Abstract
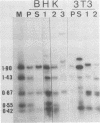
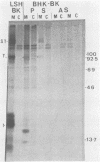
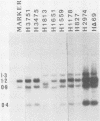
We have examined the role of the human papovavirus BK virus (BKV) tumor (T) antigen(s) in the maintenance of transformation and have identified the domain of T antigen essential for transformation. BKV-transformed BHK 21 and NIH 3T3 cells expressing antisense T-antigen RNA lose their ability to grow in soft agar, indicating the need for the continued expression of T antigen for the maintenance of the transformed phenotype. Experiments using translation termination linker insertion and deletion mutagenesis of BKV T antigen demonstrate that amino acids 356 to 384 are essential for transformation. Although BKV T antigen shares 100, 95, and 82% amino acid homology with that of simian virus 40 (SV40) for the nuclear localization signal, p53-binding domain, and DNA-binding domain, respectively, the transformation domains of BKV and SV40 T antigens share only 54% homology. Also, BKV T antigen lacks a substantial portion of the ATPase domain of SV40, and our results indicate the dispensability of the remaining portion for transformation by this protein. We suggest that the differences in the amino acids in the identified transformation domains together with the differences in the ATPase domains may account for the differences in the transformation potentials of the two proteins.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bikel I., Montano X., Agha M. E., Brown M., McCormack M., Boltax J., Livingston D. M. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell. 1987 Jan 30;48(2):321–330. doi: 10.1016/0092-8674(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Butel J. S. Role of simian virus 40 gene A function in maintenance of transformation. J Virol. 1975 Mar;15(3):619–635. doi: 10.1128/jvi.15.3.619-635.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuke W. F., Walker D. L., Peitzman L. B., Frisque R. J. Construction and characterization of hybrid polyomavirus genomes. J Virol. 1986 Dec;60(3):960–971. doi: 10.1128/jvi.60.3.960-971.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R., Lane D. P., Tjian R. Use of monoclonal antibodies as probes of simian virus 40 T antigen ATPase activity. J Biol Chem. 1981 Nov 25;256(22):11854–11858. [PubMed] [Google Scholar]
- Clark R., Peden K., Pipas J. M., Nathans D., Tjian R. Biochemical activities of T-antigen proteins encoded by simian virus 40 A gene deletion mutants. Mol Cell Biol. 1983 Feb;3(2):220–228. doi: 10.1128/mcb.3.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C. E., Murphy D., Lovett M., Rigby P. W. A fragment of the SV40 large T-antigen gene transforms. Nature. 1982 Sep 2;299(5878):59–61. doi: 10.1038/299059a0. [DOI] [PubMed] [Google Scholar]
- Dixon K., Ryder B. J., Burch-Jaffe E. Enhanced metastasis of tumours induced by a SV40 small T deletion mutant. Nature. 1982 Apr 15;296(5858):672–675. doi: 10.1038/296672a0. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Galanti N., Jonak G. J., Soprano K. J., Floros J., Kaczmarek L., Weissman S., Reddy V. B., Tilghman S. M., Baserga R. Characterization and biological activity of cloned simian virus 40 DNA fragments. J Biol Chem. 1981 Jun 25;256(12):6469–6474. [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gurney E. G., Tamowski S., Deppert W. Antigenic binding sites of monoclonal antibodies specific for simian virus 40 large T antigen. J Virol. 1986 Mar;57(3):1168–1172. doi: 10.1128/jvi.57.3.1168-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- Kimura G., Dulbecco R. A temperature-sensitive mutant of simian virus 40 affecting transforming ability. Virology. 1973 Apr;52(2):529–534. doi: 10.1016/0042-6822(73)90348-6. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Bradley M. K. The simian virus 40 large T antigen. A lot packed into a little. Mol Biol Med. 1987 Apr;4(2):63–80. [PubMed] [Google Scholar]
- Major E. O., Matsumura P. Human embryonic kidney cells: stable transformation with an origin-defective simian virus 40 DNA and use as hosts for human papovavirus replication. Mol Cell Biol. 1984 Feb;4(2):379–382. doi: 10.1128/mcb.4.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews B. J., Levine A. S., Dixon K. Deletion mutations in the small t antigen gene alter the tissue specificity of tumors induced by simian virus 40. J Virol. 1987 Apr;61(4):1282–1285. doi: 10.1128/jvi.61.4.1282-1285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo K. E., Warren R., Palmiter R. D. The mouse metallothionein-I gene is transcriptionally regulated by cadmium following transfection into human or mouse cells. Cell. 1982 May;29(1):99–108. doi: 10.1016/0092-8674(82)90094-0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenarh M., Kohler M., Aggeler G., Henning R. Structural prerequisites of simian virus 40 large T antigen for the maintenance of cell transformation. EMBO J. 1985 Nov;4(11):2941–2947. doi: 10.1002/j.1460-2075.1985.tb04027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenarh M., Vesco C., Kemmerling G., Müller D., Henning R. Regions of SV40 large T antigen necessary for oligomerization and complex formation with the cellular oncoprotein p53. FEBS Lett. 1986 Aug 11;204(1):51–55. doi: 10.1016/0014-5793(86)81386-2. [DOI] [PubMed] [Google Scholar]
