Abstract
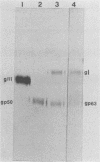
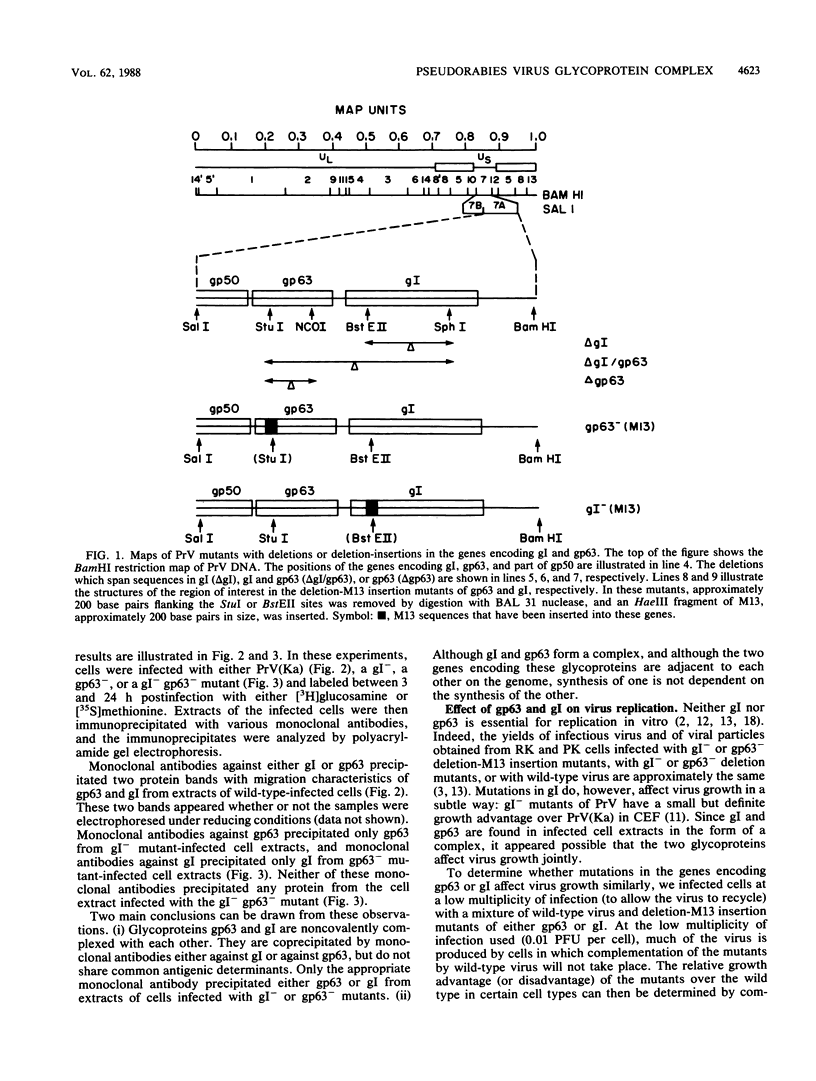
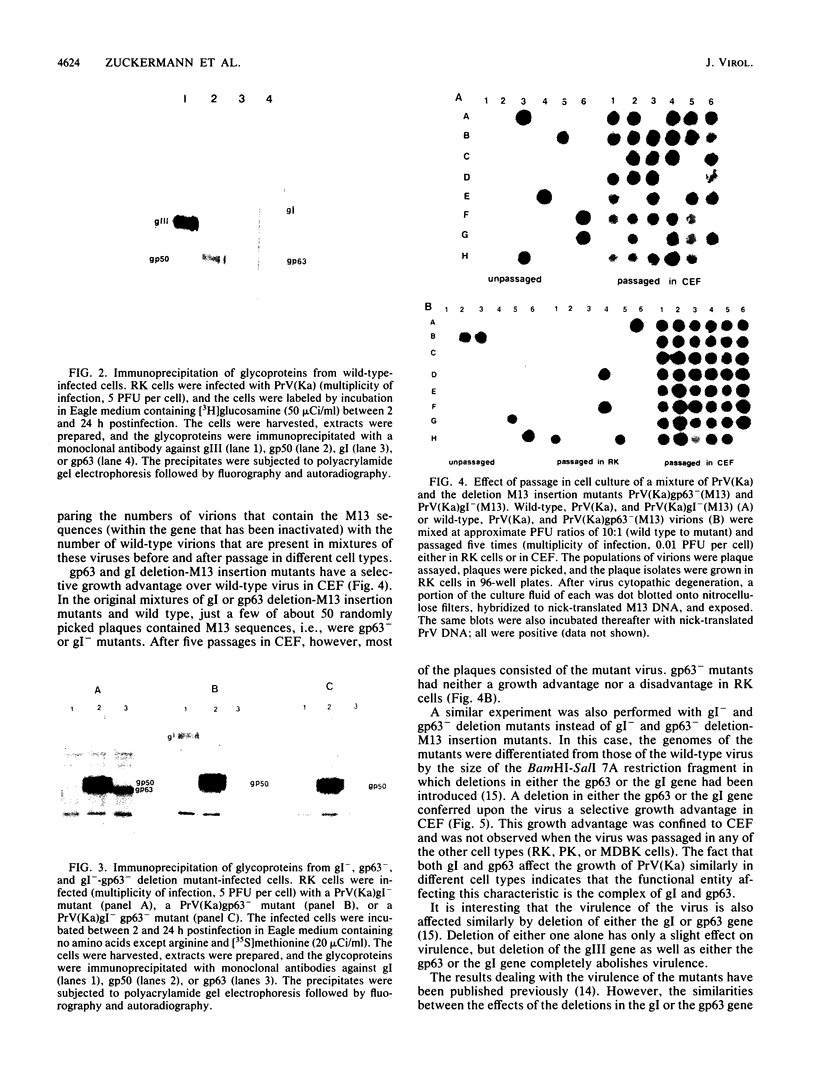
To ascertain the biological functions of different glycoproteins that are nonessential for pseudorabies virus growth in vitro, we have constructed mutants defective in one (or a combination) of these glycoproteins and have examined various aspects of their role in the infective process. We made the following two observations. (i) Glycoproteins gI and gp63 are noncovalently complexed to each other. They are coprecipitated by antisera against either one of these glycoproteins but do not share antigenic determinants: monoclonal antibodies against gp63 do not immunoprecipitate gI from extracts of gp63- mutant-infected cells, and monoclonal antibodies against gI do not immunoprecipitate gp63 from extracts of gI- mutant-infected cells. (ii) Mutants unable to synthesize either gI or gp63 have some common biological characteristics; they have a growth advantage in primary chicken embryo fibroblasts. Furthermore, we have shown previously that in conjunction with glycoprotein gIII, gI and gp63 are necessary for the expression of virulence (T. C. Mettenleiter, C. Schreurs, F. Zuckermann, T. Ben-Porat, and A. S. Kaplan, J. Virol. 62, 2712-2717, 1988). These results show that the functional entity affecting virus replication in chicken embryo fibroblasts, as well as affecting virulence, is the complex between gI and gp63. The gI-gp63 complex of pseudorabies virus does not appear to have Fc receptor activity as does its homolog, the gI-gE complex of herpes simplex virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porat T., DeMarchi J. M., Lomniczi B., Kaplan A. S. Role of glycoproteins of pseudorabies virus in eliciting neutralizing antibodies. Virology. 1986 Oct 30;154(2):325–334. doi: 10.1016/0042-6822(86)90458-7. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., DeMarchi J., Pendrys J., Veach R. A., Kaplan A. S. Proteins specified by the short unique region of the genome of pseudorabies virus play a role in the release of virions from certain cells. J Virol. 1986 Jan;57(1):191–196. doi: 10.1128/jvi.57.1.191-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feorino P. M., Shore S. L., Reimer C. B. Detection by indirect immunofluorescence of Fc receptors in cells acutely infected with Herpes simplex virus. Int Arch Allergy Appl Immunol. 1977;53(3):222–233. doi: 10.1159/000231756. [DOI] [PubMed] [Google Scholar]
