Abstract
Background and purpose
To determine the sleep response to caffeine in individuals vulnerable to stress-related sleep disturbance as measured by polysomnography.
Patients and methods
Eleven healthy individuals without insomnia scoring low (4 women, mean age=32.64 ± 15.46 years) and 10 healthy individuals also without insomnia scoring high (6 women, mean age=34.20 ± 13.73 years) on a measure of vulnerability to stress-related sleep disturbance were studied in a laboratory protocol. A moderate-low dose of caffeine (3 mg/kg) was administered 1 h prior to lights-out and compared to a counterbalanced control night with each condition separated by 1 week. Standard polysomnographic measures were assessed (i.e. total sleep time, sleep efficiency, latency to persistent sleep, and sleep stage percentages) for both control and caffeine nights.
Results
There were no between-group differences in sleep on the control night. Importantly, individuals reporting vulnerability to stress-related sleep disturbance had significantly prolonged latency to persistent sleep in response to the caffeine challenge (interaction; P <0.05).
Conclusion
Normal sleepers with an identified vulnerability to stress-induced sleep disturbance exhibited greater objectively verifiable sleep-reactivity in response to a caffeine challenge compared to non-vulnerable individuals. These results suggest that the construct of individual differences in vulnerability to sleep disturbance applies to a pharmacological ‘stressor’ (i.e. caffeine) as well as to previously assessed stressors such as a first-night effect. This finding provides further support for generalized trait vulnerability by demonstrating a sleep disturbance to a wake-promoting pharmacological challenge in specific a priori identified individuals.
Keywords: Insomnia, Predisposition, First-night effect, Hyperarousal, Sleep disturbance, Arousal
1. Introduction
Insomnia is now recognized as one of the most prevalent [1,2] and costly [3,4] sleep disorders. Criteria for insomnia in the diagnostic and statistical manual of mental disorders (DSM-IV) include a patient report of difficulty falling asleep, staying asleep, or having non-restorative sleep for a period of at least 4 weeks, associated with impairment in daytime functioning (e.g. occupational, social, other). In addition, these symptoms cannot be accounted for by another medical or psychiatric condition. Studies have begun to document a high degree of morbidity associated with insomnia including reductions in quality of life that are comparable to other chronic illnesses such as congestive heart failure and depression [5,6].
Although there are a number of well established options available for insomnia treatment [7], data regarding the etiology of this disorder remain sparse. However, there is an increasing body of literature regarding the psychological and physiological differences between insomniacs and good sleepers. Several studies have demonstrated a greater prevalence of intrusive thoughts, worry, and rumination [8], poor sleep hygiene [9,10], dysregulation of the hypothalamic pituitary axis [11], elevations in sympathetic activity [12], and metabolic rate [13], increased daytime alertness [14], and elevated high-frequency electroencephalographic (EEG) activity [15–17] in patients with insomnia compared to good sleepers.
Clearly, these data represent a growing body of evidence supporting the association between both cognitive and physiological measures of hyperarousal and insomnia. However, an important question remains. Specifically, what is the temporal relationship between hyperarousal and the development of insomnia as well as the progression of the disorder? Laboratory studies have demonstrated the ability to produce sleep disturbance in healthy good sleepers similar to that of insomniacs through experimental challenges [18,19]. These findings, along with converging evidence for an elevation in physiological and cognitive arousal measures in insomnia patients, are a first step in determining whether elevated arousal in insomnia represents the underlying pathophysiology of the disorder or a concomitant process resulting from the sleep disturbance itself.
An alternative approach to answering these questions is to study specific aspects of sleep-related processes prior to the onset of the disorder. Although this approach presents many challenges, such as identifying individuals with a ‘high risk’ for developing insomnia, it has produced promising results. We have recently developed [20] and longitudinally validated [21] the Ford Insomnia Response to Stress Test (FIRST), a measure to identify individuals predisposed to sleep disturbance and the subsequent development of chronic insomnia. This nine-item self-report instrument assesses the potential likelihood that an individual will experience sleep disturbance following various stressful events/situations. The FIRST was designed to assess sleep-related ‘reactivity’. While insomniacs do score high on this measure [22], it is not intended to identify insomniacs per se, but rather to determine individuals who may be ‘at risk’ for developing insomnia in the future. Although, as might be expected, scores are elevated in insomniacs [22], preliminary data in non-insomniacs suggests that individuals scoring high on this measure have a greater risk for the development of insomnia over the course of the next year [21]. This instrument has facilitated research on individuals at risk for insomnia to be carried out prior to the development of the disorder.
