The aldol reaction is one of the most widely utilized C-C bond-forming reactions in organic chemistry, because of the importance of β–hydroxy carbonyl compounds in many natural products.1 Recently, several groups have reported a direct catalytic enantioselective version without resorting to pre-activation of the pronucleophile using biological catalysts2a–g and non-biological transition-metal catalysts,2h–j including our own dinuclear Zn-complex 2.2h,3 Typically, however, successful donors have included simple ketones, such as methyl, and hydroxylmethyl ketones and limited progress has been made in catalytic enantioselective aldol reactions of more functionalized or functionalizable nucleophiles, such as methyl vinyl ketone (MVK). Enantio-enriched β–hydroxyketones derived from MVK are particularly interesting, because stereocenters created in the aldol reactions can be propagated further in a sequence of highly diastereoselective transformations, where MVK functions as a bifunctional building block (Scheme 1).
Scheme 1.
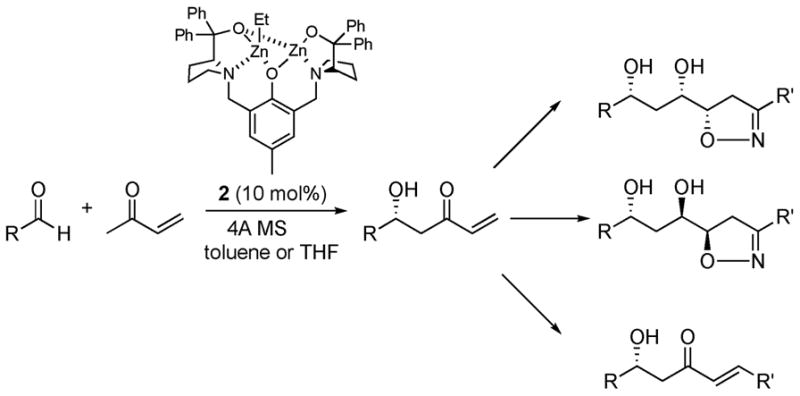
Utility of Zn-Aldol Addition
Despite tremendous synthetic potential,4 base instability of MVK and its aldol products has hampered its development.5 To the best of our knowledge, there is no general asymmetric aldol reaction of MVK reported to date. Herein we report an atom-economical and direct route to the hydroxy ketones from MVK as well as synthetic applications of resulting hydroxy enones.
Initially, on the basis of the promising catalytic activity displayed by dinuclear Zn-complex 2,3 we set out to examine the reaction of MVK as an aldol donor. The pre-cataylst was prepared as previously reported by treating ligand 1 with 2 equiv of Et2Zn in THF at RT. Subjection of this pre-catalyst (10 mol %) to a mixture of MVK, cyclohexane carboxaldehyde, 4A molecular sieve, and i-propanol (5 equiv) in THF at −35 °C led to irreproducible yields of the desired aldol product (10–30 %) and dehydration product, resulting from removal of β–hydroxy stereocenter (60–80%). Although the reaction was highly enantioselective (90–95 %ee), the product profile was time-dependent and more dehydration byproduct formed upon longer reaction time. The effect of additives and change of reaction parameters (temperature/time) did not much improve the result. On the other hand, we found that there is significant negative nonlinear effect in accord with an aggregation effect.6
Along this line, the effect of concentration and solvent was examined. Gratifyingly, we observed an improved yield of the desired aldol addition vs. elimination (entry 1, Table 1) with excellent enantioselectivity by using an increased concentration of MVK: Catalyst (10 mol %) prepared in THF or toluene (0.5 mL) was added to a combination of freshly distilled MVK (1 mL), cyclohexane carboxaldehyde (0.5 mmol), i-PrOH (5 equiv), and 4A molecular sieve. The reaction was generally faster in THF or (Method A, table 1) than in toluene (Method B), though toluene had better control over the elimination problem. As shown in Table 1, a variety of aliphatic aldehydes led to the corresponding β-hydroxy vinyl ketones in reproducible yields and high enantioselectivities. In the case of α– or β-hydroxyaldehydes, the presence of bulkier, non-coordinating silyl protecting group seem to give better turnover as well as better chiral recognition (entry 3 vs. 4 and entry 8 vs. 9). Entry 7 deals with the issue of catalyst-controlled diastereo-selectivity. In a matched case (entry 7a), exclusive Felkin-Anh product (1,2-anti-diol) was obtained with excellent selectivity and good yield, where intrinsic bias is reinforced by the catalyst in the same direction. In a mismatched case (entry 7b), 1,2-syn-diol formed with catalyst-controlled diastereoselectivity (dr 7/1) in modest yield. Some β-branched aldehydes can also be employed to give modest yield and excellent % ee (entry 10), though α-branched aliphatic aldehydes gave better results. As shown in Fig. 1, the reaction shows a slight negative non-linear effect.