- Hampl H., Ben-Porat T., Ehrlicher L., Habermehl K. O., Kaplan A. S. Characterization of the envelope proteins of pseudorabies virus. J Virol. 1984 Nov;52(2):583–590. doi: 10.1128/jvi.52.2.583-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Feenstra V. Identification of a novel herpes simplex virus type 1-induced glycoprotein which complexes with gE and binds immunoglobulin. J Virol. 1987 Jul;61(7):2208–2216. doi: 10.1128/jvi.61.7.2208-2216.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Frame M. C., Ligas M. W., Cross A. M., Stow N. D. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J Virol. 1988 Apr;62(4):1347–1354. doi: 10.1128/jvi.62.4.1347-1354.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R., Peitchel R., Goldman J. N., Goldman M. An IgG-Fc receptor induced in cytomegalovirus-infected human fibroblasts. J Immunol. 1976 Mar;116(3):772–777. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- McTaggart S. P., Burns W. H., White D. O., Jackson D. C. Fc receptors induced by herpes simplex virus. I. Biologic and biochemical properties. J Immunol. 1978 Aug;121(2):726–730. [PubMed] [Google Scholar]
- Mettenleiter T. C., Lomniczi B., Sugg N., Schreurs C., Ben-Porat T. Host cell-specific growth advantage of pseudorabies virus with a deletion in the genome sequences encoding a structural glycoprotein. J Virol. 1988 Jan;62(1):12–19. doi: 10.1128/jvi.62.1.12-19.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukàcs N., Rziha H. J. Pseudorabies virus avirulent strains fail to express a major glycoprotein. J Virol. 1985 Oct;56(1):307–311. doi: 10.1128/jvi.56.1.307-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Schreurs C., Zuckermann F., Ben-Porat T., Kaplan A. S. Role of glycoprotein gIII of pseudorabies virus in virulence. J Virol. 1988 Aug;62(8):2712–2717. doi: 10.1128/jvi.62.8.2712-2717.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Schreurs C., Zuckermann F., Ben-Porat T. Role of pseudorabies virus glycoprotein gI in virus release from infected cells. J Virol. 1987 Sep;61(9):2764–2769. doi: 10.1128/jvi.61.9.2764-2769.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Kaplan A. S., Ben-Porat T., Lomniczi B. Role of a structural glycoprotein of pseudorabies in virus virulence. J Virol. 1987 Dec;61(12):4030–4032. doi: 10.1128/jvi.61.12.4030-4032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M., Shigeta S. Appearance of immunoglobulin G Fc receptor in cultured human cells infected with varicella-zoster virus. Infect Immun. 