In a previous study, we demonstrated the existence of a trait vulnerability to hyperarousal and its association with elevated polysomnographically (PSG) measured sleep disturbance in response to the stress of a first night in the laboratory as predicted by the FIRST [20]. However, further studies addressing the beginning stages of the evolution of insomnia are needed. Specifically, the extent to which this trait vulnerability is generalizable to other stressors or challenges beyond those previously assessed has yet to be determined. Furthermore, the characteristic emotional reactivity typically associated with insomnia [23], sometimes alluded to as a ‘predisposing’ factor [24], has rarely been tested using a physiologic challenge.
In 2003, Bonnet and Arand [25], assessed the sleep-related response of individuals who demonstrated differentially poor sleep on laboratory adaptation and found that these individuals also had poor sleep in response to caffeine administration and a circadian phase-shift challenge. This demonstrationed the widely held belief that some individuals have a trait vulnerability to situational insomnia. The present study was aimed at comparing the sleep-related reactivity of individuals “a priori” hypothesized to be vulnerable to developing insomnia compared with normal healthy sleepers using low-moderate nocturnal caffeine (3 mg/kg).
2. Methods
2.1. Subjects
Participants were recruited from individuals who had participated in previous sleep center protocols as well as from media advertisements (newspaper and television). The study sample included two groups of individuals, none of whom met diagnostic criteria for insomnia. The exclusion of insomnia diagnosis and other subject selection criteria was made based on systematic clinical evaluation/screening of study participants initially by phone by a trained rater subjects were further interviewed (structure clinical interview for Diagnostic and Statistical Manual of Mental Disorders—fourth edition (DSM-IV)) by trained personnel in the laboratory. Subjects were excluded if they endorsed difficulty falling asleep, staying asleep, or having non-restorative sleep for 1 month or more at any time during their lifetime.
Habitual sleep information was determined by self-report on a standard sleep questionnaire regarding behavior during the previous week. Participants were restricted to those who reported a habitual (past week) bedtime between 9 p.m. and 1 a.m. and wake time between 6 and 9 a.m., reported spending at least 7 h/night in bed, reported no more than three awakenings per night, and reported ‘refreshing sleep’ based on a sleep quality rating (four-point Likert Scale). Following a PSG screening night, individuals with an apnea–hypopnea or periodic limb movement index ≥five per hour of sleep were excluded. No subjects met either of these criteria on PSG screening. Participation was restricted to individuals who regularly drank at least two caffeinated beverages per week (upper limit of three caffeinated beverages per day).
Only individuals scoring less than seven on the Hamilton Depression Rating Scale (HDRS) were allowed to participate. Individuals currently using hypnotics or other psychotropic medications, or those currently engaged in shift work were excluded. Any individual with a reported current or previous history of any significant psychiatric or medical disorder was also excluded from participation based on telephone interview and laboratory evaluations as noted above. Smokers were excluded from participation.
Thus, individuals in each group were healthy normal volunteers without significant illnesses. A total of 30 individuals met initial screening criteria, but nine individuals did not complete the study for a variety of reasons. Specifically, one subject dropped out due to pregnancy, one could not swallow the caffeine pills, five were discontinued due to noncompliance with some aspect of the protocol, one subject did not feel comfortable sleeping in the laboratory, and one subject had unreadable PSG screening data. Using a median split on FIRST scores, individuals were separated into two groups: those scoring low (≤ 18) on the FIRST scale (n = 11, 4 women, mean age = 32.64 ± 15.46 years) and those scoring high (> 18) on this measure (n = 10, 6 women, mean age = 34.20 ± 13.73 years). There were no significant differences in age or gender distribution between the groups (P>0.05). There are no questions on the FIRST addressing an individual’s sleep-response to caffeine. All procedures were approved by the institutional review board and informed consent was obtained from all participants. Individuals were paid for study participation.
