Abstract
Three-component coupling reactions between trialkylphosphines, methyl propiolates, and aldehydes produced 1:1:1 dipolar adducts in moderate-to-excellent yields. The product phosphonium enolate zwitterions were isolated as crystalline solids. X-ray crystallographic analyses of these single crystals established unequivocally the dipolar structures of these tetravalent phosphonium enolate zwitterions. Because phosphonium enolates are the first key intermediates in the nucleophilic phosphine-mediated catalysis of α,β-unsaturated carbonyl compounds, this study provides crucial insight into the mechanisms of Morita–Baylis–Hillman-type reactions.
Phosphine-catalyzed reactions of electron-deficient alkenes have emerged as powerful tools for the preparation of biologically and medicinally useful compounds.1,2 Common to these transformations is the generation of a putative dipolar phosphonium enolate through the addition of a tertiary phosphine to an electrophilic alkene. Focusing on the use of allenoates as substrates, we have explored the various reactions available for the key zwitterionic intermediates and have developed new methods for the syntheses of tetrahydropyridines, dihydropyrroles, dioxanes, and pyrones.3 In those studies, the reaction pathways of the proposed intermediates could be controlled by varying the nature of the electrophile4 or the structure of the initial zwitterionic adduct by using a bulky phosphine.3d Although this approach has proved fruitful, leading to the discovery of an array of new reactions, we and others have never directly observed any of the conjectured zwitterionic intermediates.5 Given the pivotal mechanistic roles played by phosphonium enolate intermediates, knowledge of their structures and reactivities will greatly benefit the further development of phosphine-catalyzed reactions. Herein, we report the syntheses of stable tetravalent phosphonium enolates through simple one-pot, three-component processes and the X-ray crystallographic characterization of these dipolar intermediates.
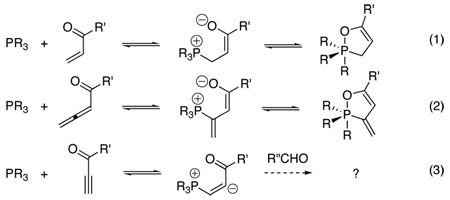
Structural studies of tetravalent phosphonium zwitterions are complicated by the ability of their phosphorous atoms to adopt multiple valence structures. In particular, β-phosphonium enolates arising from the addition of trivalent phosphines to α,β-unsaturated carbonyl compounds are unstable and exist mainly as isomeric pentavalent phosphoranes (eqs 1 and 2).6 For this reason, whereas a number of 1,2-λ5-oxaphospholenes have been characterized well,7 direct observation of tetravalent phosphonium enolates has remained an elusive goal, even though the mechanistic implications for various phosphine-catalyzed processes would be immense.8
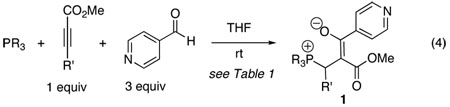
Following our interest in the chemistry of vinyl phosphonium enolates (eq 2) vis-à-vis that of alkyl derivatives (eq 1),2, 3 we pondered the reactivity of a further type of vinyl phosphonium zwitterion: one derived from an electron-deficient alkyne (eq 3). We were particularly intrigued to examine its potential to undergo addition to an aldehyde—and to determine the structure of any such adduct. We were pleased to find that the three-component coupling reaction of PMe3, methyl phenylpropiolate, and 4-pyridinecarboxaldehyde proceeded smoothly in THF at room temperature to provide yellow crystals of a 1:1:1 adduct 1a (R = Me; R′ = Ph) in 83% yield (eq 4, Table 1). The 1H, 13C, and 31P NMR spectral data of 1a suggested a dipolar structure. Most notably, the 31P NMR spectrum exhibited a diagnostic signal for tetravalent phosphorus at +16.0 ppm (entry 1).9 The reaction of PBu3 provided a similar dipolar adduct 1b (31P NMR: δ= +32.2 ppm) in 91% yield under otherwise identical reaction conditions (entry 2). This reaction proved to be a general one for a range of methyl propiolates of varying steric and electronic nature (entries 2–5).
Table 1.
