Abstract
This study examined the effects of endogenous overexpression of laminin-8 on angiogenesis and wound healing in primary human dermal microvascular endothelial cells (HDMECs). HDMECs expressed laminin-8 and laminin-10, but no other laminins, as determined by radioimmunoprecipitation assay using a panel of antibodies to individual laminin chains. To study laminin-8 function, full-length human laminin α4 cDNA was retrovirally transferred to HDMEC, and specific overexpression of laminin-8 was verified by Western blot. Laminin-8 overexpression promoted endothelial cell spreading and migration in scratch assays and accelerated angiogenic tubule formation in collagen gel overlay assays. Strong inhibitory effect of β1 integrin and weak inhibition by αvβ3 integrin antibodies were observed in laminin-8-stimulated cell migration, but only β1 integrin antibodies affected tubule formation. These studies suggest that laminin-8 overexpression may prove to be a useful method to engineer HDMECs to promote angiogenesis and wound repair.
INTRODUCTION
Laminins are a family of large, extracellular heterotrimeric basement membrane glycoproteins (Timpl and Brown, 1994; Engvall and Wewer, 1996). Laminins demonstrate specific tissue distributions, and tissue specificity of laminin function is achieved through the diversity of combinations of laminin α, β, and γ chains (Burgeson et al., 1994). To date, five α chains, three β chains, and three γ chains have been identified (Table 1). The α chains contain a globular (G) domain of about 100 kDa at their C-terminus, which functions as a primary binding site for integrin cell surface receptors (Timpl et al., 2000).
Table 1.
Nomenclature of currently identified laminins
| Name | Composition | Tissue distribution | References |
|---|---|---|---|
| Laminin-1 | α1,β1,γ1 | Epithelium | Timpl et al. (1979) |
| Laminin-2 | α2,β1,γ1 | Muscle, nerve | Ehrig et al. (1990) |
| Laminin-3 | α1,β2,γ1 | Neuromuscular junction | Hunter et al. (1989) |
| Laminin-4 | α2,β2,γ1 | Ligaments | Engvall et al. (1990) |
| Laminin-5 | α3,β3,γ2 | Epithelium | Marinkovich et al. (1992b) |
| Laminin-6 | α3,β1,γ1 | Epithelium | Marinkovich et al. (1992a) |
| Laminin-7 | α3,β2,γ2 | Epithelium | Champliaud et al. (1996) |
| Laminin-8 | α4,β1,γ1 | Blood vessels | Gonzales et al. (2001) |
| Laminin-9 | α4,β2,γ1 | Neuromuscular junction | Sunderland et al. (2000) |
| Laminin-10 | α5,β1,γ1 | Epithelium, blood vessels | Li et al. (2003) and Miner et al. (1995) |
| Laminin-11 | α5,β2,γ1 | Epithelium | Champliaud et al. (1996) |
| Laminin-12 | α5,β1,γ3 | Epithelium, muscle | Koch et al. (1999) |
Laminins are involved in many important biologic processes including embryogenesis, tumor invasion, tissue differentiation and wound healing (Ryan et al., 1996). The physiologic importance of the laminins in these processes is illustrated by mutations of laminin genes, which are associated with human diseases (McGowan and Marinkovich, 2000). Gene mutations that affect the α2 chain gene can lead to muscular dystrophy (Wewer and Engvall, 1996). Mutations that induce defects of α3, β3, or γ2 chains (Uitto et al., 1997) can result in severe mucocutaneous blistering diseases (junctional epidermolysis bullosa) (Marinkovich, 1999).
The process of angiogenesis shows many interesting parallels to the process of epithelial wound healing (Folkman and Shing, 1992). Each process requires the production of basement membrane components, spreading and migration of cells upon these components, and eventual assembly of the basement membrane after cell migration. Endothelial basement membranes are similar to epithelial basement membranes in that they contain a single predominant large laminin, laminin-10, as well as a smaller laminin, laminin-8 (Lefebvre et al., 1999; Gonzales et al., 2001; Sixt et al., 2001). In molecular similarity, laminin-8 (α4β1γ1) most closely resembles laminin-6 (α3β1γ1). Each molecule contains the same β1 and γ1 chains, and of all laminin chains, the α4 chain shows the highest sequence homology to the laminin α3a chain (Liu and Mayne, 1996; Iivanainen et al., 1997; Richards et al., 1997). Both laminin-6 and laminin-8 adopt a Y shape as shown by rotary shadowing electron microscopy (Marinkovich et al., 1992a; Kortesmaa et al., 2000). Like laminin α3 chain, the laminin α4 chain appears to be processed in the tissue, through proteolytic cleavage at the junction of the G3–4 domains (Talts et al., 2000).
Laminin-8, in particular its α4 G domain, has been implicated in the regulation of endothelial cell survival (DeHahn et al., 2004), as well as endothelial cell migration and adhesion (Gonzales et al., 2001; Gonzalez et al., 2002), which occurs in association with the activation of the Rac1 small GTPase (Fujiwara et al., 2004). While the use of recombinant laminin α4 G domain fragments in the studies above has proven informative, studies that address the effects of endogenous expression of laminin-8 on endothelial cell function are thus far lacking.
In the work described in this report, we have used a retroviral vector to generate microvascular endothelial cells that overexpress the laminin α4 chain, and have characterized the isoforms of the laminins synthesized. Using these cells, we have examined how this overexpression affects wound healing and angiogenesis in in vitro models. Methods to increase wound healing and angiogenesis by overexpression of laminins in vitro may increase our understanding of how to apply these procedures in vivo when these functions are defective.