2.2. Procedures
Prior to the experimental nights, participants completed an adaptation night in the laboratory, during which they were evaluated for any signs of periodic limb movements or sleep-disordered breathing using a nasal–oral thermister and leg electromyogram (EMG). Participants were then scheduled for two overnight visits, each separated by 1 week. In several cases, screening PSG bedtimes were allowed to vary according to each participant’s habitually reported bedtime although most subjects were recruited from a previous protocol with screening PSGs offset to 8.5 h time in bed. In order to standardize the experimental nights, PSGs were set to 8 h beginning at 11 p.m. (lights-out) and ending at 7 a.m. (lights-on). Caffeine was administered 60 min prior to bedtime at 10 p.m. Each recording included electroencephalograms (EEG) (C3, C4, O1, O2 referenced to contralateral ear electrodes), two electro-oculograms (EOG) (bilateral horizontal), submental EMG and electrocardiogram (ECG) (V5 lead) and were scored in 30-s epochs according to standard procedures [26]. In addition, leg movements were monitored using a bilateral tibialis EMG, and respiration was monitored using a nasal/oral thermistor. All recordings were made using Grass Heritage or Aurora digital polygraphs (Grass-Telefactor, Astromed, Inc, West Warwick, RI).
Participants were administered 3 mg/kg of caffeine in pill form on one of the two overnight laboratory visits. This dose was chosen as it has been shown to produce small to moderate disruptions of sleep initiation in healthy normal individuals [27]. Counterbalancing was incomplete due to scheduling conflicts and attrition. Overall, 12 subjects were studied with the control night as the first experimental night, while nine participants were studied with caffeine administration as the first experimental night. Subjects were instructed to eat a meal prior to coming into the laboratory at 8 p.m., and subjects did not consume any food thereafter on the laboratory nights. Subjects were given breakfast at approximately 7 a.m. Subjects were instructed to refrain from consuming any alcohol or caffeine after 5 p.m. on any laboratory night. Noise and other environmental stimuli were minimized, as is standard in the laboratory. The private sound attenuated and individually temperature-controlled bedrooms were located on a separate wing from any hospital offices and in a building that is separate from all in-patient medical facilities for the hospital.
2.3. Statistics
The primary outcome measure for the present study was latency to persistent sleep (20 continuous epochs of PSG sleep). This selection was based on its frequent use as an outcome measure of sleep onset in the clinical insomnia literature, sleep latency as a component in the diagnosis of chronic primary insomnia, and the documented effect of caffeine on sleep latency [27]. Furthermore, the moderate dose of caffeine utilized as the sleep-related challenge (3 mg/kg caffeine) has not been shown to produce significant sleep disturbance beyond a latency effect when administered prior to bedtime. Nonetheless, sleep efficiency was also tested as a secondary measure in order to facilitate comparisons with other clinical insomnia literature [28]. A two-factor mixed (Group X Caffeine) repeated measures analysis of variance (ANOVA) was used to test for differences in the sleep-related response to caffeine for latency to persistent sleep and sleep efficiency. Follow-up post-hoc t-tests were performed if an interaction was present. An alpha criterion of 0.05 was used to determine statistical significance. Potential differences in demographic variables were tested using the Student’s t-test and χ2 analysis, respectively.
3. Results
No demographic differences were found between groups in terms of age, gender, caffeine consumption, or other pertinent demographic variables (see Table 1). Table 2 contains means and standard deviations for all sleep variables by group for each condition. No differences in latency to persistent sleep were found between FIRST groups (main effect of group, F(1,19) = 3.34, P = 0.08), supporting the contention, along with habitual sleep times, that none of the participants in either group were insomniacs or demonstrated basal sleep disturbance. A main effect for caffeine was present (main effect of condition, F(1,19) = 8.97, P = 0.007), illustrating that this dose of caffeine was effective at producing prolonged sleep latency.
Table 1.
