Abstract
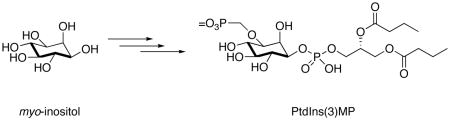
Phosphatidylinositol-3-phosphate (PtdIns(3)P) is spatial regulator of vesicular trafficking and other vital cellular processes. We describe the asymmetric total synthesis of a metabolically stabilized analogue, phosphatidylinositol-3-methylenephosphate (PtdIns(3)MP) from a differentially-protected myo-inositol. NMR studies of PtdIns(3)MP bound to the 15N-labelled FYVE domain showed significant 1H and 15N chemical shift changes relative to the unliganded protein.
Phosphoinositide (PtdInsPn) signaling networks are dynamically modulated by proteins with lipid recognition motifs as well as kinase, phosphatase, and phospholipase enzymatic activities. In particular, the 3-phosphorylated PtdInsPn lipids have been implicated as activators of protein kinase C isoforms, and are messengers in cellular signal cascades pertinent to inflammation, cell proliferation, transformation, protein kinesis, and cytoskeletal assembly.1–3 PtdIns(3)P is produced by the action of phosphoinositide-3-kinase (PI 3-K)1,4 on PtdIns, and its interactions with cognate binding proteins, kinase and phosphatases are important in cell physiology. PtdIns(3)P specifically binds FYVE domains5–7 and is involved in phagocytosis,8 membrane trafficking and protein sorting.9 PX domains also recognize PtdIns(3)P, and spatiotemporal changes mediate important aspects of cell respiration.10 The myotubularin-related (MTMR) protein family11 is comprised of PtdIns(3)P phosphatases that contribute to lipid remodeling and are mutated in genetic diseases.12
To gain deeper insights into these biological pathways, selective reagents that can interfere with ligand binding, inhibit enzyme activity, and activate protein mediated-lipid signaling are needed.13 We recently described a general approach to the synthesis of methylphosphonate, (monofluoromethyl)phosphonate, and phosphorothioate analogues of PtdIns(3)P.14 These metabolically-stabilized ligands were recognized by 15N-labeled FYVE and PX domains, and were also substrates for PIKfyve, a 5-kinase required for the formation of multivesicular bodies. We now introduce a modified synthetic route that provides access to a stabilized methylenephosphonate analogue, PtdIns(3)MP, which retains the inositol 3-oxygen as well as the dianionic head group. In this modification, a methylene bridge was inserted between the oxygen of the inositol moiety and the phosphate head group. An similar approach was also used to generate alkoxymethylene phosphonate containing geranylgeranyl protein transferase inhibitors15 and antiviral drugs,16 including anti-HIV phosphorylated nucleoside analogues.17,18 We have also used this approach to synthesize potent analogues of lysophosphatidic acid and phosphatidic acid, and these results will be presented in due course. Herein we describe the asymmetric total synthesis of the methylenephosphonate analogue of PtdIns(3)P, and we illustrate the binding of PtdIns(3)MP to the FYVE domain.
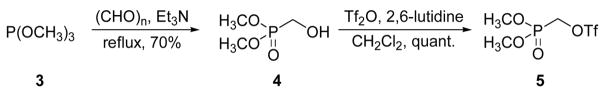
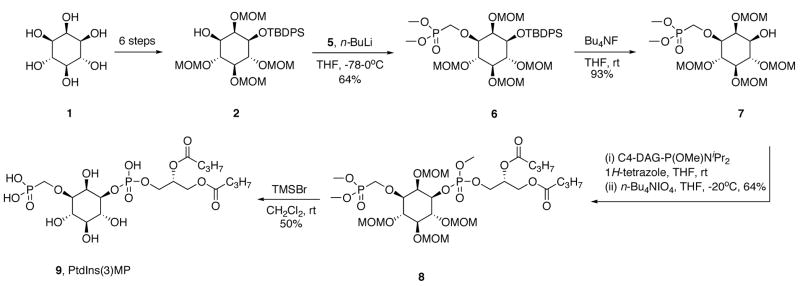
The synthetic strategy employed the simple and elegant protection scheme of Bruzik,19 in which the 1-position of myo-inositol (1) was silylated with the TBDPS group, the phosphomonoester 3-positon was protected as a benzoate group, and all remaining hydroxyl groups were protected as methoxymethyl (MOM)-ethers. Thus, 1-O-(tert-butyl-diphenylsilyl)-2,4,5,6-O-tetrakis-(methoxymethylene)-myo-inositol (2) was synthesized from myo-inositol in six steps. Installation of the methylenephosphonate moiety required the preparation (Scheme 1) of dimethyl phosphonomethyltriflate (5) following the literature route.20,21 Reaction of paraformaldehyde with dimethyl phosphite gave hydroxymethyl phosphonate 4, which was converted to triflate 5 using 2,6-lutidine as the base, and was employed without further purification.
Scheme 1.
Synthesis of dimethyl phosphonomethyltriflate (5).