Table 1.
Reactions of Various Aldehydes
| entry | RCHO | method | temp/time (h) | yielda (%) | Eeb (%) | Product |
|---|---|---|---|---|---|---|
| 1 |
|
A | −35/48 | 53 | 92 |
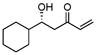
|
| B | −25/21 | 56 | 91 | |||
| 2 |
|
A | −30/48 | 57 | 77 |
|
| B | −15/7 | 74 | 86 | |||
| 3 |
|
A | −30/48 | 64 | 83 |
|
| B | −15/22 | 46 | 87 | |||
| 4 |
|
A | −15/8 | 52 | 93 |
|
| B | −15/14 | 66 | 92 | |||
| 5 |
|
A | −35/36 | 51 | 90 |
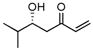
|
| B | −20/10 | 56 | 91 | |||
| 6 |
|
A | −35/36 | 47 | 83 |
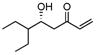
|
| B | −20/10 | 37 | 85 | |||
| 7a |
|
B | −15/17 | 59 | >99
de |
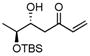
|
| 7b |
|
B | −15/18 | 33 | 71
de |
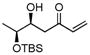
|
| 8 |
|
B | −15/15 | 33 | 44 |
|
| 9 |
|
B | −15/14 | 50 | 91 |
|
| 10 |
|
A | −35/36 | 49 | 98 |
|
All yields are for isolated pure product.
The ee’s were determined by chiral hplc.
Fig. 1.
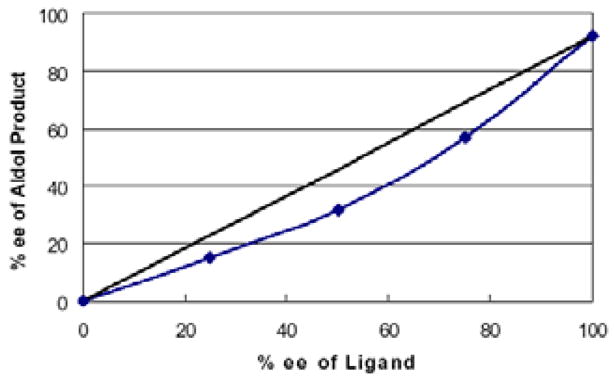
Non-linear Effect (%ee of aldol product vs. % ee of ligand 1
We envisioned propagation of the existing stereocenter created in the aldol addition through successive highly diastereoselective transformations. The obtained β-hydroxy enones were diastereo-selectively reduced to 1,3-syn (dr >99:1) or 1,3-anti diols (dr >90:10) in excellent yields using Et2B(OMe)/NaBH4 and Me4NBH(OAc)3, respectively (Chart 1).6,7 These diols were used directly without protection in a Mg-mediated diastereoselective cycloaddition of nitrile oxide.8 When the 1,3-diols were treated with 3 equiv of EtMgBr in CH2Cl2 followed by a pre-formed solution of a nitrile oxide at −25 °C, the corresponding dihydro-isoxazoles formed smoothly in excellent diastereoselectivities and good yields (Table 2). Notably, the obtained diastereoselectivity depends only on the stereocenter of the allylic alcohol in the dipolarophile and stereocenters elsewhere had no stereo-directing effect, thus providing effective method for convergent fragment coupling.
Chart 1.