Synthesis and structural data of phosphonium enolate zwitterions 1
| entry | phosphine | R′ | time (h) | temperature | yield (%)a | product | δP (ppm) | P+–O− distance (Å) | C4–C3–C6–O7 dihedral angle |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PMe3 | Ph | 12 | rt | 83 | 1a | +16.0 | 2.821 | 175.74° |
| 2 | PBu3 | Ph | 6 | rt | 91 | 1bb | +32.3 | 2.947 & 2.941 | 146.84 & 174.63° |
| 3 | PBu3 | H | 0.5 | rt | 95 | 1c | +32.4 | NA | NA |
| 4 | PBu3 | CH3 | 5.5 | rt | 33 | 1d | +35.8 | NA | NA |
| 5 | PBu3 | CO2Me | 1 | rt | 84 | 1e | +36.2 | NA | NA |
| 6 | PMe2Ph | Ph | 12 | rt | 81 | 1fb | +17.4 | 2.597 & 2.620 | 2.95 & 175.29° |
| 7 | PMePh2 | H | 12 | rt | 77 | 1g | +22.4 | NA | NA |
| 8 | PMePh2 | Ph | 72 | 80 °Cc | 0 | 1h | +16.5 | NA | NA |
| 9 | PPh3 | Ph | 168 | 80 °Cc | No reaction | NA | NA | NA | NA |
| 10 | PPh3 | H | 168 | 80 °Cc | No reaction | NA | NA | NA | NA |
Isolated yield.
Crystals of 1b and 1f contained two zwitterions per unit cell; tabulated data are presented for each molecule.
Sealed tube.
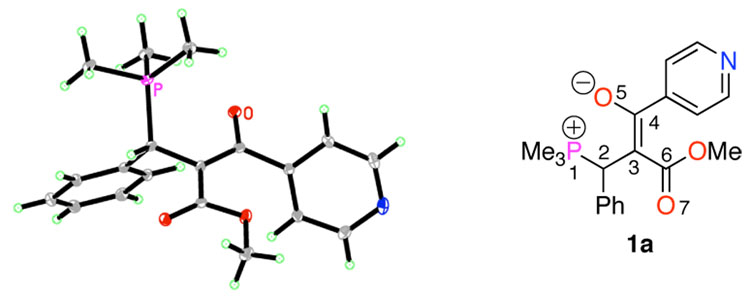
The X-ray crystallographic data for 1a reveals that the phosphorous atom exists in a tetrahedral geometry and does not bond covalently with the enolate oxygen atom, as evidenced by the P1–O5 distance of 2.821 Å (Figure 1).10,11 The C3–C4 and C4–O5 bonds (1.393 and 1.271 Å, respectively) both possess partial double bond character, with the negative charge dispersed mainly between the C3 and O5 atoms. Although the carbomethoxy group appears to contribute to the delocalization of the negative charge only to a small degree, as indicated by the C3–C6 and C6–O7 bond lengths (1.440 and 1.230 Å, respectively), its near in-plane orientation with the enolate (C4–C3–C6–O7 dihedral angle: 175.74°) suggests a possible means of stabilization of the dipolar structure.
Figure 1.
ORTEP depiction of 1a (50% thermal ellipsoids).
The crystals of 1b and 1f contained pairs of conformationally distinct non-interacting zwitterions in each unit cell. The distances between the P1 and O5 atoms in the pair of 1b zwitterions (2.947 and 2.941 Å, respectively) reflect the increased steric bulk around the phosphorus center of 1b relative to that in 1a. Again, the negative charge is localized on each C3–C4–O5 enolate moiety. In fact, in one of the two conformations, the ester is twisted out of conjugation from the enolate (C4–C3–C6–O7 dihedral angle: 146.84°; Table 1, entry 2). The more electron deficient phosphonium center in 1f resides closer to the oxygen atom than that in 1a, despite the increased steric bulk around the phosphorus atom (P1–O5 distances in 1f: 2.597 and 2.620 Å, respectively).
Our X-ray crystallographic analyses of 1a, 1b, and 1f provide the first direct experimental proof of the tetravalency of phosphorus atoms in phosphonium enolate zwitterions; previously, evidence for their structures was implied from NMR and IR spectroscopic data.8 We construe that the presence of electron-donating alkyl groups on the phosphonium centers in our zwitterions decreases their impetus for conversion to pentavalent phosphoranes. Indeed, a number of stable oxaphospholenes have been isolated containing three aryl substituents on pentavalent phosphorus atoms.12 Accordingly, we turned our attention to zwitterionic systems containing one or more aryl substituents on their phosphorus atoms. We isolated zwitterions 1f and 1g from the reactions of PMe2Ph with methyl phenylpropiolate and PMePh2 with methyl propiolate, respectively (Table 1, entries 6 and 7). The reaction of PMePh2 with methyl phenylpropiolate did produce a zwitterion that was observable in solution (NMR spectroscopy), but not isolable (entry 8). In contrast, the reactions of PPh3 did not provide any detectable zwitterions (entries 9 and 10).13 These results are consistent with our hypothesis that electron-releasing alkyl substituents on the phosphonium center play a critical role in stabilizing phosphonium enolate zwitterions.