RESULTS
Expression of laminin-8 and -10 by human dermal microvascular endothelial cells
To characterize the endogenous expression of laminins in human dermal microvascular endothelial cells (HDMECs), we performed radioimmunoprecipitation studies of conditioned medium from primary cultures of HDMECs, using a panel of specific laminin antibodies. An antibody to the laminin α5 chain precipitated only one band of high electrophoretic migration by non-reduced SDS-PAGE (Figure 1a, lane 1), whereas antibodies to laminin β1 and γ1 chains (Figure 1a, lanes 2 and 3) precipitated two laminin species, one with high molecular weight, which is same as that precipitated by laminin α5 antibody. No laminins were precipitated by antibody to laminin α2 chain or by control mouse IgG (Figure 1a, lanes 4 and 5). These results demonstrate that HDMECs produced two forms of laminin, a higher molecular weight species that contains laminin α5, β1, and γ1 chains, namely laminin-10, and another lower molecular weight species that contains β1, γ1 chains, but not the α5 or α2 chain.
Figure 1. (a) Laminin expression in primary HDMECs.
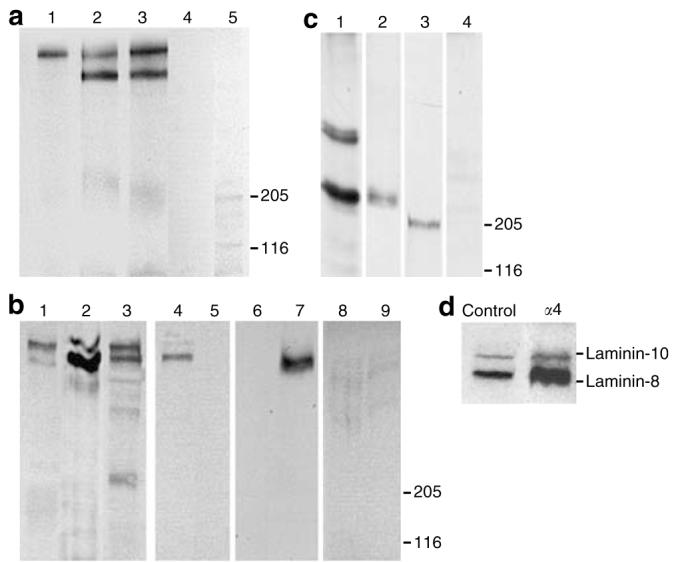
Anti-laminin α5 mAb 4C7 (lane 1), anti-laminin γ1 mAb 2E8 (lane 2), anti-laminin β1 mAb 545 (lane 3), anti-laminin α2 chain antibody 5H2 (lane 4), or mouse IgG (lane 5) were used to immunoprecipitate laminins from HDMEC medium. Precipitated proteins were washed extensively, then dissolved in sample buffer and separated by SDS-PAGE under non-reducing conditions on a 3–5% acrylamide gel, and then visualized by autoradiography. (b) Western blot analysis of HDMEC laminins. Conditioned medium of LSV5 keratinocytes (lanes 1, 4, 6, and 8) or HDMECs (lanes 2, 5, 7, and 9) or HDMEC matrix (lane 3) was separated by non-reduced SDS-PAGE, transferred to nitrocellulose, and analyzed with the following primary antibodies: human placental laminin pAb (lanes 1–3), laminin-5/6 pAb (lanes 4 and 5), laminin α4 pAb (lanes 6 and 7), non-immune rabbit sera (lanes 8 and 9). (c) Reduced SDS-PAGE analysis of HDMEC laminins. Conditioned medium of HDMECs was separated on 5% acrylamide gels by reduced SDS-PAGE and analyzed by Western blot with the following antibodies: human placental laminin pAb (lane 1), Engelbaeth-Holm-Swarm sarcoma laminin pAb (lane 2), laminin α4 pAb (lane 3), and non-immune rabbit sera (lane 4). (d) Non-reduced Western blot of conditioned HDMEC-LACZ (control) or HDMEC-α4(α4) medium using human placental laminin pAb. Positions of laminin-10 and -8 are shown to the right.
To further identify the lower molecular weight laminin, identified in Figure 1a, conditioned serum-free HDMEC medium was concentrated and examined by Western blot. As the lower molecular weight band seen in HDMEC medium was of the same size as that of laminin-6, we examined in parallel the serum-free conditioned medium of LSV5 epithelial cells. These cells fail to produce laminin-5, but produce laminin-6, which contains an α3 chain, and which is immunologically distinct but structurally similar to laminin α4 chain. (Marinkovich et al., 1993). A polyclonal antibody to human placental laminin, which reacts with laminin β1 and γ1 chains, identified a pair of laminin species in conditioned medium of each cell type and in HDMEC matrix (Figure 1b, lanes 1-3). Laminin-5/6 polyclonal antibody recognized the lower band in LSV5 medium, identifying it as laminin-6, but failed to recognize the lower band in HDMEC medium (Figure 1b, lanes 4 and 5). In contrast, a polyclonal antibody against the laminin α4 chain recognized the lower laminin band in HDMEC medium, but not in LSV5 medium (Figure 1b, lanes 6 and 7). These results show that the smaller laminin species produced by HDMECs contains an α4, but not an α3 chain.
HDMECs laminins were then analyzed by Western blot under reducing conditions using several antibodies (Figure 1c). An antibody directed against a whole molecule preparation of human placental laminin, which was affinity purified with monoclonal antibody (mAb) 4C7, specific to the laminin α5 chain, reacts with bands in the 210 and 220 kDa range, which represent the β1 and γ1 laminin chains. In addition, this antisera reacts with a tight doublet in the 400 kDa range, which represents laminin α5 chain (Figure 1c, lane 1). A polyclonal antibody directed against a laminin preparation from the mouse Engelbaeth-Holm-Swarm sarcoma tumor, which contains laminin-1, but no laminin-10, recognized β1 and γ1 chains in HDMEC medium, but no reactivity in the 400 kDa range could be demonstrated (Figure 1c, lane 2). Finally, antisera specific to laminin α4 chain recognized an approximately 200 kDa band in conditioned HDMEC medium (Figure 1c, lane 3). These results show that HDMEC laminins contain α4β1γ1 and α5β1γ1 chains, but no detectable α1, α2, or α3 chains.
