Abstract
Fluorogenic analogues of phosphatidylcholine and lysophosphatidylcholine, DDPB and lysoDDPB, were synthesized by an enzyme-assisted strategy. The analogues were evaluated as substrates for phospholipases C and D, and lysophospholipase D. DDPB was cleaved by bacterial and plant phospholipase D (PLD) enzymes and represents the first direct fluorogenic substrate for real-time measurement of PLD activity. Both fluorogenic substrates have potential in screening for PLD and PC-PLC inhibitors, and for monitoring spatiotemporal changes in PLD activity in cells.
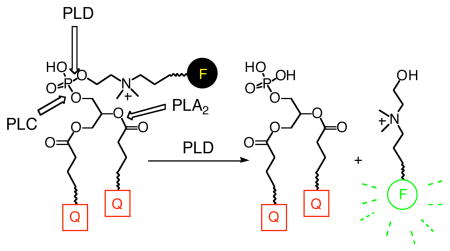
PLC, PLD, and lysoPLD are three phospholipases that catalyze hydrolysis of phospholipid (PL) head groups. PLC catalyzes the hydrolysis of the PL phosphodiester glyceryl P-O bond to give diacylglycerol and a phosphomonoester. PLD cleaves the head group P-O bond of the phosphodiester linkage, to produce phosphatidic acid (PA) and an alcohol. LysoPLD catalyzes the same reaction as PLD, but is selective for lysophospholipids. Most PLDs also catalyze transphosphatidylation,1 in which a primary alcohol replaces water as the cleaving nucleophile.
PLC isozymes selectively hydrolyze PLs with either inositol or choline headgroups. There are at least eleven different phosphoinositide-selective PLCs (PI-PLC),2 two putative phosphatidylcholine- selective PLCs (PC-PLCs)3 in mammals, and one PC-PLC in plants.4
PLD isozymes found in mammals are involved in a diversity of normal and disease-related biological processes.5–7 The PLD enzymes found in mammals, plants, and some bacteria are called “HKD PLD” because they share a consensus amino acid sequence (HxKx4Dx6GG/S), and employ a common catalytic mechanism involving a covalent histidine intermediate.8 The “non-HKD PLD” enzymes lack an HKD consensus sequence, and have a catalytic mechanism in which metal ions are required to position the PL substrate and activate the attacking nucleophile.9 S. chromofuscus PLD (scPLD) is an archetypical non-HKD PLD. Similar to the non-HKD PLD, lysoPLD requires metal ions for catalysis and does not contain an HKD sequence.10 The lysoPLD autotaxin, a nucleotide pyrophosphatase/phosphodiesterase, catalyzes hydrolysis via an active site threonine.11
HKD PLDs can be detected in cell biological studies by inhibition with exogenous ethanol, 1-propanol, or 1-butanol. Biochemical assays employ radioactive12 or fluorescent13 PLs, requiring extraction, separation, and detection. Alternatively, PLD products can be detected in vitro using direct14 or indirect15–18 methods. There are also indirect assays for PC-PLC18, 19 and lysoPLD;20 in particular, p-nitrophenylphosphate analogues have also been used for monitoring non-HKD PLD and lysoPLD,21, 22 and PC-PLC23 in vitro. Fluorogenic substrates for lysoPLD have recently been described.24,25
We report here the synthesis of fluorogenic PC and lysoPC analogues that contain a fluorescence quencher (dabcyl, a.k.a p-methyl red) at each acyl chain terminus, and a fluorophore appended to the PL head group through a choline-mimetic linker. These PL analogues, denoted DDPB and lysoDDPB, were evaluated in microtiter plate assays as substrates for lysoPLD, scPLD, PLC, and phospholipase A2 (PLA2), as well as several commercially-available HKD PLD enzymes.