The alkoxide of protected inositide 2 (n-BuLi15, −78°C) was alkylated with triflate 5 in 64% yield (Scheme 2). Use of NaH or t-BuOK as bases did not significantly improve the yield and resulted in greater decomposition of the starting material. The TBDPS group was removed by treating intermediate 6 with t-BuNH4F·H2O. The resulting alcohol 7 was coupled with the di-butanoylglyceryl phosphoramidite14 in the presence of 1H-tetrazole, followed by mild oxidation with n-BuNIO414 to give fully protected PtdIns(3)MP (8). The removal of phosphate and hydroxyl protecting groups of 8 was accomplished under strictly anhydrous conditions with 20 eq of fresh TMSBr (CH2Cl2, rt, 1 h). After concentration in vacuo, the residue was dissolved in a 90% aq. CH3OH and stirred for 40 min to hydrolyze the silyl phosphate esters. We found that under these conditions, not only were the phosphates deprotected, but all MOM groups were also removed. After complete evaporation of the organic solvent in vacuo, the crude compound was dissolved in water and passed through a short column of acidic Dowex ion-exchange resin to yield final product in >98% purity.
Scheme 2.
Synthesis of PtdIns(3)MP (9).
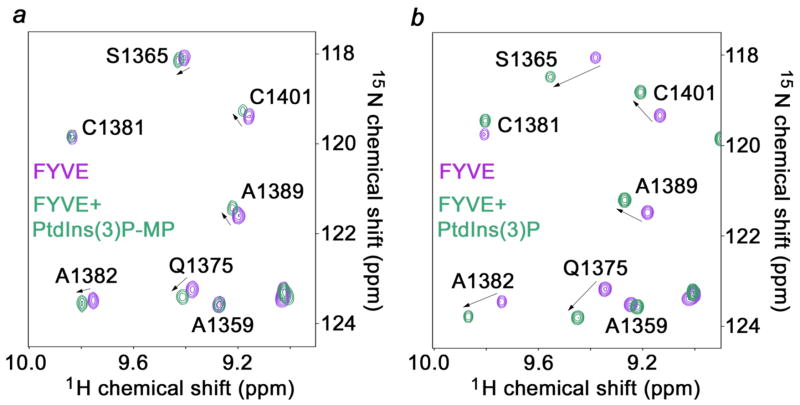
In endosomal membranes, PtdIns(3)P is specifically recognized by a number of protein binding partners including FYVE and PX domains. We next investigated the interactions of human EEA1 FYVE and yeast Vam7 PX domains with PtdIns(3)MP by NMR spectroscopy. Significant changes were observed in 1H and 15N resonances in the FYVE domain when titrating in dibutanoyl PtdIns(3)MP (9) (Figure 1a). These perturbations were of reduced magnitude, but paralleled the chemical shift changes apparent in the complex of the FYVE domain with dibutanoyl-PtdIns(3)P (Figure 1b). Thus, the PtdIns(3)MP analogue and native lipid are accommodated by the same binding pocket consisting of four Arg and two His residues of the FYVE domain. Based on 1H and 15N chemical shift changes the FYVE domain affinity for PtdIns(3)MP was calculated to be 3.8 ± 0.5 mM (see Supplementary Figure 2 in Supporting Information). To put this in perspective, the local concentration of PtdIns(3)MP in early endosomal membranes is quite high (~ 200 μM)22 and the FYVE – dibutanoyl-PtdIns(3)P affinity is 135 μM under similar experimental conditions.23 A similar experiment with the PX domain showed a much weaker binding without significant chemical shift changes. Addition of up to 11.3 mM (57-fold excess) of PtdIns(3)MP to a 0.2 mM PX domain sample induced no noticeable resonance changes. For comparison, the Kd of the PX domain for dibutanoyl-PtdIns(3)P under these conditions is approximately 300 μM (M. Cheever, T. Kutateladze, M. Overduin, unpublished results). This result may be attributed to the difference in the binding modes for the two complexes. PtdIns(3)P inserts its 3-phosphate between two loops and an a-helix of the PX domain.24 However, in the case of the FYVE domain, PtdIns(3)P occupies a shallow pocket in a side-on orientation.25 Apparently the extended methylenephosphonate group is too bulky to be accommodated in the PX domain binding pocket. Thus, PtdIns(3)MP is the first analogue of PtdIns(3)P that shows discrimination in its protein-ligand interactions, suggesting that it could be useful in selectively perturbing distinct PtdIns(3)P signaling pathways.
Figure 1.
Binding of PtdIns(3)P and PtdIns(3)MP to the FYVE domain. 1H-15N Heteronuclear single quantum coherence (HSQC) NMR spectra of 0.2 mM EEA1 FYVE domain before and after addition of (a) dibutanoyl-PtdIns(3)MP (9) and (b) dibutanoyl-PtdIns(3)P.