Demographic variables
| Variable | Low FIRST group (n=11) | High FIRST group (n=10) |
|---|---|---|
| Age | 32.64±15.46 | 34.20±13.73 |
| Gender | 4F | 6F |
| Daily caffeine | 1.4±0.96 | 1.5±1.09 |
| Alcohol consumption | 0.39±0.46 | 0.37±0.40 |
| HDRS | 0.18±0.60 | 0.6±1.58 |
| Habitual TIB | 7.27±0.75 | 7.45±0.76 |
| Sleep quality | 3.27±0.47 | 3.0±0.0 |
There were no statistically significant between group differences in any of the variables (P>0.05); daily caffeine and alcohol consumption are shown in ‘cups’ and ‘drinks’ per day, respectively; HDRS, Hamilton depression rating scale score (including sleep items); TIB, time in bed assessed by questionnaire referenced over the week prior to the study; sleep quality (Likert scale: 1 = not refreshing at all; 2 = somewhat, 3 = pretty refreshing, 4 = very refreshing).
Table 2.
Polysomnographic (PSG) sleep data of individuals with high and low scores on the Ford insomnia response to stress test (FIRST) during each experimental night
| PSG variable | Control night
|
Caffeine night
|
||
|---|---|---|---|---|
| Low FIRST | High FIRST | Low FIRST | High FIRST | |
| LPS (min) | 17.5±14.3 | 18.2±18.8 | 24.5±26.8 | 64.5±57.6* |
| SE % | 88.1±10.5 | 87.6±6.0 | 79.5±18.2 | 74.3±19.7 |
| Stage 1% | 9.6±11.1 | 7.9±4.1 | 9.6±7.0 | 10.9±7.5 |
| Stage 2% | 49.5±7.0 | 51.4±5.5 | 50.5±8.5 | 52.5±6.0 |
| Stage 3–4% | 19.9±10.4 | 19.6±8.8 | 20.8±13.0 | 15.6±8.7 |
| REM % | 21.0±5.1 | 21.0±3.3 | 19.1±5.9 | 21.0±5.1 |
P<0.05 interaction and vs. control night. Data are presented as means ± SD; LPS, latency to persistent sleep (i.e. first 20 continuous epochs of sleep); REM, rapid eye movement; SE, sleep efficiency.
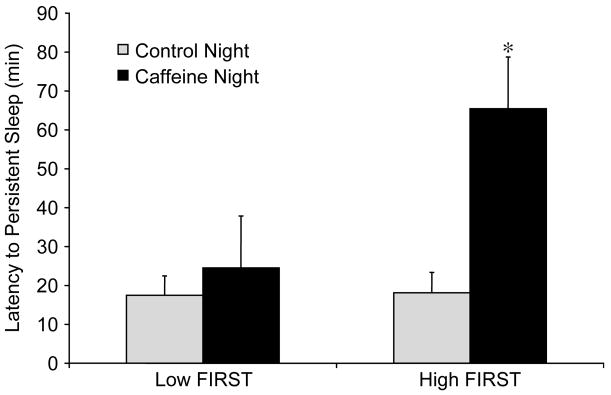
Importantly, there was a significant interaction (group by condition, F(1,19) = 4.90, P = 0.04) (Fig. 1), suggesting an effect of caffeine at this moderate-low dose only in the vulnerable group (High FIRST). Post-hoc t-tests showed that the High FIRST group responded to caffeine with an increased latency to sleep (P < 0.02), while the Low FIRST group did not (P = 0.46). The effect size calculated for the High FIRST group in terms of the effects of caffeine in standard deviation units (Cohen’s d) was d = 1.21.
Fig. 1.
Mean ± SEM of latency to persistent sleep (LPS) in groups with low and high scores on the Ford Insomnia Response to Stress Test (FIRST) during the No caffeine and caffeine night. *P=0.04 vs. Low FIRST caffeine group.
No differences in sleep efficiency were found between FIRST groups (main effect of group, F(1,19) = 0.28, P = 0.60=n.s.), further supporting the contention that none of the participants in either group were insomniacs. A main effect of caffeine was found (Main effect of condition, F(1,19) = 10.39, P = 0.004), also providing evidence for the impact of caffeine on sleep efficiency, although much of this effect was likely accounted for by the impact of nocturnal caffeine administration on sleep onset latency as described above. No interaction was present for sleep efficiency (Interaction F(1,19) = 0.48, P = 0.50).
While the results of additional analyses regarding PSG variables did not reach statistical significance (Table 2), there was a trend for slow wave sleep to be reduced in the High FIRST group on the caffeine administration night, while the other group was comparable on this sleep parameter across the two nights.