Stable overexpression of laminin-8 in primary cultures of HDMEC
To further evaluate the functional role of laminin-8 in HDMEC, full-length human laminin α4 or β-galactosidase cDNA was overexpressed in HDMECs using retroviral vectors, which have been shown to produce durable expression in mammalian cells. Using human placental laminin polyclonal antibody (pAb), Western blot analysis of HDMECs, overexpressing the full-length laminin α4 cDNA (HEMEC-α4), revealed an increased expression of laminin-8, without a significant change in laminin-10 (Figure 1d) or total protein expression as evaluated by Coomassie Brilliant Blue staining (not shown).
Effect of laminin-8 overexpression on HDMEC Morphology
Figure 2 illustrates the effect of overexpression of laminin-8 on cell morphology, Factor 8, platelet-endothelial cell adhesion molecule and collagen synthesis. Normal HDMECs clearly display two characteristic cell types in cell culture; epithelioid cells are arranged in islands, which are surrounded by spindle-shaped cells. These two normal cell populations have been more fully characterized previously (Lipton et al., 1991). Overexpression of laminin-8 clearly promoted a population of cells with a uniform epithelioid morphology (Figure 2b). These cells were transduced with the laminin α4 chain retroviral vector and selected with Blasticidin, and considered the pure population harboring the α4 vector, while control cells transfected with retroviral vector alone showed typically two types of morphology, epithelioid and spindle shaped (Figure 2a). PECAM and Factor 8 expression were equivalent in both control and in cells overexpressing laminin-8 with the typical decrease in Factor 8 in spindle-shaped populations (Figure 2c). A major difference in the two populations is the marked increase in type IV collagen deposition in the underlying matrix (Figure 2h), suggesting that the synthesis of laminin-8 influences type IV collagen deposition, a major constituent of the endothelial basement membrane.
Figure 2. Laminin-8 promotes epithelial morphology of HDMECs.

HDMECs were transduced (b, d, f,and h) with full-length laminin α4 chain sense cDNA or (a, c, e, and g) with retroviral vector alone as control and selected with Blasticidin. (a) Phase-contrast photography revealed typical mixture of epithelioid and spindled cells. (b) Laminin α4 sense-transduced cells were of epithelioid morphology. Both cell types were examined by immunohistochemistry using antibodies directed to (c and d) von Willebrand factor, (e and f) PECAM, and (g and h) type IV collagen. Bar = 20 μm.
Effects of laminin-8 overexpression on angiogenesis
We further examined the effects of laminin-8 on endothelial tubule formation (Figure 3a-d). HDMECs-α4 (Figure 3a) showed a more rapid tubule formation at 4 hours in collagen overlay assay, compared to control cells (Figure 3b). There was no significant difference at 24 hours (Figure 3c and d), suggesting that laminin-8 promotes HDMEC tubule formation at early stage. The subsequent study of tubule formation inhibition in HDMECs-α4 using anti-integrin antibodies revealed that the tubule formation could be partially blocked by antibodies directed to α2 or α6 integrins at 4 and 8 hours, and almost completely blocked by using α2 and α6 integrins together or β1 integrin at 8 hours (Figure 3e-i), indicating that α2β1 and α6β1 integrins play major roles in promoting angiogenesis.
Figure 3. (a-d) Laminin-8 promotes HDMEC tubule formation.

(a, c) HDMECs-α4, (b, d) HDMEC-LACZ were evaluated by tubule/vessel formation collagen overlay assay and observed by phase-contrast microscopy at (a, b) 4 hours and (c, d) 24 hours. (e—i) HDMEC tubule formation via α2β1 and α6β1 integrins. HDMECs-α4 were grown to confluence, and incubated with integrin antibodies overnight prior to collagen gel overlay and observed at 4, 8, and 24 hours. Antibody of (f) α2 integrin or (g) α6 integrin alone could partially block tubule formation at 8 hours, (i) β1 integrin alone or (h) α2 and α6 integrins together could completely block the tubule formation at 8 hours. Panel e is control with mouse IgG. Bar = 200 μm.
Furthermore, laminin-8 overexpression greatly promoted HDMECs spreading and migration during scratch assays. At 24 hours, HDMECs-α4 consistently filled the gap almost completely, while control cells consistently failed to cover most of the scratched surface (Figure 4a-d). To test which integrin was involved in the process of migration, we added several integrin-blocking antibodies to the growth medium of HDMECs-α4 after scratching. The study revealed that the migration of HDMECs-α4 during wound healing could be blocked by α6 or β1 integrin antibody, and partially by αvβ3 antibody (Figure 4e-j), suggesting α6β1 integrin might play an important role in the migration of HDMECs on laminin-8.
Figure 4. (a-d) Laminin-8 promotes HDMEC migration.

(b, d) HDMEC-α4 or (a, c) HDMEC-LACZ were grown to confluence in 35 mm dishes. Cross-shaped scratches were made and cells were analyzed at (a, b) 0 and (c, d) 24 hours. (e—j) Antibody inhibition of HDMEC migration. HDMECs-α4 cells were evaluated by scratch assay at (e) 0 or (f—j) 24 hours in the presence of inhibitory antibodies to integrins (g) α6, (h) αvβ3, (i) β1, (j) β1 and αvβ3 together or (b) mouse IgG. Bar = 200 μm.
As previous reports have focused mainly on recombinant α4 G domain in cell-based assays, we wished to determine whether there were any functional differences between endogenous full-length whole molecule protein and the G domain alone. We examined cell migration in a transwell assay with recombinantly produced α4 G1—3 domain protein (Figure 5a). The G1—3 is the region of the laminin α4 G domain, which remains after processing occurs (Talts et al., 2000). Laminin α4 G domain coated at a concentration of 10 μg/ml supported rapid attachment and spreading of HDMEC cells (Figure 5b), and the integrins that mediated this attachment were subsequently analyzed (Figure 5c). The α3 integrin-blocking antibody showed no effect on attachment, whereas both α2 and α6 antibodies showed partial inhibition. Approximately 40% inhibition of attachment was seen with αvβ3 blocking antibody LM-609, whereas greater inhibition was noted with β1 integrin antibody P5D2. The β1 and αvβ3 inhibitory antibodies combined together served to totally inhibit HDMEC attachment. While mAb P5D2 against β1 integrin did not have a significant inhibiting effect on HDMEC migration, mAb LM-609 against αvβ3 integrin blocked migration almost completely (Figure 5d).