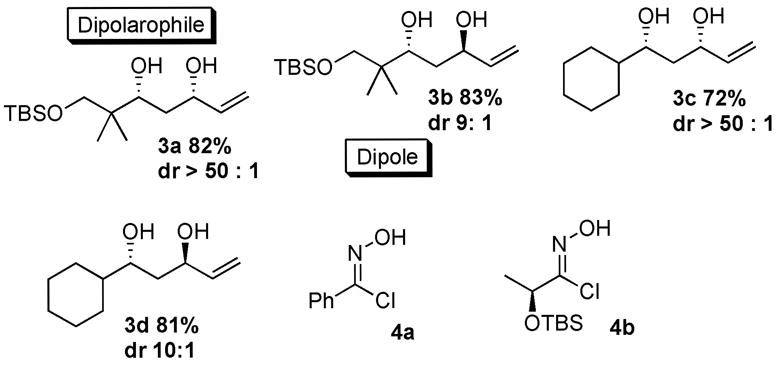
Cycloaddition Partners
Table 2.
Mg-mediated Cycloaddition of Nitrile Oxide
| Dipolaro-phile | dipole | condition | yield(dr)a | product |
|---|---|---|---|---|
| 3a | 4a | −25 °C, 10 h | 59 % (>95:5) |
|
| 3b | 4a | −20 °C, 9 h | 71 % (>95:5) |
|
| 3c | 4b | −25 °C, 11 h | 69 % (>95:5) |
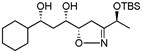
|
| 3d | 4b | −30 °C, 18 h | 60 % (>95:5) |
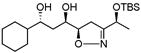
|
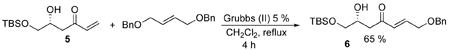
In contrast to alkynylmethyl ketones,3e vinylmethyl ketone donors require their use in excess, thus necessitating readily available donors. Resolution of this issue resides in the recent developments in the cross-metathesis reaction which allows a liberal modification on the terminal olefin end also reinforcing the bifunctionality of MVK as a synthetic building block.9 As shown in eq. 1, the desired vinyl-modified ketone 6 was obtained from 5 in 65 % yield with excellent E/Z selectivity (>15:1).
(1).
In conclusion, we have demonstrated dinuclear Zn-complex 2 catalyze aldol reaction of methyl vinyl ketone in good yield and excellent %ee. The resulting product could be transformed via stereoselective reactions into a variety of useful intermediates, showcasing MVK as a useful bifunctional building block.
Supplementary Material
Experimental procedures for the preparation of new compounds as well as characterization data are included (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the National Science Foundation and the National Institutes of Health, General Medical Sciences (GM-13598), for their generous support of our programs. Mass spectra were provided by the Mass Spectrometry Regional Center of the University of California-San Francisco, supported by the NIH Division of Research Resources.
References
- 1.(a) Roush WR. In: Comprehensive Organic Synthesis. Trost BM, Fleming I, Heathcock CH, editors. Vol. 2 Pergamon; Oxford, U.K: 1991. [Google Scholar]; (b) Carreira EM. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 3. Springer; Heidelberg: 1999. p. 998. [Google Scholar]; (c) Yamamoto Y, Asao N. Chem Rev. 1993;93:2207. [Google Scholar]; (d) Marshall JA. Chem Rev. 1996;96:31. doi: 10.1021/cr950037f. [DOI] [PubMed] [Google Scholar]; (e) Mahrwald R. Chem Rev. 1999;99:1095. doi: 10.1021/cr980415r. [DOI] [PubMed] [Google Scholar]
- 2.For biological catalysts, see Wong C-H. Enzyme Catalysis in Organic Synthesis. 2. Vol. 2. 2002. p. 931.Curtis ADM. Biotechnology. 2000;8b:5.Matsumoto K, Shimagaki M, Nakata T, Oishi T. Tetrahedron Lett. 1993;34:4935.Bednarski MD, Simon ES, Bischofberger N, Fessner WD, Kim MJ, Lees W, Saito T, Waldmann H, Whitesides GM. J Am Chem Soc. 1989;111:627.Fessner WD, Sinerius G, Schneider A, Dreyer M, Schulz GE, Badia J, Aguilar J. Angew Chem, Int Ed. 1991;30:555.List B, Shabat D, Barbas CF, III, Lerner RA. Chem Eur J. 1998;4:881.Hoffmann T, Zhong G, List B, Shabat D, Anderson J, Gramatikova S, Lerner RA, Barbas CF., III J Am Chem Soc. 1998;120:2768.. For non-biological catalyst, see Trost BM, Ito H. J Am Chem Soc. 2000;122:12003.List B, Lerner RA, Barbas CF., III J Am Chem Soc. 2000;122:2395.Kumagai N, Matsunaga S, Yoshikawa N, Ohshima T, Shibasaki M. Org Lett. 2001;3:1539. doi: 10.1021/ol015878p.