- Moran E. A region of SV40 large T antigen can substitute for a transforming domain of the adenovirus E1A products. Nature. 1988 Jul 14;334(6178):168–170. doi: 10.1038/334168a0. [DOI] [PubMed] [Google Scholar]
- Oren M., Maltzman W., Levine A. J. Post-translational regulation of the 54K cellular tumor antigen in normal and transformed cells. Mol Cell Biol. 1981 Feb;1(2):101–110. doi: 10.1128/mcb.1.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnani M., Negrini M., Reschiglian P., Corallini A., Balboni P. G., Scherneck S., Macino G., Milanesi G., Barbanti-Brodano G. Molecular and biological properties of BK virus-IR, a BK virus variant isolated from a human tumor. J Virol. 1986 Aug;59(2):500–505. doi: 10.1128/jvi.59.2.500-505.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pater A., Pater M. M. Transformation of primary human embryonic kidney cells to anchorage independence by a combination of BK virus DNA and the Harvey-ras oncogene. J Virol. 1986 May;58(2):680–683. doi: 10.1128/jvi.58.2.680-683.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pater M. M., Pater A., di Mayorca G., Beth E., Giraldo G. BK virus-transformed inbred hamster brain cells: status of viral DNA in subclones. Mol Cell Biol. 1982 Jul;2(7):837–844. doi: 10.1128/mcb.2.7.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucha E., Kalderon D., Harvey R. W., Smith A. E. Simian virus 40 origin DNA-binding domain on large T antigen. J Virol. 1986 Jan;57(1):50–64. doi: 10.1128/jvi.57.1.50-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas J. M., Peden K. W., Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Fareed G. C. Transformation of human embryonic kidney cells by human papovarirus BK. J Virol. 1979 Feb;29(2):763–769. doi: 10.1128/jvi.29.2.763-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K., Major E. O., Lampert M. Association of cellular 56,000- and 32,000-molecular-weight protein with BK virus and polyoma virus t-antigens. J Virol. 1981 Mar;37(3):1090–1093. doi: 10.1128/jvi.37.3.1090-1093.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Takemoto K. K., Martin M. A. Relationship between the methionine tryptic peptides of simian virus 40 and BK virus tumor antigens. J Virol. 1977 Oct;24(1):319–325. doi: 10.1128/jvi.24.1.319-325.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. A., Khoury G., Jay G., Howley P. M., Scangos G. A. Early regions of JC virus and BK virus induce distinct and tissue-specific tumors in transgenic mice. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8288–8292. doi: 10.1073/pnas.83.21.8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano K. J., Galanti N., Jonak G. J., McKercher S., Pipas J. M., Peden K. W., Baserga R. Mutational analysis of simian virus 40 T antigen: stimulation of cellular DNA synthesis and activation of rRNA genes by mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):214–219. doi: 10.1128/mcb.3.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Takemoto K. K., Linke H., Miyamura T., Fareed G. C. Persistent BK papovavirus infection of transformed human fetal brain cells. I. Episomal viral DNA in cloned lines deficient in T-antigen expression. J Virol. 1979 Mar;29(3):1177–1185. doi: 10.1128/jvi.29.3.1177-1185.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T. H., Wallis J., Levine A. J. Identification of the p53 protein domain involved in formation of the simian virus 40 large T-antigen-p53 protein complex. J Virol. 1986 Sep;59(3):574–583. doi: 10.1128/jvi.59.3.574-583.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornow J., Polvino-Bodnar M., Santangelo G., Cole C. N. Two separable functional domains of simian virus 40 large T antigen: carboxyl-terminal region of simian virus 40 large T antigen is required for efficient capsid protein synthesis. J Virol. 1985 Feb;53(2):415–424. doi: 10.1128/jvi.53.2.415-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble W. S., Johnson P. W., Hozumi N., Roder J. C. Inducible cellular transformation by a metallothionein-ras hybrid oncogene leads to natural killer cell susceptibility. Nature. 1986 Jun 19;321(6072):782–784. doi: 10.1038/321782a0. [DOI] [PubMed] [Google Scholar]
- Uchida S., Watanabe S., Aizawa T., Furuno A., Muto T. Polyoncogenicity and insulinoma-inducing ability of BK Virus, a human Papovavirus, in Syrian golden hamsters. J Natl Cancer Inst. 1979 Jul;63(1):119–126. [PubMed] [Google Scholar]
- Vass-Marengo J., Ratiarson A., Asselin C., Bastin M. Ability of a T-antigen transport-defective mutant of simian virus 40 to immortalize primary cells and to complement polyomavirus middle T in tumorigenesis. J Virol. 1986 Sep;59(3):655–659. doi: 10.1128/jvi.59.3.655-659.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Soeda E., Uchida S., Yoshiike K. DNA rearrangement affecting expression of the BK virus transforming gene. J Virol. 1984 Jul;51(1):1–6. doi: 10.1128/jvi.51.1.1-6.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Wold M. S., Li J. J., Kelly T. J. Initiation of simian virus 40 DNA replication in vitro: large-tumor-antigen- and origin-dependent unwinding of the template. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3643–3647. doi: 10.1073/pnas.84.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA: complete nucleotide sequence of a human tumor virus. Science. 1979 Oct 26;206(4417):456–462. doi: 10.1126/science.228391. [DOI] [PubMed] [Google Scholar]