Figure 5. Functional analysis of recombinant laminin α4 G domain.

(a) Conditioned medium of laminin α4 G domain, expressing 293-EBNA cells (lanes 1 and 3) or control parental 293-EBNA cells (lanes 2 and 4) was purified by FLAG antibody affinity chromatography, separated by SDS-PAGE, and stained with Coomassie Blue total protein stain (lanes 1 and 2) or with anti-laminin α4 pAb (lanes 3 and 4). (b) Improved spreading and flattening of HDMECs on G1–3 coated culture surfaces. (c) The attachment of endothelial cells to laminin α4G domain via β1 and αvβ3 integrins. The 96-well plates were pre-coated with 10 μg/ml laminin α4G1-3 domain protein or BSA as control, and then utilized in HDMEC colorimetric cell attachment assays in the presence of 15 μg/ml of each of the various integrin-blocking antibodies as indicated under the bars. The attached cells were stained with 0.1% crystal violet and analyzed by colorimetric measurement at OD570. (d) Laminin α4 G domain promotes HDMEC migration via αvβ3 integrin. The bottoms of Costar migration chamber were pre-coated with BSA (left bar) or laminin α4 G1-3 protein (all other condition shown on the right), HDMECs were then plated on the top of the migration chambers and immerged in the growth medium containing mouse IgG 15 μg/ml (control) or the indicated antibodies at the same concentration, and cells were counted in the lower chamber after 24 hours.
DISCUSSION
As a first step in these studies, it was necessary to verify which laminins were produced by primary HDMEC and to analyze their deposition into matrix. Laminin-10 and -8 were both produced by these cells, and laminin-8 appeared to be significantly deposited onto the culture substrate. This is consistent with previous results, which have shown localization of laminin α4 chain in endothelial cell cultures by indirect immunofluorescent microscopy (Gonzales et al., 2001). It is possible that sequences on the α4 chain could enhance interaction with tissue culture plastic or other matrix molecules. In particular, we noted increased extracellular type IV collagen deposition in cells overexpressing laminin-8. The increased deposition of collagen IV is probably due to the increased deposition of laminin-8. A recent knockout mouse model showed that the deletion of the laminin α4 chain leads to the dramatically reduced deposition of type IV collagen and nidogen in the basement membrane zone of blood vessel and impaired microvessel maturation, suggesting that laminin 8 is required for the organized assembly of type IV collagen and nidogen into the capillary basement membrane zone and is a central player in microvessel growth (Thyboll et al., 2002). An early study found that laminin appeared before collagen IV (Leivo et al., 1980). In cultured cells, inactivation of laminin synthesis led to abolished deposition of type IV collagen and nidogen, failure of basement membrane zone formation, and loss of cell polarity (De Arcangelis et al., 1996). It is possible that laminin-8 bound to the cell surface integrin receptor can in turn stabilize type IV collagen of organization in the extracellular matrix. Alternatively, laminin-8 binding to its cell surface ligands could also trigger signaling pathways, which influence the expression of collagen IV and other extracellular matrix molecules.
Laminins are large multifunctional proteins, which are deposited into the basement membrane and into attachment structures in a precise manner. While studies that examine laminin-8 deposited onto a culture substrate can be utilized to explain some aspects of laminin-8 function, it is likely that more dynamic processes such as migration, and tubule formation require precise coordination of cell movement and laminin-8 secretion. Accordingly, studies of laminin-8 overexpression provide information that may not be obtained with studies of purified proteins. It is known that laminin α chain synthesis is the rate-limiting step in the assembly of several types of laminins, including laminin-1 and laminin-5 (Yurchenco and O’Rear, 1994; Matsui et al., 1995). The significant overexpression of laminin-8, which we achieved after transduction of laminin α4 chain cDNA to HDMECs, is consistent with the hypothesis that laminin α4 chain expression is the rate-limiting step in laminin-8 synthesis.
The role of laminins in tubule formation has been previously studied, when it was believed that laminin-1 was the primary endothelial laminin (Grant et al., 1994). The current study is the first to show that laminin-8 promotes tubule formation in endothelial cells. In this process, α2β1 and α6β1 integrins appear to play a significant role, as evidenced by our antibody inhibition studies. Thus, several stages of angiogenesis appear to be significantly influenced by laminin-8, including cell attachment, cell migration and tubule formation. Targeting of laminin α chains in inducing overexpression may be of use in future applications of genetic therapy in vivo.
Previous studies have produced some conflicting results, showing either that endothelial cells bind to laminin-8 via β1-containing integrins (Kortesmaa et al., 2000; Talts et al., 2000), or that αvβ3 integrin is the primary receptor for laminin-8 in endothelial cells (Gonzales et al., 2001). From our studies, it is clear that HDMECs utilize both β1 integrins as well as αvβ3 integrin in their interactions with laminin-8. Specifically, each of the integrins binds to the region of the G1–3 domain of the α4 chain. In the case of migration, we have certainly been able to show changes in the utilization of the two principal laminin-8 binding integrins, on purified versus endogenously synthesized laminin-8. While αvβ3 integrin appears to play the more important role in HDMEC migration on purified laminin-8 α4 G domain in the transwell chambers, α6β1 integrin appears to play a more significant role in the utilization of endogenously synthesized laminin-8 during scratch migration assays. Other studies have suggested that recombinant α4 G domain does not have identical functional properties compared to the native laminin-8 molecule (Talts et al., 2000). It is possible that the endogenous laminin whole molecule has different tertiary structural conformation, compared with α4 G domain alone, and that a cooperative interaction of these integrins, in a process such as cell migration, occurs only on native full-length laminin-8.