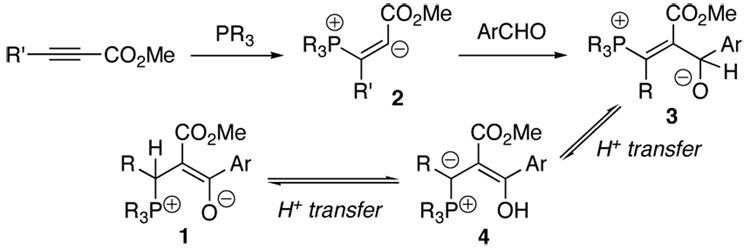
We propose that zwitterion 1 is formed through conjugate addition of the phosphine to the propiolate and subsequent nucleophilic addition of the vinyl anion 2 to 4-pyridinecarboxaldehyde (Scheme 1).14 The resulting zwitterion 3, upon proton transfer, forms the ylide 4. Another proton transfer process affords the zwitterion 1.15
Scheme 1.
Proposed mechanism for the formation of 1
This paper describes the synthesis of stable phosphonium enolate zwitterions, which have been proposed as intermediates in Morita–Baylis–Hillman (MBH) reactions, through novel three-component coupling reactions between tertiary phosphines, alkynoates, and aldehydes. We report, for the first time, the isolation and X-ray crystallographic characterization of such phosphonium enolate zwitterions, establishing the tetravalent nature of their phosphorous atoms unequivocally. These structures, which stand in contrast to those of the well-established pentavalent 1,2-λ5-oxaphospholenes, might explain the instability and high reactivity of phosphonium enolate zwitterions in MBH-type reactions. Our future efforts will focus on exploring the synthetic utility of zwitterions 1 and on extending this study to novel catalytic processes involving phosphonium enolates.
Supplementary Material
Representative experimental procedures and spectral data for all new compounds (PDF). Crystallographic data for compounds 1a, 1b, and 1f (CIF). This information is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgment
We thank UCLA and the NIH (R01GM071779) for funding. C.E.H. is a recipient of a USPHS national research service award (GM08496). We thank Dr. Saeed Khan for performing the crystallographic analyses. O.K. thanks Dr. Chulbom Lee for helpful discussions.
References
- 1.For reviews on the Morita–Baylis–Hillman reaction, see: Basavaiah D, Rao AJ, Satyanarayana T. Chem. Rev. 2003;103:811. doi: 10.1021/cr010043d.Langer P. Angew. Chem., Int. Ed. 2000;39:3049. doi: 10.1002/1521-3773(20000901)39:17<3049::aid-anie3049>3.0.co;2-5.Ciganek E. Org. React. 1997;51:201.
- 2.For reviews on phosphine-catalyzed reactions of activated allenes, see: Lu X, Du Y, Lu C. Pure Appl. Chem. 2005;77:1985.Methot JL, Roush WR. Adv. Synth. Catal. 2004;346:1035.Lu X, Zhang C, Xu Z. Acc. Chem. Res. 2001;34:535. doi: 10.1021/ar000253x.
- 3.(a) Zhu X-F, Lan J, Kwon O. J. Am. Chem. Soc. 2003;125:4716. doi: 10.1021/ja0344009. [DOI] [PubMed] [Google Scholar]; (b) Zhu X-F, Henry CE, Kwon O. Tetrahedron. 2005;61:6276. [Google Scholar]; (c) Zhu X-F, Henry CE, Wang J, Dudding T, Kwon O. Org. Lett. 2005;7:1387. doi: 10.1021/ol050203y. [DOI] [PubMed] [Google Scholar]; (d) Zhu X-F, Schaffner A-P, Li RC, Kwon O. Org. Lett. 2005;7:2977. doi: 10.1021/ol050946j. [DOI] [PubMed] [Google Scholar]; (e) Tran YS, Kwon O. Org. Lett. 2005;7:4289. doi: 10.1021/ol051799s. [DOI] [PubMed] [Google Scholar]
- 4.Dudding T, Kwon O, Mercier E. Org. Lett. 2006;8:3643. doi: 10.1021/ol061095y. [DOI] [PubMed] [Google Scholar]
- 5.Attempts to isolate or observe the 1:1 dipolar adducts of various trialkylphosphines and allenoates led only to oligomerization of the allenoates and recovery of the phosphines.