1979 Nov;26(2):770–774. doi: 10.1128/iai.26.2.770-774.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Glycoprotein gE of herpes simplex virus type 1: effects of anti-gE on virion infectivity and on virus-induced fc-binding receptors. J Virol. 1982 Jan;41(1):129–136. doi: 10.1128/jvi.41.1.129-136.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Gierman T. M., Post L. E. Deletions in vaccine strains of pseudorabies virus and their effect on synthesis of glycoprotein gp63. J Virol. 1986 Dec;60(3):1166–1169. doi: 10.1128/jvi.60.3.1166-1169.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovskis E. A., Timmins J. G., Post L. E. Use of lambda gt11 to isolate genes for two pseudorabies virus glycoproteins with homology to herpes simplex virus and varicella-zoster virus glycoproteins. J Virol. 1986 Oct;60(1):185–193. doi: 10.1128/jvi.60.1.185-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A. A., Teschner M., Sethi K. K., Brandis H. Appearance of IgG (Fc) receptor(s) on cultured human fibroblasts infected with human cytomegalovirus. J Immunol. 1976 Jul;117(1):253–258. [PubMed] [Google Scholar]
- Rea T. J., Timmins J. G., Long G. W., Post L. E. Mapping and sequence of the gene for the pseudorabies virus glycoprotein which accumulates in the medium of infected cells. J Virol. 1985 Apr;54(1):21–29. doi: 10.1128/jvi.54.1.21-29.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. K., Whealy M. E., Watson R. J., Enquist L. W. Pseudorabies virus gene encoding glycoprotein gIII is not essential for growth in tissue culture. J Virol. 1986 Sep;59(3):635–645. doi: 10.1128/jvi.59.3.635-645.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs C., Mettenleiter T. C., Zuckermann F., Sugg N., Ben-Porat T. Glycoprotein gIII of pseudorabies virus is multifunctional. J Virol. 1988 Jul;62(7):2251–2257. doi: 10.1128/jvi.62.7.2251-2257.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Wathen L. M. Characterization and mapping of a nonessential pseudorabies virus glycoprotein. J Virol. 1986 Apr;58(1):173–178. doi: 10.1128/jvi.58.1.173-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland D., St Jeor S., Rapp F. The development by cytomegalovirus-infected cells of binding affinity for normal human immunoglobulin. J Immunol. 1976 Jun;116(6):1566–1570. [PubMed] [Google Scholar]
- Westmoreland D., Watkins J. F. The IgG receptor induced by herpes simplex virus: studies using radioiodinated IgG. J Gen Virol. 1974 Jul;24(1):167–178. doi: 10.1099/0022-1317-24-1-167. [DOI] [PubMed] [Google Scholar]