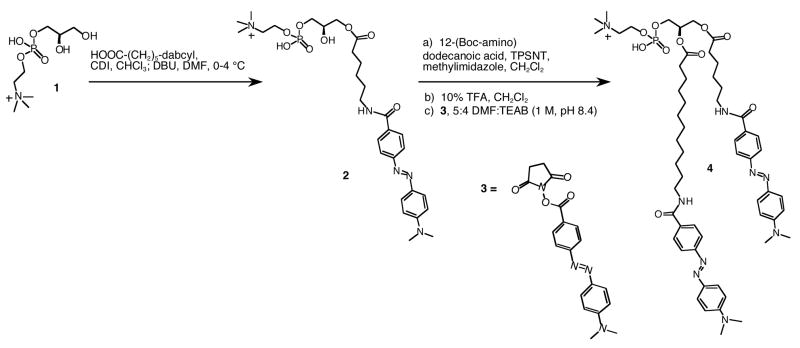
DDPB and lysoDDPB were synthesized efficiently as illustrated in Schemes 1 and 2. The lysophosphatidylcholine intermediate 2 containing the shorter dabcyl acyl chain was obtained a selective monoacylation of the commercially-available glycerol phosphocholine, 1, followed by installation of the longer-chain dabcyl quencher at the sn-2 position (4, Scheme 1). While the longer, dodecanoyl acyl chain at sn-2 improved the lipophilicity of the analogue over compounds with two hexanoyl chains, adding a second C12 linker at sn–1 afforded a less soluble analog that did not exhibit either improved micelle insertion or in vitro enzyme activity (data not shown).
Scheme 1.
Synthesis of di-dabcyl intermediate 4
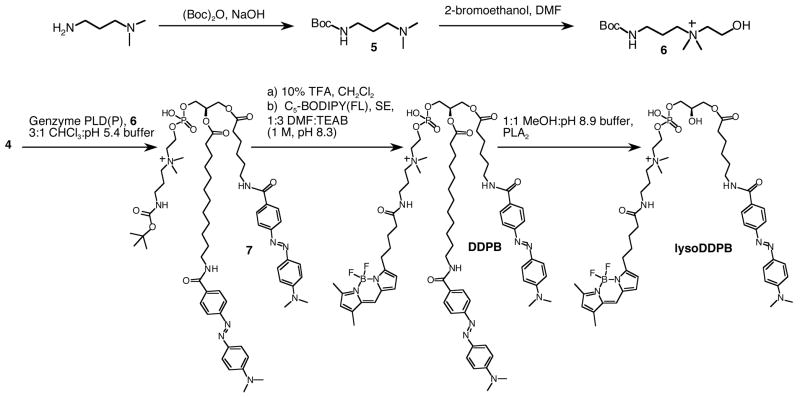
Scheme 2.
Synthesis of DDPB and lysoDDPB
The key step was the modification of the head group of intermediate 4 by transphosphatidylation, using Genzyme PLD(P) and a designed “choline-like” primary alcohol 6, prepared as shown in Scheme 2. Transphosphatidylation has been exploited previously26–28 to generate PL analogues with both natural and unnatural head groups. In this case, the head group remodeling allowed the installation of a phosphodiester linkage bearing an internal quaternary amine similar to choline as well as a protected primary amine that could be used for further conjugation reactions (7, Scheme 2). The primary amine was deprotected and allowed to react with an activated ester of BODIPY-FL (Molecular Probes) to give the fluorogenic PC analogue DDPB (Scheme 2). Removal of the sn-2 ester of DDPB by cobra venom PLA2 gave lysoDDPB, a fluorogenic lysoPC analogue.
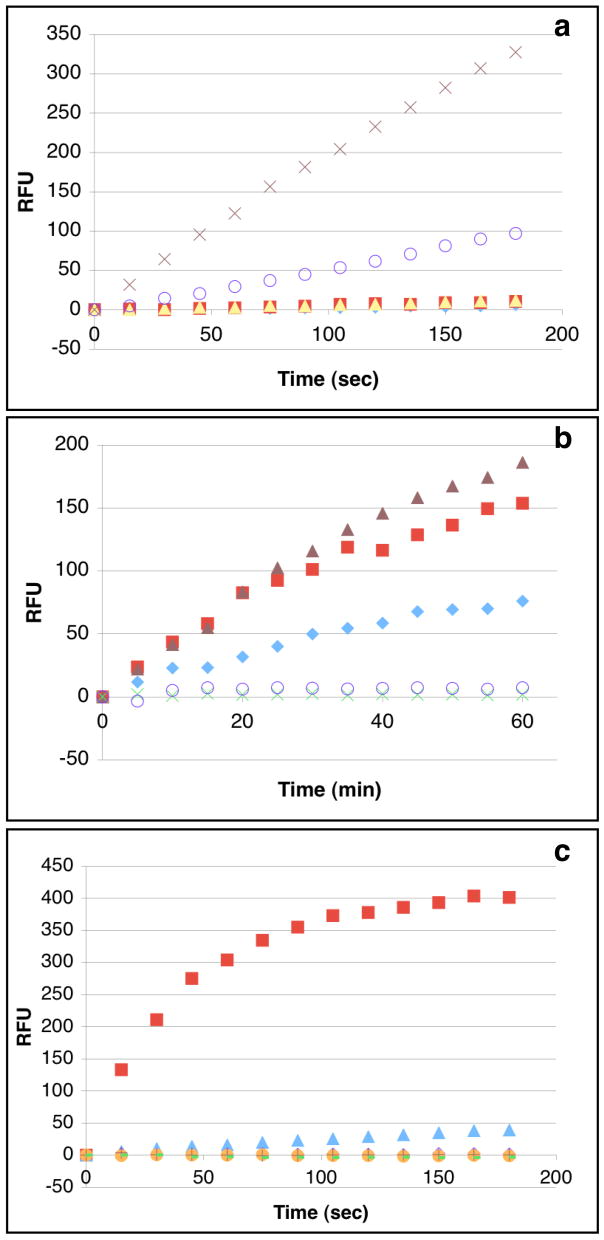
Mixed micelles containing DDPB or lysoDDPB in Triton X-100 (reduced) were incubated with commercial PLDs from various sources for 3 min in buffers near each enzyme’s pH optimum. Over this period, DDPB (Figure 1a) gave robust fluorescence increases with the enzyme used for its synthesis, Genzyme PLD(P), and with scPLD. LysoDDPB (Supporting information, Figure 2) over 3 min, gave a comparable signal with scPLD, but only a very small response to Genzyme PLD(P). Over 60 min (Figure 1b), DDPB mixed micelles with HKD PLD from peanut, cabbage, and