- 6.For the first report of a five-coordinate phosphorus-containing molecule, PPh5, see: Wittig G, Rieber M. Naturwissenschaften. 1948;35:345.For reviews, see: Gillespie P, Ramirez F, Ugi I, Marquarding D. Angew. Chem., Int. Ed. 1973;12:91.Kumara Swamy KC, Satish Kumar N. Acc. Chem. Res. 2006;39:324. doi: 10.1021/ar050188x.
- 7.(a) Ramirez F, Madan OP, Heller SR. J. Am. Chem. Soc. 1965;87:731. [Google Scholar]; (b) Gorenstein D, Westheimer FH. J. Am. Chem. Soc. 1970;92:634. [Google Scholar]; (c) Buono G, Peiffer G. Tetrahedron Lett. 1972;13:149. [Google Scholar]; (d) Buono G, Llinas JR. J. Am. Chem. Soc. 1981;103:4532. [Google Scholar]; (e) McClure CK, Jung K-Y. J. Org. Chem. 1991;56:867. [Google Scholar]; (f) McClure CK, Mishra PK, Grote CW. J. Org. Chem. 1997;62:2437. doi: 10.1021/jo962144v. [DOI] [PubMed] [Google Scholar]; (g) Fürmeier S, Lau MML, Lie Ken Jie MSF, Lützen A, Metzger JO. Eur. J. Org. Chem. 2003:4874. [Google Scholar]
- 8.One exception is noted from Ramirez’s studies, in which the 1:1 adduct formed between a trialkylphosphine and 3-benzylidene-2,4-pentanedione was reported to be a dipolar ion: Ramirez F, Pilot JF, Smith CP. Tetrahedron. 1968;24:3735.Ramirez F, Pilot JF, Madan OP, Smith CP. J. Am. Chem. Soc. 1968;90:1275.
- 9.Hudson HR, Dillon KB, Walker BJ. 31P NMR Data of Four Coordinate Phosphonium Salts and Betaines. In: Tebby JC, editor. Handbook of Phosphorus-31 Nuclear Magnetic Resonance Data. Boca Raton: CRC Press; 1991. pp. 181–226. [Google Scholar]
- 10.To the best of our knowledge, this phosphonium enolate zwitterion is the first to have been characterized using X-ray crystallography.
- 11.The average trigonal bipyramidal phosphorane P–O distances measured from 20 crystalline organic compounds are 1.689 ± 0.024 Å(axial) and 1.619 ± 0.024 Å (equatorial); see: Allen FH, Kennard O, Watson DG, Brammer L, Orpen AG, Taylor R. J. Chem. Soc., Perkin Trans. 1987;2:S1.
- 12.(a) Vedejs E, Steck PL. Angew. Chem., Int. Ed. 1999;38:2788. doi: 10.1002/(sici)1521-3773(19990917)38:18<2788::aid-anie2788>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]; (b) Yavari I, Alizadeh A. Synthesis. 2004:237. [Google Scholar]
- 13.This situation is reminiscent of the stabilization of betaine intermediates in Wittig reactions employing PMe3 rather than PPh3; see: Wittig G, Rieber M. Liebigs Ann. Chem. 1949;562:177.
- 14.(a) Nozaki K, Sato N, Ikeda K, Takaya H. J. Org. Chem. 1996;61:4516. doi: 10.1021/jo951828k. [DOI] [PubMed] [Google Scholar]; (b) Bhuvan Kumar NN, Chakravarty M, Kumara Swamy KC. New J. Chem. 2006;30:1614. [Google Scholar]
- 15.Trace amounts (0.5–7%) of a product containing two units from 4-pyridinecarboxaldehyde and one from methyl propiolate were isolated from several of the reactions. The formation of this product can be rationalized from consideration of our proposed mechanism; see the Supporting Information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative experimental procedures and spectral data for all new compounds (PDF). Crystallographic data for compounds 1a, 1b, and 1f (CIF). This information is available free of charge via the Internet at http://pubs.acs.org.