Supplementary Material
Experimental details for the synthesis and characterization of new compounds and protocols for the 1H and 15N NMR binding measurement. This material is available free of charge via the Internet at http://pubs.acs.org
Acknowledgments
We thank the NIH (Grant NS 29632 to G.D.P.) and the American Cancer Society (T.G.K.) for financial support of this work.
References
- 1.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 2.Yin HL, Janmey PA. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 3.Wymann MP, Bjorklof K, Calvez R, Finan P, Thomas M, Trifilieff A, Barbier M, Altruda F, Hirsch E, Laffargue M. Biochem Soc Trans. 2003;31:275–280. doi: 10.1042/bst0310275. [DOI] [PubMed] [Google Scholar]
- 4.Anderson KE, Jackson SP. Int J Biochem Cell Biol. 2003;35:1028–1033. doi: 10.1016/s1357-2725(02)00270-4. [DOI] [PubMed] [Google Scholar]
- 5.Kutateladze T, Ogburn K, Watson W, de Beer T, Emr S, Burd C, Overduin M. Molec Cell. 1999;3:805–811. doi: 10.1016/s1097-2765(01)80013-7. [DOI] [PubMed] [Google Scholar]
- 6.Gaullier JM, Simonsen A, D’Arrigo A, Bremnes B, Stenmark H, Aasland R. Nature. 1998;394:432–433. doi: 10.1038/28767. [DOI] [PubMed] [Google Scholar]
- 7.Kutateladze T. BBA - Mol Cell Biol Lipids. 2006 in press. [Google Scholar]
- 8.Gillooly DJ, Simonsen A, Stenmark H. J Cell Biol. 2001;155:15–17. doi: 10.1083/jcb.200109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petiot A, Faure J, Stenmark H, Gruenberg JJ. Cell Biol. 2003;162:971–979. doi: 10.1083/jcb.200303018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato T, Overduin M, Emr SD. Science. 2001;294:1881–1885. doi: 10.1126/science.1065763. [DOI] [PubMed] [Google Scholar]
- 11.Wishart MJ, Dixon JE. Trends Cell Biol. 2002;12:579–585. doi: 10.1016/s0962-8924(02)02412-1. [DOI] [PubMed] [Google Scholar]
- 12.Laporte J, Bedez R, Bolino A, Mandel JL. Hum Mol Genet. 2003;12:R285–R292. doi: 10.1093/hmg/ddg273. [DOI] [PubMed] [Google Scholar]
- 13.Prestwich GD. Chem & Biol. 2004;11:619–637. doi: 10.1016/j.chembiol.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Lee SA, Kutateladze TG, Sbrissa D, Shisheva A, Prestwich GDJ. Am Chem Soc. 2006;128:885–897. doi: 10.1021/ja0554716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minutolo F, Antonello M, Barontini S, Bertini S, Betti L, Danesi R, Gervasi G, Giannaccini G, Papi C, Placanic G, Rapposelli S, Macchia M. Il Farmaco. 2004;59:887–892. doi: 10.1016/j.farmac.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 16.De Clercq E, Holy A. Nat Rev Drug Discov. 2005;4:928–940. doi: 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]
- 17.Jie L, Van Aerschot A, Balzarini J, Janssen G, Busson R, Hoogmartens J, De Clercq E, Hardewijn PJ. Med Chem. 1990;33:241–245. doi: 10.1021/jm00171a023. [DOI] [PubMed] [Google Scholar]
- 18.Van Aeroschot A, Jie L, Herdewijn P. Tetrahedron Lett. 1991;32:1905–1908. [Google Scholar]
- 19.Kubiak RJ, Bruzik KS. J Org Chem. 2003:960–968. doi: 10.1021/jo0206418. [DOI] [PubMed] [Google Scholar]
- 20.Phillion DP, Andrew SS. Tetrahedron Lett. 1986;27:1477–1480. [Google Scholar]
- 21.Hamilton CJ, Roberts SM. J Chem Soc, Perkin Trans 1. 1999:1051–1056. [Google Scholar]
- 22.Stenmark H, Gillooly DJ. Semin Cell Dev Biol. 2001;12:193–199. doi: 10.1006/scdb.2000.0236. [DOI] [PubMed] [Google Scholar]
- 23.Lee SA, Eyeson R, Cheever ML, Geng J, Verkhusha VV, Burd C, Overduin M, Kutateladze TG. PNAS. 2005;102:13052–13057. doi: 10.1073/pnas.0503900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bravo J, Karathanassis D, Pacold CM, Pacold M, Ellson CD, Anderson KE, Butler P, Lavenir I, Perisic O, Hawkins PT, Stephens L, Williams R. Molecular Cell. 2001;8:829–839. doi: 10.1016/s1097-2765(01)00372-0. [DOI] [PubMed] [Google Scholar]
- 25.Kutateladze T, Overduin M. Science. 2001;291:1793–1796. doi: 10.1126/science.291.5509.1793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental details for the synthesis and characterization of new compounds and protocols for the 1H and 15N NMR binding measurement. This material is available free of charge via the Internet at http://pubs.acs.org