Streptomyces PMF (BIOMOL) evolved fluorescence to a degree approaching that observed in 3 min with scPLD, but bee and cobra venom sPLA2 did not generate a fluorescent signal over the same time period, consistent with the retention of an intramolecular quencher even after cleavage of the sn-2 quenched acyl chain. Longer incubation time with lysoDDPB mixed micelles (Supporting information, Figure 3) only amplified fluorescence from Genzyme PLD(P). These results support the idea that lysophospholipids are substrates for scPLD,14 but are not substrates for HKD PLD.29,30
Figure 1.
Fluorescence evolution (λEx/λEm = 500/530 nm) during a) 3 min incubation of DDPB mixed micelles with PLD from various sources (
 = peanut PLD;
= peanut PLD;
 = cabbage PLD;
= cabbage PLD;
 = Strep. PMF PLD;
= Strep. PMF PLD;
 = Genzyme PLD(P);
= Genzyme PLD(P);
 = scPLD), b) 60 min incubation of DDPB mixed micelles with PLD and PLA2 from various sources (
= scPLD), b) 60 min incubation of DDPB mixed micelles with PLD and PLA2 from various sources (
 = peanut PLD;
= peanut PLD;
 = cabbage PLD;
= cabbage PLD;
 = Strep. PMF PLD;
= Strep. PMF PLD;
 = cobra venom PLA2;
= cobra venom PLA2;
 = bee venom PLA2), and c) 3 min incubation of DDPB or lysoDDPB mixed micelles with PC-PLC or PI-PLC from B. cereus or C. perfringens (
= bee venom PLA2), and c) 3 min incubation of DDPB or lysoDDPB mixed micelles with PC-PLC or PI-PLC from B. cereus or C. perfringens (
 = B. cereus PC-PLC + DDPB;
= B. cereus PC-PLC + DDPB;
 = B. cereus PC-PLC + lysoDDPB;
= B. cereus PC-PLC + lysoDDPB;
 = C. perfmgens PC-PLC + DDPB;
= C. perfmgens PC-PLC + DDPB;
 = C. perfringens PC-PLC + lysoDDPB;
= C. perfringens PC-PLC + lysoDDPB;
 = B. cereus PI-PLC + DDPB;
= B. cereus PI-PLC + DDPB;
 = B. cereus PI-PLC + lysoDDPB).
= B. cereus PI-PLC + lysoDDPB).
Next, mixed micelles of the PC analogues were tried as substrates for PC-PLC from Bacillus cereus and Clostridium perfringens and PI-PLC from B. cereus (Figure 1c). Over 3 min, DDPB gave an overwhelmingly large fluorescence response with B. cereus PC-PLC and virtually none with B. cereus PI-PLC or C. perfringens PC-PLC. LysoDDPB gave a small but detectable signal with B. cereus PC-PLC, but none with the other PLC tested.