4. Discussion
This study served to extend our previous findings in individuals identified as having a vulnerability to sleep disturbance [20]. Specifically, the current data demonstrate that individuals who report a vulnerability to stress-induced sleep disturbance also show elevated sleep-related reactivity in terms of their polysomnographic response to the effects of a pharmacological challenge (3 mg/kg caffeine). This is an important extension in that it supports, along with our previous work, the possibility that individual vulnerability to sleep disturbance may be mediated by an underlying physiological reactivity to stimuli that cross broad categories of psychological and physiological challenges to the sleep system. Furthermore, the relatively low dose of caffeine used in the present study, equal to approximately two cups of coffee, suggests that for a subset of individuals, small otherwise benign stimuli may trigger insomnia. This notion is consistent with the observation that some individuals (~22%) are unable to identify a specific stressor that they believe can account for the onset of their insomnia symptoms [29]. An important result of the present study is that following nocturnal caffeine administration, the vulnerable population had a higher sleep latency (~60 min) than is typically reported for chronic insomniacs [30] and longer than that found in studies using similar doses of caffeine in healthy subjects [27].
The present results are consistent with those of Bonnet and Arand 2003 [25], who found that subjects who had poor sleep on a laboratory adaptation night also showed consistent sleep disturbance following caffeine administration and a circadian phase-shift challenge. Our results compliment those findings using a mild stressor and show that such vulnerable individuals can be identified a priori. The importance of the generalizability of the current study as well as the previous research regarding vulnerability to acute sleep disturbance is apparent when considering that it provides evidence for an overall vulnerability or predisposition to sleep disturbance rather than a distinct vulnerability to a specific stressor. While it is possible that some individuals will react more or less prominently to a specific stressor, our current data and that of others [32] imply that there may be individuals who have a trait vulnerability to sleep disturbance from a very broad range of challenges to the sleep system. These findings suggest why multiple stressors across an array of categories such as medical or psychiatric disease and psychosocial stress may trigger chronic insomnia in some individuals but not others [31–33]. As the current data and other research [25] has suggested, there are specific individual differences responsible for the sleep-response to stress [7,20] and these differences may be markedly important in terms of the longitudinal development of insomnia [21].
As noted by Spielman et al. [34], it is likely that it is the interaction of individual vulnerability with specific triggers in the environment that serves to produce chronic insomnia. The high variability in the High FIRST group suggests that the predisposition to insomnia may lie on a continuum and that a specific cut point for such a construct may be less important than the degree of inherent vulnerability. Thus, a greater degree of inherent vulnerability to acute sleep disturbance may convey a proportionally larger risk for the eventual development of chronic insomnia. The current results provide further support for the existence of a generalized individual vulnerability or predisposition to insomnia and emphasize the need and value of future work in this area.
Several limitations of the present study should be noted. First, due to the absence of a ‘placebo’ condition, we cannot fully determine the exact nature of the underlying precipitant/trigger of the sleep disruption in the High FIRST group. For example, simply the act of consuming a pill prior to bedtime that is anticipated to be sleep-disruptive (i.e. a ‘threat’) may have induced enough cognitive arousal to delay sleep onset to the extent found in this study. However, such an effect did not occur in the Low FIRST group, suggesting that regardless of whether the effect was mediated by an underlying physiological response to the caffeine or a cognitive response to the ingestion of a pill, High FIRST individuals were more responsive. The differentiation of a pharmacological from a psychological effect was not the question of the present study. However, other studies consistent with the current results suggest that the differential response is not likely due solely to a pharmacological effect [20,25]. Specifically, the current work and previous studies showing that certain ‘vulnerable’ individuals respond with sleep disturbance to the stress of a first-night effect, phase-shift challenges, and pharmacological challenges provide convincing evidence that this is likely a generalizable trait associated with vulnerability to multiple and distinct stressors. While we have demonstrated the ability to predict this elevated sleep response, the mechanism(s) of this predisposition to sleep disturbance remain unknown. Although our sample size was small relative to most clinical trials in insomnia, the sample was clearly defined and such reductions in power would only serve to reduce the ability to detect an effect if one was present. This reduction of power may account for the absence of an effect with regard to sleep efficiency, as the effect was in the expected direction. Ongoing personal or work-related stress was not assessed in the present study and should be considered in future research. Finally, as only one dose of caffeine was used, we had no ability to determine any potential dose interaction with vulnerability. This issue may have important implications in terms of environment-patient interactions such as an individual’s response to varying degrees of stress.