MATERIALS AND METHODS
Antibodies
Polyclonal antibodies directed to human placental laminin β1 and γ1 chain complex were purchased from Chemicon International Inc. (Temecula, CA). mAbs 4C7, 545, and 2E8, directed against human laminin α5, β1, and γ1 chain, respectively, were generously donated by Dr Eva Engvall (Burnam Institute, La Jolla, CA). Type IV collagen mAb was purchased from Research and Diagnostic Systems (Berkeley, CA), von Willebrand factor antibody from DAKO Corporation (Carpinteria, CA), and PECAM-1 antibody from Endogen (Woburn, MA). Antibody specific against laminin α4 chain (Pierce et al., 1998) was kindly provided by Dr Jeff Miner (Washington University, St Louis, MO). Human placental laminin pAb were purchased from Chemicon (Temecula, CA); laminin-5/6 pAb, Engelbaeth-Holm-Swarm sarcoma laminin pAb was described previously (Marinkovich et al., 1992b). The antibodies used for different laminin chains are summarized in Table 2. mAbs against various integrin subunits including α2 (clone P1E6), α6 (clone GoH3), β1 (clone P5D2), and αvβ3 (clone LM-609) were purchased from Chemicon International Inc. For integrin antibody-blocking analyses in our cell-based assays, we used a concentration of 15 μg/ ml. A variety of reported studies including our own experiences indicate that maximal integrin antibody inhibition is usually achieved in the range of 10–15 μg/ml (Russell et al., 2003). We tried a higher concentration of 30 μg/ml for our antibodies and did not see any additional inhibitory effect.
Table 2.
Summary of antibodies used for laminins or laminin chains
| Antigen | Antibody | Resource/reference |
|---|---|---|
| β1 and γ1 chain | pAb placental laminin | Chemicon |
| α5, β1 γ1 chain | pAb placental laminin | Chemicon |
| α4 chain | pAb α4 | Pierce et al. (1998) |
| Laminin-1 (α1, β1, γ1 | pAb Engelbaeth-Holm-Swarm sarcoma laminin |
Research diagnostics |
| Laminin-5(α3, β3 γ2) | pAb laminin 5/6 | Marinkovich et al.(1992b) |
| Laminin-6(α3, β1 γ1) | pAb laminin 5/6 | Marinkovich et al.(1992a) |
| α5 chain | mAb 4C7 | Tiger et al. (1997) |
| β1 chain | mAb 545 | Marinkovich et al. (1992a) |
| γ1 chain | mAb 2E8 | Engvall et al. (1986) |
| α2 chain | mAb 5H2 | Leivo et al. (1989) |
Cell cultures
Primary HDMECs were isolated from neonatal human foreskin from normal circumcisions as described previously (Normand and Karasek, 1995). Foreskin tissues were obtained using an exempted protocol approved by Stanford University Institutional Review Board and in accordance with the Declaration of Helsinki Principles. HDMECs were cultured in 1% gelatin-coated tissue culture dishes and maintained in a 37°C, 5% CO2 incubator with Iscove’s medium from Gibco BRL (Grand Island, NY) supplemented with 8% fetal bovine serum, 2% human prepartum maternal serum, 2.0 × 10-4M dibutyryl cyclic adenosine monophosphate, 3.0 × 10-5M isobutyl methylxanthine, 0.84 × 10-4M hypoxanthine, and 1.33 × 10-5M thymidine. At passage 1, HDMECs were purified through Ulex europeus-coated Dynal beads according to the manufacturer’s instruction (Dynal Inc., NY), with greater than 95% of purity were achieved as determined by staining for von Willebrand factor, PECAM, and type IV collagen. Third to fourth passage HDMECs were used for the experiments. LSV5 cells were cultured in serum-free keratinocyte growth medium (Clonetics, San Diego, CA) and were generously provided by Dr Jean-Paul Ortonne, Nice, France. LSV5 cells are an SV40 virus-immortalized cell line of skin keratinocytes, from an epidermolysis bullosa patient, which do not produce laminin-8 and -10 and are deficient in laminin-5 protein but have increased the secretion of laminin-6 (Miquel et al., 1996).
Immunostaining for endothelial cell marker expression
The expression of several antigens in HDMECs was examined by immunostaining with specific antibodies as described previously (Zhou et al., 2000). Briefly, cells were fixed in 100% cold methanol for 20 minutes, blocked with 3% H2O2 for 30 minutes to inhibit endogenous peroxidase activity, and incubated with primary antibodies at 4°C overnight. Immunoperoxidase activity was determined using the Vectastain ABC kit and VIP kit (Vector Lab. Inc., Burlingame, CA) according to manufacturer’s instruction. A Zeiss Axioskop II microscope with a digital camera (Zeiss, Hallbergmoos, Germany) was used to capture the images. The quantitation was performed using Zeiss molecular image system AxioVision 3.0 software (issued 10/2,000). A total of 18 areas were analyzed for each condition (two independent cultures were used for each condition, three pictures were taken for each culture, and three areas were analyzed for each picture). The intensity of staining was expressed as mean gray scale value of the staining per pixel (or square picture element). Statistical differences between groups were determined by Student’s t-test. P-value <0.05 is considered as significant.
Immunoprecipitation assay
Purified HDMECs were pulse labeled with 35S methionine/cysteine (Amersham Biosciences, Piscataway, NJ) in methionine- and cysteine-deficient medium, and conditioned medium was collected and immunoprecipitated with antibodies directed to laminin subunits: laminin α5 mAb 4C7, laminin γ1 mAb 2E8, laminin β1 mAb 545, laminin α2 chain mAb 5H2, or mouse IgG as control. Precipitated proteins were washed, dissolved in sample buffer, and separated by SDS-PAGE under non-reducing conditions on a 3–5% acrylamide gel, and then visualized by autoradiography (Marinkovich et al., 1992b).
