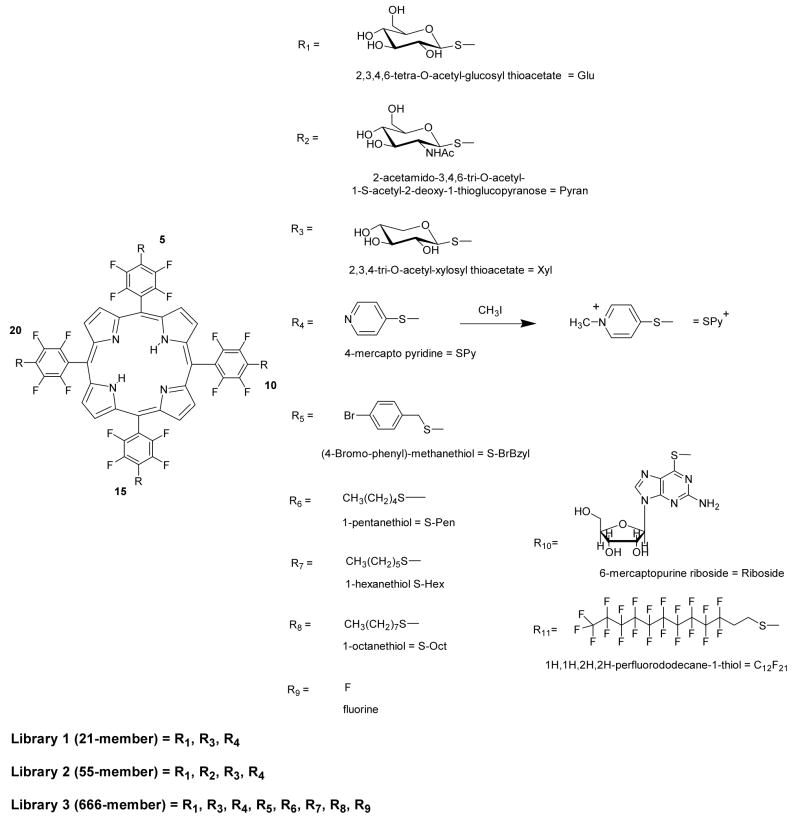
Abstract
The four para fluoro groups on 5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorophenyl)-porphyrin (TPPF20) are known to react with a variety of nucleophiles, but the reaction conditions for this substitution reaction depend on the nature of the nucleophiles, e.g. primary amines versus thiols. Glycosylated derivatives of this core porphyrin have been shown to be effective photodynamic agents in the induction of necrosis or apoptosis in several cancer cell lines. The present report demonstrates that TPPF20 can be used as a core platform to efficiently generate a variety of solution phase combinatorial libraries. The focused combinatorial libraries have substituents that are chosen from a set of motifs known to bind biopolymers such as DNA, be taken up by cancer cells, or to render the compounds amphipathic. Incubation of a breast cancer cell line with these solution phase libraries, followed by cell lyses and extraction, affords a selection assay. MALDI mass spectrometry of the extracts allows identification of the molecules taken up by the cells. Cell binding assays of the winning compounds synthesized directly, indicate that both glycosylation and amphipathicity are key properties since neither tetraglycosylated porphyrins nor those with four polar groups are selected to the same extent. In addition, photodynamic efficacy was evaluated.
Keywords: solution phase combinatorial library, porphyrin, corrole, photodynamic therapy, cell binding assays, fluorescence
Introduction
Peripheral substituents on the porphyrin core can be used both to modulate the photophysical properties and impart desired chemical characteristics to the macrocycle. For example these substituents can: direct the self-assembly or self-organization of the molecules into solid state materials,1–4 target the chromophore to various biological materials such as cells,5–7 modulate the electronic properties such as absorption and emission,8–10 serve as recognition motifs for small molecules,11 and serve as a scaffold for further chemical modifications.6, 12, 13 Porphyrinic systems coordinating redox active metals can be used as oxidation catalysts,14–19 and metalloporphyrins have wide applications as selective and cross reactive sensors.20–23 Herein we report the synthesis of a library of porphyrins in solution, and selection by a binding assay, in this case to a cancer cell line, that demonstrates the feasibility of this approach for the discovery of new porphyrinic materials.
