation in DNA replication and damage checkpoint pathways. Genes Dev. 10 395–406. [DOI] [PubMed] [Google Scholar]
- Taylor, S. D., H. Zhang, J. S. Eaton, M. S. Rodeheffer, M. A. Lebedeva et al., 2005. The conserved Mec1/Rad53 nuclear checkpoint pathway regulates mitochondrial DNA copy number in Saccharomyces cerevisiae. Mol. Biol. Cell 16 3010–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinei, M., I. Berniakovich, P. G. Pelicci and M. Giorgio, 2006. Mitochondrial DNA copy number is regulated by cellular proliferation: a role for Ras and p66(Shc). Biochim. Biophys. Acta 1757 624–630. [DOI] [PubMed] [Google Scholar]
- Tsang, W. Y., and B. D. Lemire, 2002. Mitochondrial genome content is regulated during nematode development. Biochem. Biophys. Res. Commun. 291 8–16. [DOI] [PubMed] [Google Scholar]
- van den Heuvel, S., 2005. Cell-cycle regulation, pp. 1–16 in WormBook. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Weinert, T. A., G. L. Kiser and L. H. Hartwell, 1994. Mitotic checkpoint genes in budding yeast and the dependence of mitosis on DNA replication and repair. Genes Dev. 8 652–665. [DOI] [PubMed] [Google Scholar]
- Zhao, X., E. G. Muller and R. Rothstein, 1998. A suppressor of two essential checkpoint genes identifies a novel protein that negatively affects dNTP pools. Mol. Cell 2 329–340. [DOI] [PubMed] [Google Scholar]
- Zheng, P., D. S. Fay, J. Burton, H. Xiao, J. L. Pinkham et al., 1993. SPK1 is an essential S-phase-specific gene of Saccharomyces cerevisiae that encodes a nuclear serine/threonine/tyrosine kinase. Mol. Cell. Biol. 13 5829–5842. [DOI] [PMC free article] [PubMed] [Google Scholar]