Transduction of endothelial cells using retroviral vector
Full-length human laminin α4 cDNA was assembled from overlapping cDNA clones generously donated by Drs Alan Richards and Michael Pope (Strangeway Research, Cambridge, UK) and ligated into retroviral vector Lazarus retrovirus, which carries a Blasticitin resistance gene as a selection marker (Deng et al., 1998). Endothelial cells were grown to 20–40% confluence in six-well culture plate and retrovirally transduced with LACZ (β-galactosidase) cDNA (Deng et al., 1998) or full-length laminin α4 cDNA. Briefly, the cells were incubated in the growth medium supplemented with 5 μg/ml of polybrene for 10 minutes prior to infection, overlaid 3 ml/well of viral supernatant supplemented with 5 μg/ml of polybrene, centrifuged at 32°C, 1,000 r.p.m. for 1 hour, incubated at 32°C for additional 2 hours, and then replaced with fresh growth medium. At 48–72 hours after infection, cells were selected with 5 μg/ml of Blasticidin for 5–7 days until it reached 100% pure population harboring the viral structure (as indicated by no viable cells remaining in normal control dish). The initial positive transduction before selection, at an efficiency of 35–40%, and pure positive population after selection were confirmed by staining for β-galactosidase activity in the vector. The expression of laminin-8 was confirmed by Western blot analysis.
Endothelial cell scratch assay
Scratch migration assay was performed as previously described (Russell et al., 2003) with the following modifications. Cells were grown to confluency in a six-well culture plate. Then, a cross-shaped wound (no cell zone) was made by a uniformly placed scratch among the cells with a pipette tip. The floating cells or cells detached from the dish by scratch were washed off and the remaining cells were continually incubated with growth medium in the presence of mitomycin C at 10 μg/ml (Sigma, St Louis, MO). For the antibody inhibition assay, antibodies were added to the growth medium immediately after scratch. The migration of the cells (or the gap filling) was recorded at 12, 24, and 36 hours (only showed 24 hours as representative) through a phase-contrast microscopy, quantified by the time and percentage of the wound gap covered by the cells that migrated in and filled up the gap, and quantified using NIH image software Image J Program (public domain, http://rsb.info.nih.gov/ij/). The center of the cross (where the two scratch lines meet) was used for the positioning. Three independent experiments were performed with each in duplicates, a total of six measurements was obtained for each treatment. The results were analyzed by Student’s t-test. P-value ≤0.05 was significant.
Endothelial cell tubule formation/collagen gel overlay assay
Endothelial cell tubule formation was adapted from a previous method (Kubota et al., 1988), with minor modifications. Confluent endothelial cells cultured in six-well plate were overlaid with 2 ml of collagen solution of 1:1 mixture of Vitrogen 100 (Angiotech BioMaterials, Palo Alto, CA) and 2 × Iscove’s medium (Life Technologies Inc., Carlsbad, CA) and neutralized with 0.5 N NaOH. Solidification of the collagen gel occurred after incubation at 37°C for 30 minutes. Tubule formations were observed at 0, 4, 8, and 24 hours time points. For tubule formation inhibition assay, antibodies directed against various integrins were added to the cell culture medium at 15 μg/ml 14 hours prior to the collagen overlay assay.
Recombinant laminin α4 subdomain expression and purification
Subdomain construct of laminin α4G1–3 was produced in a mammalian expression system utilizing 293 kidney epithelial cells. The vector, pCEP-Pu/Flag, originally from pCEP4 (Invitrogen, Carlsbad, CA), is a puromycin-resistant EBNA episomal vector and engineered to produce an 8-amino-acid Flag-tag. The transfection was performed with Lipofectamine (GibcoBRL) according to the manufacturer’s instruction. After 48 hours, the cells were selected with 10 μg/ml of puromycin, and stable transfectants were used for protein expression. The expressed and secreted fusion protein was collected from cell growth medium and purified through an anti-Flag antibody affinity column according to the manufacturer’s instruction (Sigma). The purified protein was confirmed by Western blot with anti-Flag polyclonal antibody (Sigma).
Laminin α4G1–3 protein was used at a concentration of 10 μg/ml for cell attachment and transwell migration assays. Previous reported studies (Russell et al., 2003) including our own experiences indicate that optimal cell attachment or migration is usually achieved when coating with the protein at 10 μg/ml overnight. Lower protein concentration would result in the less cell adhesion or migration, while no significant increase was observed with higher concentrations.
Endothelial cell transwell migration assay
Eight micrometer pore size Costar transwell migration chambers (Corning, NY) in 24-well plate were used for migration analysis with laminin α4 G domain protein. Briefly, the bottom of each chamber was pre-coated with 250 μl of protein solution at concentration of 10 μg/ml and incubated at 4°C overnight. Subsequently, remaining protein-binding sites were blocked with 0.5 ml/chamber of 50 mg/ml BSA in both the top and bottom of the chamber. Then, 750 μl of medium was added to each well, and 100 μl of endothelial cells at 2 × 106/ml was plated on the top of the chamber and incubated at 37°C for desired time period. For the migration-blocking assay, blocking antibody was added to the medium at 15 μg/ml prior to plating the cells. Cells were fixed with 3% paraformaldehyde at room temperature for 15 minutes and stained with 0.1% crystal violet. The stain was rinsed off thoroughly with water, cells remaining in the top of the migration chamber were removed by lightly swabbing, and stained cells adhering to the coated bottom of the chamber were counted under a phase-contract microscope. The results were analyzed by Student’s t-test. P-value ≤0.05 was significant.
Endothelial cell attachment assay
The 96-well plates were pre-coated with 100 μl/well of protein solution of 10 μg/ml at 4°C overnight and blocked with 100 μl of 20 mg/ml BSA in phosphete-buffered saline at 37°C for 1 hour. Then, HDMECs of 100 μl/well at 6 × 105/ml were added to plate and incubated at 37°C. One hour later, medium and floating cells were removed, and attached cells were fixed with 1.25% glutaraldehyde solution for 20 minutes at room temperature, stained with 0.1% crystal violet, solubilized with 100 μl/well of 10% acetic acid for 10 minutes, and the colorimetric reading at optical density 570 was measured with a spectrophotometric plate reader. For antibody-blocking assay, cells were incubated with blocking antibodies at 15 μg/ml for 20 minutes prior to the plating.