We anticipated that lysoDDPB would be accepted as a fluorogenic substrate by lysoPLD, but neither lysoDDPB-nor DDPB-containing mixed micelles produced fluorescence when assayed in the presence of FBS, an abundant source of lysoPLD activity,31 even after 60 min incubation. On the other hand, the commercial lysoPLD substrates pNP-TMP32 and FS-325 both generated significant UV and fluorescence signals, respectively (Supporting information, Figure 4). LysoDDPB was assayed with 50% FBS at 37 °C, but no fluorescence increase was observed even when the concentration of lysoDDPB was quadrupled and the FBS concentration increased to 80% (data not shown). Incubation of lysoDDPB-containing micelles with up to 2 μg of venom from Loxosceles reclusa, another known source of lysoPLD,33 failed to show a fluorescence increase (data not shown).
The inability of lysoPLD to hydrolyze lysoDDPB was unexpected, considering the close structural similarity among lysoPC, lysoDDPB and FS-3 (Supporting information, Figure 4). The phosphate linker of FS-3, in spite of its lack of a quaternary amine, is nearly twice the length of that in lysoDDPB. This extra length may give the bulky fluorescent group of FS-3 flexibility to avoid unfavorable interactions in the binding site. Increasing the chain length between the phosphate and the fluorophore in future fluorogenic analogues of lysoPC could test this supposition.
DDPB and lysoDDPB were both designed to be PC-like, amphiphilic probes that could be used in living cells by inserting into lipid bilayers; neither is freely soluble in water. DDPB was further engineered to have fluorescence quenchers at the end of each acyl chain in order to prevent fluorescence increases resulting from cellular PLA2 or PLA1 activity. Indeed, no fluorescent signal was observed even after incubation of DDPB with venom PLA2 at 37 °C for 1 hr (Figure 1c).
Given the well-established importance of the HKD PLDs and the growing importance of PC-PLC in cell signaling, DDPB and lysoDDPB have the potential to be valuable tools in cellular and molecular biology. The utility of these fluorogenic substrates with mammalian HKD PLD has been observed and will be reported elsewhere (M. Hogdkin, personal communication). Both DDPB and lysoDDPB are cell-permeant (data not shown) and may be applicable for cell-based assays, as previously described for PLA2.34,35 Further studies examining the use of DDPB and lysoDDPB in living systems are merited and will be reported in due course.
Supplementary Material
Figures 2, 3, and 4, full experimental details, and NMR and MS spectroscopic data for the characterization of DDPB and lysoDDPB and their intermediates are available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank C. Ferguson (Echelon Biosciences, Inc., an Aeterna Zentaris company), for providing a gift of FS-3. The Center for Cell Signaling, a Utah Center of Excellence (1997-2002) and the NIH (Grants HL070231 and NS29632 to GDP) provided financial support.
References
- 1.Yang SR, Freer S, Benson AA. J Biol Chem. 1967;242:477–484. [PubMed] [Google Scholar]
- 2.Bradshaw RA, Dennis EA. Handbook of cell signaling. Academic Press; Amsterdam; Boston: 2004. [Google Scholar]
- 3.Ramoni C, Spadaro R, Menegon M, Podo F. J Immunol. 2001;167:2642–2650. doi: 10.4049/jimmunol.167.5.2642. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura Y, Awai K, Masuda T, Yoshioka Y, Takamiya K, Ohta H. J Biol Chem. 2005;280:7469–76. doi: 10.1074/jbc.M408799200. [DOI] [PubMed] [Google Scholar]
- 5.Foster DA, Xu L. Mol Cancer Res. 2003;1:789–800. [PubMed] [Google Scholar]
- 6.Freyberg Z, Siddhanta A, Shields D. Trends Cell Biol. 2003;13:540–546. doi: 10.1016/j.tcb.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert ML, Quon MJ, Reed BC, Dikic I, Farese RV. J Biol Chem. 2001;276:35537–35545. doi: 10.1074/jbc.M106042200. [DOI] [PubMed] [Google Scholar]