The present results show that individuals who are highly reactive to sleep-disrupting stimuli can be identified a priori in the absence of insomnia symptoms. These findings may also inform our understanding of chronic insomnia. First, this study demonstrates that the vulnerability to stress-related sleep disturbance seen in some individuals may also extend to pharmacological reactivity. Specifically, these individuals may have an inherently high reactivity to many if not all sleep-related challenges. This notion is supported by our previous work which showed that High FIRST individuals have greater acute sleep disturbance during a first night in the laboratory where cognitive awareness of the challenge was clearly apparent [20]. In another previous study, which did include a placebo condition, results were similar in that vulnerable individuals (albeit defined differently) were differentially more responsive to caffeine, suggesting that at least part of the vulnerability to sleep disturbance may include a purely physiological component [25]. The comparatively larger effects on sleep seen in that study are also consistent with this interpretation as the caffeine dose used was higher in the previous study (400 mg). Further research on pre-morbid insomniacs will be needed to determine if physiological hyper-reactivity is present prior to any signs of cognitive reactivity or if these characteristics have a similar temporal evolution during the development of chronic insomnia.
Acknowledgments
This study was supported by a National Institute of Mental Health Grant: MH68372 to CLD. The authors would like to thank the technical staff of Henry Ford Hospital Sleep Center for their invaluable assistance in the completion of the present study. In addition, we would like to thank Holly Scofield, Tara McClure, and Eric Myers for their dedication to the completion of the overall project and for editorial comments on the manuscript.
Disclosure statement: This study was supported by a National Institute of Mental Health Grant: MH-068372 Awarded to Dr Drake. No significant financial interest/other relationship to disclose. This was not an industry-supported study.
References
- 1.Ohayon MM. Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res. 1997;31(3):333–46. doi: 10.1016/s0022-3956(97)00002-2. [DOI] [PubMed] [Google Scholar]
- 2.Leger D, Guilleminault C, Dreyfus JP, et al. Prevalence of insomnia in a survey of 12,778 adults in France. J Sleep Res. 2000;9(1):35–42. doi: 10.1046/j.1365-2869.2000.00178.x. [DOI] [PubMed] [Google Scholar]
- 3.Leger D, Levy E, Paillard M. The direct costs of insomnia in France. Sleep. 1999;22(Suppl 2):S394–S401. [PubMed] [Google Scholar]
- 4.Walsh JK, Engelhardt CL. The direct economic costs of insomnia in the United States for 1995. Sleep. 1999;22(Suppl 2):S386–S93. [PubMed] [Google Scholar]
- 5.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51(3):229–35. [PubMed] [Google Scholar]
- 6.Hatoum HT, Kong SX, Kania CM, Wong JM, Mendelson WB. Insomnia, health-related quality of life and healthcare resource consumption. A study of managed-care organisation enrollees. Pharmacoeconomics. 1998;14(6):629–37. doi: 10.2165/00019053-199814060-00004. [DOI] [PubMed] [Google Scholar]
- 7.Drake CL, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: an overview. Depress Anxiety. 2003;18(4):163–76. doi: 10.1002/da.10151. [DOI] [PubMed] [Google Scholar]
- 8.Harvey AG, Tang NK, Browning L. Cognitive approaches to insomnia. Clin Psychol Rev. 2005;25(5):593–611. doi: 10.1016/j.cpr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Jefferson C, Drake C, Scofield H, et al. Sleep hygiene practices in a population-based sample of insomniacs. Sleep. 2005;28(5):611–5. doi: 10.1093/sleep/28.5.611. [DOI] [PubMed] [Google Scholar]