ACKNOWLEDGMENTS
This work was funded through the Office of Research and Development, Palo Alto Veterans Affairs Health Care Service, NIH Grants P01 AR 44-012 and R01-47223-01 (M.P.M.) as well as NIH F32 AR08576 and R03 AR048648 (J.L.) and a grant from the Ludwig Foundation (M.A.K.).
Abbreviations
- G
globular
- HDMEC
human dermal microvascular endothelial cell
- mAb
monoclonal antibody
Footnotes
CONFLICT OF INTEREST The authors state no conflict of interest.
REFERENCES
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, et al. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–11. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Champliaud MF, Lunstrum GP, Rousselle P, Nishiyama T, Keene DR, Burgeson RE. Human amnion contains a novel laminin variant, laminin 7, which like laminin 6, covalently associates with laminin 5 to promote stable epithelial—stromal attachment. J Cell Biol. 1996;132:1189–98. doi: 10.1083/jcb.132.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Arcangelis A, Neuville P, Boukamel R, Lefebvre O, Kedinger M, Simon-Assmann P. Inhibition of laminin alpha 1-chain expression leads to alteration of basement membrane assembly and cell differentiation. J Cell Biol. 1996;133:417–30. doi: 10.1083/jcb.133.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHahn KC, Gonzales M, Gonzalez AM, Hopkinson SB, Chandel NS, Brunelle JK, et al. The alpha4 laminin subunit regulates endothelial cell survival. Exp Cell Res. 2004;294:281–9. doi: 10.1016/j.yexcr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Deng H, Choate KA, Lin Q, Khavari PA. High-efficiency gene transfer and pharmacologic selection of genetically engineered human keratinocytes. Biotechniques. 1998;25:274–80. doi: 10.2144/98252gt02. [DOI] [PubMed] [Google Scholar]
- Ehrig K, Leivo I, Argraves WS, Ruoslahti E, Engvall E. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci USA. 1990;87:3264–8. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E, Davis GE, Dickerson K, Ruoslahti E, Varon S, Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite promoting site. J Cell Biol. 1986;103:2457–65. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E, Earwicker D, Haaparanta T, Ruoslahti E, Sanes JR. Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul. 1990;1:731–40. doi: 10.1091/mbc.1.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E, Wewer UM. Domains of laminin. J Cell Biochem. 1996;61:493–501. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C493::AID-JCB2%3E3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–4. [PubMed] [Google Scholar]
- Fujiwara H, Gu J, Sekiguchi K. Rac regulates integrin-mediated endothelial cell adhesion and migration on laminin-8. Exp Cell Res. 2004;292:67–77. doi: 10.1016/j.yexcr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Gonzales M, Herron GS, Nagavarapu U, Hopkinson SB, Tsuruta D, et al. Complex interactions between the laminin alpha 4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo. Proc Natl Acad Sci USA. 2002;99:16075–80. doi: 10.1073/pnas.252649399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M, Weksler B, Tsuruta D, Goldman RD, Yoon KJ, Hopkinson SB, et al. Structure and function of a vimentin-associated matrix adhesion in endothelial cells. Mol Biol Cell. 2001;12:85–100. doi: 10.1091/mbc.12.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DS, Kibbey MC, Kinsella JL, Cid MC, Kleinman HK. The role of BMZ in angiogenesis and tumor growth. Path Res Pract. 1994;190:854–63. doi: 10.1016/S0344-0338(11)80989-1. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989;338:229–34. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Iivanainen A, Kortesmaa J, Sahlberg C, Morita T, Bergmann U, Thesleff I, et al. Primary structure, developmental expression, and immunolocalization of the murine laminin alpha4 chain. J Biol Chem. 1997;272:27862–8. doi: 10.1074/jbc.272.44.27862. [DOI] [PubMed] [Google Scholar]
- Koch M, Olson PF, Albus A, Jin W, Hunter DD, Brunken WJ, et al. Characterization and expression of the laminin gamma3 chain: a novel, non-basement membrane-associated, laminin chain. J Cell Biol. 1999;145:605–18. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortesmaa J, Yurchenco P, Tryggvason K. Recombinant laminin-8 (alpha(4)beta(1)gamma(1)). Production, purification, and interactions with integrins. J Biol Chem. 2000;275:14853–9. doi: 10.1074/jbc.275.20.14853. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–98. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre O, Sorokin L, Kedinger M, Simon-Assmann P. Developmental expression and cellular origin of the laminin alpha2, alpha4, and alpha5 chains in the intestine. Dev Biol. 1999;210:135–50. doi: 10.1006/dbio.1999.9270. [DOI] [PubMed] [Google Scholar]
- Leivo I, Engvall E, Laurila P, Miettinen M. Distribution of merosin, a laminin-related tissue-specific basement membrane protein, in human Schwann cell neoplasms. Lab Invest. 1989;61:426–32. [PubMed] [Google Scholar]
- Leivo I, Vaheri A, Timpl R, Wartiovaara J. Appearance and distribution of collagens and laminin in the early mouse embryo. Dev Biol. 1980;76:100–14. doi: 10.1016/0012-1606(80)90365-6. [DOI] [PubMed] [Google Scholar]
- Li J, Tzu J, Chen Y, Zhang YP, Nguyen NT, Gao J, et al. Laminin-10 is crucial for hair morphogenesis. EMBO J. 2003;22:2400–10. doi: 10.1093/emboj/cdg239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton BH, Bensch KG, Karasek MA. Microvessel endothelial cell transdifferentiation: phenotype characterization. Differentiation. 1991;46:117–33. doi: 10.1111/j.1432-0436.1991.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Liu JG, Mayne R. The complete cDNA coding sequence and tissue-specific expression of the mouse laminin alpha4 chain. Matrix Biol. 1996;15:433–7. doi: 10.1016/s0945-053x(96)90162-6. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP. Update on inherited bullous dermatoses. Dermatol Clin. 1999;17(vii):473–85. doi: 10.1016/s0733-8635(05)70102-9. [DOI] [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Burgeson RE. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem. 1992b;267:17900–6. [PubMed] [Google Scholar]