There are several therapeutic applications of porphyrins.24–35 In terms of photodynamic therapeutics (PDT), the applications of porphyrins and related macrocycles to the treatment of cancers and as potential antibacterial or antiviral compounds36–41 arise from the high triplet quantum yields of the free bases and some metalloporphyrins. The main mechanism for the therapeutic activity of these compounds is that the triplet state of the chromophore acts as a photosensitizer in the formation of singlet oxygen. Singlet oxygen reacts with near diffusion limited kinetics with a variety of biomolecules, such as those containing double bonds, aromatic moieties, sugars, and phosphate esters. There are other potential therapeutic applications of porphyrins including treatment of stroke by iron porphyrins acting as superoxide dismutases,16, 42, 43 and as potential agents that prevent the misfolding of the prion proteins that cause scrapies and Creutzfeldt-Jakob syndrome.44–48
Depending on the intended application and the modality of use, some of the goals of current research on the therapeutic applications of porphyrinoids are, broadly, to make compounds that: (a) are more selective to target cells or tissues, (b) have greater triplet quantum yields, (c) are good two-photon absorbers, (d) have increased optical density in the 650–850 nm region where bio-fluids absorb light less, (e) cross the blood brain-barrier, (f) selectively bind to target bacteria, viruses, infected cells, (g) selectively bind to target proteins to prevent misfolding or to otherwise modulate activity, and (g) have different catalytic activities, e.g. dismutases. There is vigorous research on other applications of porphyrins and metalloporphyrins as sensors, catalysts, electronic, and luminescent materials. The vast majority of this research focuses on the design and synthesis of the peripheral substituents on the porphyrin to affect the desired chemical or materials properties. Thus there is an obvious need for the rapid, parallel synthesis of porphyrinoids with diverse peripheral substituents.
A complex mixture of monomers and oligomers of free base hematoporphyrin, Photofrin, is widely used in the treatments of a variety of cancers, and other applications are in the pipeline.49–52 Because of the huge market as an anti-cancer treatment and the many other possible therapeutic applications, there are numerous labs exploring the design and efficacy of new porphyrinic derivatives for PDT applications. One widely claimed rational for this research is that a major disadvantage of Photofrin is that it is a mixture. In contrast, we hypothesized that the efficacy of Photofrin as an anti-cancer treatment is due to the fact that it is a mixture.6, 53 The role of a mixture of hydrophobic porphyrins for PDT applications has also been considered.54 This hypothesis would indicate that the various Photofrin components partition into different parts of the diseased tissues and bind to different cellular components (e.g. cell walls, mitochondria, proteins, other organelles). When cell death is the intended outcome, this multi-pronged attack reduces the ability of the cell machinery to simultaneously repair the damage caused by singlet oxygen-induced oxidative stress at multiple sites. Secondly, the broad range of cancers that are effectively treated with Photofrin may be a result of the partitioning of different components into different cancer tissues and cancer cells. Thus, it is unlikely that one pure compound will be as broadly applicable to as wide a variety of diseased tissues and as effective as Photofrin. However, pure compounds that are selective to cancer cells or other diseased tissues, which also can be made in high yields, have significant advantages over the currently used mixtures, e.g. less cross reactivity to non-cancerous cells and lower dosages. This necessitates discovery of specific cell recognition motifs as substituents on the porphyrinoid core. A cocktail of these porphyrins targeted to a specific cell type or tissue then may have significant advantages over current technologies.
For example, previous reports on non-hydrolysable glycosylated porphyrins reveals that the sugars direct the macrocycles to cancer cells because of the significantly increase metabolic needs of these cells and the increased number of sugar transporters found on cancer cells.5, 6 A simple, high-yield procedure for making non-hydrolysable thio- glycosylated porphyrins based on the TPPF20 core was reported. It was shown that a glucose derivative was 2–5 times more active as a PDT agent than a galactose derivative on a breast cancer cell line known to express glucose transporters, MDA-MB-231. The tetraglucose derivative was also shown to induce apoptosis under low light at low porphyrin concentrations.6, 55
In contrast to appending porphyrins with one or more substituents designed to meet a given criteria, a combinatorial approach can be used to rapidly discover new substituents, combinations of substituents, and isomers for a given application. We have shown that large solution phase combinatorial libraries of porphyrins can be screened for DNA binding by running the library down a chromatography column of DNA adsorbed onto glass fibers.53 From a library of 1540 compounds, 4–6 members were selected as the best binders to DNA. A significant finding was that amphipathic molecules with two polar groups and two positively charged groups on the same side of the macrocycle have substantially greater affinity to DNA than the widely studied tetra cationic tetrakis(4-N-methylpyridinium)porphyrin. Photo-induced cleavage assays using plasmid DNA further indicated the activity of the winning compounds. This study synthesized a mixture of porphyrins from the corresponding aryl aldehydes and pyrrole, which necessitated purification of the library from the side products of the reaction and subsequent deprotection of the substituents.
One advantage of solution phase libraries includes the straightforward synthetic chemistry around a multitopic core.53, 56–66 Another advantage is that artifacts arising from the confounding roles of surface chemistry and energetics are eliminated from the selection process. Characterization of these libraries is straight forward using HPLC and electrospray mass spectrometry.53 Disadvantages can arise from the identification of selected compounds, but again the sensitivity of mass spectrometry can be exploited for this purpose.