- 3.For aldol reactions: Trost BM, Ito H, Silcoff ER. J Am Chem Soc. 2001;123:3367. doi: 10.1021/ja003871h.Trost BM, Silcoff ER, Ito H. Org Lett. 2001;3:2497. doi: 10.1021/ol0161211.Trost BM, Yeh VSC. Org Lett. 2002;4:3513. doi: 10.1021/ol026665i.Trost BM, Fettes A, Shireman BT. J Am Chem Soc. 2004;126:2660. doi: 10.1021/ja038666r. Henry reactions: Trost BM, Yeh VSC. Angew Chem Int Ed. 2002;41:861. doi: 10.1002/1521-3773(20020301)41:5<861::aid-anie861>3.0.co;2-v.Trost BM, Yeh VSC, Ito H, Bremeyer N. Org Lett. 2002;4:2621. doi: 10.1021/ol020077n.Mannich-type reactions: Trost BM, Terrell LR. J Am Chem Soc. 2003;125:338. doi: 10.1021/ja028782e.Diol desymmetrization: Trost BM, Mino T. J Am Chem Soc. 2003;125:2410. doi: 10.1021/ja029708z.
- 4.(a) Joo JM, Kwak HS, Park JH, Song JH, Lee E. Bioorg Med Chem Lett. 2004;14:1905. doi: 10.1016/j.bmcl.2004.01.088. [DOI] [PubMed] [Google Scholar]; (b) Trost BM, Hanson PR. Tetrahedron Lett. 1994;35:8119. [Google Scholar]; (c) Boukouvalas J, Fortier G, Radu II. J Org Chem. 1998;63:916. [Google Scholar]
- 5.One account was described on Mukaiyama-type aldol reaction of MVK, where instability was noted for the adduct derived from benzaldehyde and MVK: Denmark SE, Stavenger RA. J Am Chem Soc. 2000;122:8837. However, for a successful reaction using lanthanides see Hong BC, Chin S-F. Synth Commmun. 1997;27:1191. Antibody catalyzed retro-aldol reaction (kinetic resolution) of racemic β-hydroxy ketone has recently been published. However, no enantioselectivity was observed for β-hydroxy ketone derived from MVK: Baker-Glenn C, Hodnett N, Reiter M, Ropp S, Ancliff R, Gouverneur V. J Am Chem Soc. 2005;127:1481. doi: 10.1021/ja043925d.
- 6.See Supporting Information for details.
- 7.(a) Chen KM, Hardtmann GE, Prasad K, Repic O, Shapiro MJ. Tetrahedron Lett. 1994;28:155. [Google Scholar]; (b) Evans DA, Chapman KT, Carreira EM. J Am Chem Soc. 1988;110:3560. [Google Scholar]
- 8.(a) Kanemasa S, Masaki N, Kamimira A, Kenzi H. J Am Chem Soc. 1994;116:2324. [Google Scholar]; (b) Bode JW, Fraefel N, Muri D, Carreira EM. Angew Chem Int Ed. 2001;40:2082. doi: 10.1002/1521-3773(20010601)40:11<2082::AID-ANIE2082>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 9.For cross metathesis, see Connon SJ, Blechert S. Angew Chem, Int Ed. 2003;42:1900. doi: 10.1002/anie.200200556.Chatterjee AK, Morgan JP, Scholl M, Grubbs RH. J Am Chem Soc. 2000;122:3783.Chatterjee AK, Choi TL, Sanders DP, Grubbs RH. J Am Chem Soc. 2003;125:11360. doi: 10.1021/ja0214882.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures for the preparation of new compounds as well as characterization data are included (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.