- Marinkovich MP, Lundstrum GP, Keene DR, Burgeson RE. The dermal—epidermal junction of human skin contains a novel laminin variant. J Cell Biol. 1992a;119:695–703. doi: 10.1083/jcb.119.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich MP, Verrando P, Keene DR, Meneguzzi G, Lunstrum GP, Ortonne JP, et al. The basement membrane proteins kalinin and nicein are structurally and immunologically identical. Lab Invest. 1993;69:295–9. [PubMed] [Google Scholar]
- Matsui C, Wong CK, Nelson CF, Bauer EA, Hoeffler WK. The assembly of laminin 5 subunits. J Biol Chem. 1995;270:23496–503. doi: 10.1074/jbc.270.40.23496. [DOI] [PubMed] [Google Scholar]
- McGowan KA, Marinkovich MP. Laminins and human disease. Microsc Res Technol. 2000;51:262–79. doi: 10.1002/1097-0029(20001101)51:3<262::AID-JEMT6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Miner JH, Lewis RM, Sanes JR. Molecular cloning of a novel laminin chain, alpha5, and widespread expression in adult mouse tissues. J Biol Chem. 1995;270:28523–6. doi: 10.1074/jbc.270.48.28523. [DOI] [PubMed] [Google Scholar]
- Miquel C, Gagnoux-Palacios L, Durand-Clement M, Marinkovich P, Ortonne JP, Meneguzzi G. Establishment and characterization of cell line LSV5 that retains the altered adhesive properties of human junctional epidermolysis bullosa keratinocytes. Exp Cell Res. 1996;224:279–90. doi: 10.1006/excr.1996.0138. [DOI] [PubMed] [Google Scholar]
- Normand J, Karasek MA. A method for the isolation and serial propagation of keratinocytes, endothelial cells, and fibroblasts from a single punch biopsy of human skin. In vitro Cell Dev Biol Anim. 1995;31:447–55. doi: 10.1007/BF02634257. [DOI] [PubMed] [Google Scholar]
- Pierce RA, Griffin GL, Mudd MS, Moxley MA, Longmore WJ, Sanes JR, et al. Expression of laminin α3, α4, and α5 chains by alveolar epithelial cells and fibroblasts. Am J Respir Cell Mol Biol. 1998;19:237–44. doi: 10.1165/ajrcmb.19.2.3087. [DOI] [PubMed] [Google Scholar]
- Richards A, Luccarini C, Pope FM. The structural organisation of LAMA4, the gene encoding laminin α4. Eur J Biochem. 1997;248:15–23. doi: 10.1111/j.1432-1033.1997.t01-1-00015.x. [DOI] [PubMed] [Google Scholar]
- Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, et al. Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci. 2003;116:3543–56. doi: 10.1242/jcs.00663. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Christiano AM, Engvall E, Wewer UM, Burgeson RE. The functions of laminins: lessons from in vivo studies. Matrix Biol 1996. 1996:369–81. doi: 10.1016/s0945-053x(96)90157-2. [DOI] [PubMed] [Google Scholar]
- Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM. Endotheial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood—brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol. 2001;153:933–46. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland WJ, Son YJ, Miner JH, Sanes JR, Carlson SS. The presynaptic calcium channel is part of a transmembrane complex linking a synaptic laminin (alpha4beta2gamma1) with non-erythroid spectrin. J Neurosci. 2000;20:1009–19. doi: 10.1523/JNEUROSCI.20-03-01009.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talts JF, Sasaki T, Miosge N, Gohring W, Mann K, Mayne R, et al. Structural and functional analysis of the recombinant G domain of the laminin alpha4 chain and its proteolytic processing in tissues. J Biol Chem. 2000;275:35192–9. doi: 10.1074/jbc.M003261200. [DOI] [PubMed] [Google Scholar]
- Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, et al. Deletion of the laminin alpha4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22:1194–202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiger CF, Champliaud MF, Pedrosa-Domellof F, Thornell LE, Ekblom P, Gullberg D. Presence of laminin alpha5 chain and lack of laminin alpha1 chain during human muscle development and in muscular dystrophies. J Biol Chem. 1997;272:28590–5. doi: 10.1074/jbc.272.45.28590. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC. The laminins. Matrix Biol. 1994;14:275–81. doi: 10.1016/0945-053x(94)90192-9. [DOI] [PubMed] [Google Scholar]
- Timpl R, Rohde H, Robey Gehron P. Laminin-a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–7. [PubMed] [Google Scholar]
- Timpl R, Tisi D, Talts JF, Andac Z, Sasaki T, Hohenester E. Structure and function of laminin LG modules. Matrix Biol. 2000;19:309–17. doi: 10.1016/s0945-053x(00)00072-x. [DOI] [PubMed] [Google Scholar]
- Uitto J, Pulkkinen L, McLean WH. Epidermolysis bullosa: a spectrum of clinical phenotypes explained by molecular heterogeneity. Mol Med Today. 1997;3:457–65. doi: 10.1016/s1357-4310(97)01112-x. [DOI] [PubMed] [Google Scholar]
- Wewer UM, Engvall E. Merosin/laminin-2 and muscular dystrophy. Neuromusc Disord. 1996;6:409–18. doi: 10.1016/s0960-8966(96)00384-7. [DOI] [PubMed] [Google Scholar]
- Yurchenco PD, O’Rear JJ. Basement membrane assembly. In: Ruoslahti E, Engvall E, editors. Methods of enzymology. Vol. 245. Academic Press; San Diego: 1994. pp. 489–518. [DOI] [PubMed] [Google Scholar]
- Zhou L, Dosanjh A, Chen H, Karasek M. Divergent effects of extracellular oxygen on the growth, morphology, and function of human skin microvascular endothelial cells. J Cell Physiol. 2000;182:134–40. doi: 10.1002/(SICI)1097-4652(200001)182:1<134::AID-JCP15>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]