- 10.LeBourgeois MK, Giannotti F, Cortesi F, et al. The relationship between reported sleep quality and sleep hygiene in Italian and American adolescents. Pediatrics. 2005;115(Suppl 1):257–65. doi: 10.1542/peds.2004-0815H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86(8):3787–94. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 12.Bonnet MH, Arand DL. Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med. 1998;60(5):610–5. doi: 10.1097/00006842-199809000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Bonnet MH, Arand DL. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18(7):581–8. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 14.Stepanski E, Zorick F, Roehrs T, Young D, Roth T. Daytime alertness in patients with chronic insomnia compared with asymptomatic control subjects. Sleep. 1988;11(1):54–60. doi: 10.1093/sleep/11.1.54. [DOI] [PubMed] [Google Scholar]
- 15.Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25(6):630–40. [PubMed] [Google Scholar]
- 16.Freedman RR. EEG power spectra in sleep-onset insomnia. Electroencephalogr Clin Neurophysiol. 1986;63(5):408–13. doi: 10.1016/0013-4694(86)90122-7. [DOI] [PubMed] [Google Scholar]
- 17.Perlis ML, Smith MT, Andrews PJ, et al. Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24(1):110–7. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 18.Vitiello MV, Prinz PN, Halter JB. Sodium-restricted diet increases nighttime plasma norepinephrine and impairs sleep patterns in man. J Clin Endocrinol Metab. 1983;56(3):553–6. doi: 10.1210/jcem-56-3-553. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet MH, Arand DL. The consequences of a week of insomnia. Sleep. 1996;19(6):453–61. doi: 10.1093/sleep/19.6.453. [DOI] [PubMed] [Google Scholar]
- 20.Drake CL, Richardson G, Roehrs T, et al. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–91. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 21.Drake C, Jefferson C, Roehrs T, et al. Vulnerability to chronic insomnia: a longitudinal population-based prospective study. Sleep. 2004;27(Abstract Supplement):A270–A1. [Google Scholar]
- 22.Jefferson C, Roehrs T, Roth T. Sleep reactivity to stress in insomniacs. In: Chokroverty S, editor. Congress of the World Association of Sleep Medicine WASM. Berlin: Elsevier; 2005. [Google Scholar]
- 23.Stepanski E, Glinn M, Zorick F, et al. Heart rate changes in chronic insomnia. Stress Med. 1994;10:261–6. [Google Scholar]
- 24.Kales A, Vgontzas AN. Predisposition to and development and persistence of chronic insomnia: importance of psychobehavioral factors. Arch Intern Med. 1992;152(8):1570–2. [PubMed] [Google Scholar]
- 25.Bonnet MH, Arand DL. Situational insomnia: consistency, predictors, and outcomes. Sleep. 2003;26(8):1029–36. doi: 10.1093/sleep/26.8.1029. [DOI] [PubMed] [Google Scholar]
- 26.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring systems for sleep stages of human subjects. Los Angles: BIS/BRI, UCLA; 1968. [DOI] [PubMed] [Google Scholar]
- 27.Karacan I, Thornby JI, Anch M, et al. Dose-related sleep disturbances induced by coffee and caffeine. Clin Pharmacol Ther. 1976;20(6):682–9. doi: 10.1002/cpt1976206682. [DOI] [PubMed] [Google Scholar]
- 28.Roth T. Measuring treatment efficacy in insomnia. J Clin Psychiatry. 2004;65(Suppl 8):8–12. [PubMed] [Google Scholar]
- 29.Bastien CH, Vallieres A, Morin CM. Precipitating factors of insomnia. Behav Sleep Med. 2004;2(1):50–62. doi: 10.1207/s15402010bsm0201_5. [DOI] [PubMed] [Google Scholar]
- 30.Walsh JK, Vogel GW, Scharf M, et al. A five week, polysomnographic assessment of zaleplon 10 mg for the treatment of primary insomnia. Sleep Med. 2000;1(1):41–9. doi: 10.1016/s1389-9457(99)00006-4. [DOI] [PubMed] [Google Scholar]
- 31.Ohayon MM, Caulet M, Lemoine P. Comorbidity of mental and insomnia disorders in the general population. Compr Psychiatry. 1998;39(4):185–97. doi: 10.1016/s0010-440x(98)90059-1. [DOI] [PubMed] [Google Scholar]
- 32.Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc. 2005;53(Suppl 7):S264–S71. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- 33.Katz DA, McHorney CA. Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med. 1998;158(10):1099–107. doi: 10.1001/archinte.158.10.1099. [DOI] [PubMed] [Google Scholar]
- 34.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541–53. [PubMed] [Google Scholar]