- 8.Muller G, Petty S. Lipases and phospholipases in drug development: from biochemistry to molecular pharmacology. Wiley-VCH; Weinheim: 2004. p. xvii.p. 336. [Google Scholar]
- 9.Zambonelli C, Roberts MF. Prog Nucleic Acid Res Mol Biol. 2005;79:133–181. doi: 10.1016/S0079-6603(04)79003-0. [DOI] [PubMed] [Google Scholar]
- 10.Gijsbers R, Ceulemans H, Stalmans W, Bollen M. J Biol Chem. 2001;276:1361–1368. doi: 10.1074/jbc.M007552200. [DOI] [PubMed] [Google Scholar]
- 11.Stefan C, Jansen S, Bollen M. Trends Biochem Sci. 2005;30:542–550. doi: 10.1016/j.tibs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Gutowski S, Singer WD, Sternweis PC. Methods Enzymol. 2002;345:328–334. doi: 10.1016/s0076-6879(02)45026-4. [DOI] [PubMed] [Google Scholar]
- 13.Xie Y, Meier KE. Methods Enzymol. 2002;345:294–305. doi: 10.1016/s0076-6879(02)45024-0. [DOI] [PubMed] [Google Scholar]
- 14.Mezna M, Lawrence AJ. Anal Biochem. 1994;218:370–376. doi: 10.1006/abio.1994.1194. [DOI] [PubMed] [Google Scholar]
- 15.Kinkaid AR, Wilton DC. Biochem Soc Trans. 1997;25:S595. doi: 10.1042/bst025s595. [DOI] [PubMed] [Google Scholar]
- 16.Hergenrother PJ, Haas MK, Martin SF. Lipids. 1997;32:783–788. doi: 10.1007/s11745-997-0101-5. [DOI] [PubMed] [Google Scholar]
- 17.Petersen G, Chapman KD, Hansen HS. J Lipid Res. 2000;41:1532–1538. [PubMed] [Google Scholar]
- 18.Zhou M, Zhang C, Haugland RP. Proc SPIE. 2000;3926:166–171. [Google Scholar]
- 19.Wilton DC. Biochem J. 1991;276(Pt 1):129–133. doi: 10.1042/bj2760129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umezu-Goto M, Kishi Y, Taira A, Kama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H. J Cell Biol. 2002;758:227–33. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gijsbers R, Aoki J, Arai H, Bollen M. FEES Lett. 2003;538:60–64. doi: 10.1016/s0014-5793(03)00133-9. [DOI] [PubMed] [Google Scholar]
- 22.Cha J, Lee J, Koh EH, Choi MU. Bull Korean Chem Soc. 1994;75:1001–1003. [Google Scholar]
- 23.Kurioka S, Matsuda M. Anal Biochem. 1976;75:281–289. doi: 10.1016/0003-2697(76)90078-6. [DOI] [PubMed] [Google Scholar]
- 24.Takakusa H, Kikuchi K, Urano Y, Sakamoto S, Yamaguchi K, Nagano T. J Am Chem Soc. 2002;124:1653–1657. doi: 10.1021/ja011251q. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson CG, Bigman CS, Richardson RD, van Meeteren LA, Moolenaar WA, Prestwich GD. Org Lett. 2006 doi: 10.1021/ol060414i. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Arrigo P, de Ferra L, Piergianni V, Selva A, Servi S, Strini A. J Chem Soc, Perkin Trans 1. 1996;21:2651–2656. [Google Scholar]
- 27.Wang P, Schuster M, Wang Y-R, Wong CH. J Am Chem Soc. 1993;115:10487–10491. [Google Scholar]
- 28.Pisch S, Bornscheuer UT, Meyer HH, Schmid RD. Tetrahedron. 1991;53:14627–14634. [Google Scholar]
- 29.Ryu SB, Karlsson BH, Ozgen M, Palta JP. Proc Natl Acad Set USA. 1997;94:12717–12721. doi: 10.1073/pnas.94.23.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryu SB, Palta JP. J Lipid Res. 2000;41:940–944. [PubMed] [Google Scholar]
- 31.Umezu-Goto M, Kishi Y, Taka A, Kama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, Arai H. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Decker K, Bischoff E. FEES Lett. 1972;21:95–98. doi: 10.1016/0014-5793(72)80172-8. [DOI] [PubMed] [Google Scholar]
- 33.Lee S, Lynch KR. Biochem J. 2005;391:317–323. doi: 10.1042/BJ20050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng L, Manabe K, Shope JC, Widmer S, DeWald DB, Prestwich GD. Chem Biol. 2002;9:795–803. doi: 10.1016/s1074-5521(02)00168-0. [DOI] [PubMed] [Google Scholar]
- 35.Rose TM, Prestwich GD. ACS Chem Biol. 2006;1:83–91. doi: 10.1021/cb5000014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures 2, 3, and 4, full experimental details, and NMR and MS spectroscopic data for the characterization of DDPB and lysoDDPB and their intermediates are available free of charge via the Internet at http://pubs.acs.org.