Herein we present a significantly improved route to solution phase combinatorial libraries of porphyrins that uses TPPF20 as a core platform. One advantage is that TPPF20 is commercially available and can be made in large 100 g batches in the laboratory. By appending substituents to TPPF20, this approach eliminates the need to remove the tarry side products of the porphyrin synthesis,53 and obviates the need for most protecting groups.13, 55 We demonstrate the chemistry by forming focused solution phase libraries of this porphyrin bearing functionalities known to be important for PDT, e.g. sugars, cationic groups, and moieties that impart amphipathic solubility properties. The reagents used herein are also commercially available and can be made in large quantities. As a proof-of-concept, these solution phase libraries are screened for binding to the MDA-MB-231 breast cancer cells by incubating the cells, washing away the unbound materials, and identifying the absorbed porphyrins in cell extracts by MALDI mass spectrometry. The winning compounds were made by directed synthesis. Fluorescence microscopy based cell-binding assays and photodynamic efficacy of the winning compounds made from the TPPF20 platform and from the original solution phase libraries were compared to the tetra(thioglucose) derivative (TPPF16-Glu4) standard and confirm the selection criteria.
Results and Discussion
Nucleophilic substitution reactions of the four para fluoro groups on TPPF20 are well documented.6, 12, 13, 67 The reactivity of the para fluoro group on the pentafluorophenyl moiety varies significantly with the specific primary nucleophile used, where softer nucleophiles are more reactive than harder nucleophiles, HS-CH2R > H2N-CH2R ≫ HO-CH2R. As expected, secondary nucleophiles are much less reactive than similar primary derivatives. For example, primary amine derivatives are efficiently synthesized in N-methylpyrrolidone and microwave heating for about 12 minutes,12 and thiol derivatives are made in DMF under an inert atmosphere at room temperature for 8–24 hrs.13 The yields of both of these reactions can exceed 90%, and are tolerant of other functional groups such as alcohols. Thus TPPF20 can serve as a suitable platform for combinatorial chemistry as long as one class of nucleophile is used, since the reaction conditions vary with nucleophile type and degree of steric crowding.
A survey of reactions with primary thiols allowed development of a list of reagents with similar reactivities. For example, the overnight reaction of TPPF20 with six equivalents of 6-mercaptopurine riboside in DMF with DEA at room temperature results only in a low yield (< 10%) of the tetrasubstituted product. But when the amount of 6-mercaptopurine riboside was increased to 16 equivalent (4 equivalent to each p-fluorine on the porphyrin), and the reaction allowed to proceed for five days, 5,10,15,20-tetrakis-[4-(6′-thio-purineriboside)-2,3,5,6-tetrafluorophenyl)]porphyrin (TPPF16-Ribo4) is isolated in about 38% yield. The major side product was the partially reacted 5-(pentafluorophenyl)-10,15,20-tris-[4-(6′-thiopurineriboside)-2,3,5,6-(tetrafluorophenyl)]porphyrin (30% yield). Also, since fluorous alkanes can be used as identifiers in combinatorial libraries, the coupling of a long perfluoroalkyl structure possessing a thiol tail to TPPF20 was examined. 1H,1H,2H,2H-perfluorododecane-1-thiol in DMF was first dissolved in a DMF/ethyl acetate/DEA (4:4:1 v/v) solvent mixture, and followed by the addition of the porphyrin dissolved in minimum amount of DMF. The ratio of porphyrin to thio reagent was 1:10. Nitrogen is then passed through this solution for about 2 minutes and the product precipitates. The yield of the desired tetra substituted product, 5,10,15,20-tetrakis-[4-(1′-thio-1′H,1′H,2′H,2′H-perfluorododecane)-2,3,5,6-tetrafluorophenyl]porphyrin (TPPF16-C12F21), is greater than 95%. ESI-MS of the product mixture showed no peaks correspond to the tri-substituted or partially substituted porphyrins suggesting the excellent efficiency and the importance of the solvent system for this particular reaction. Thiopyridine was used as it can be further modified to form cationic species, e.g. the methylpyridinium.
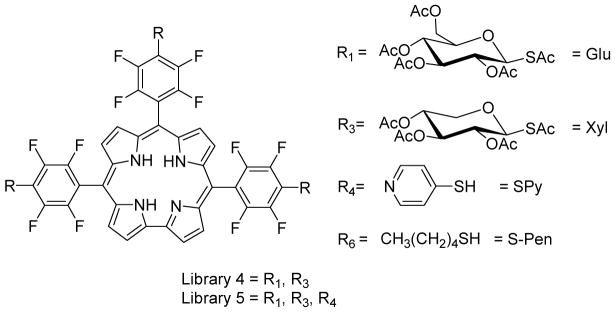
A 21-member porphyrin combinatorial library: L-1
The efficiency of the nucleophilic substitution of the 4-fluoro group by various thio compounds indicated that TPPF20 would be a good platform for the formation of core-centered combinatorial libraries. Based on the PDT activity of the aforementioned thioglycosylated derivatives and the amphipathic solubility of pyridyl moieties, small 21 member libraries focusing on different thiosugars and pyridine were synthesized and characterized to assure this strategy results in a statistical distribution of products. When one equivalent of TPPF20 is stirred with 1.4 equivalents of each of the three thiols (2,3,4,6-tetra-O-acetyl-glucosylthioacetate, 2,3,4-tri-O-acetylxylosylthioacetate and 4-mercaptopyridine; a 1:4.2 ratio of TPPF20 to thiol, or 1:1.05 per para-fluoro group) and 20 equivalents of diethylamine (DEA) at room temperature in DMF for 24 hrs, a statistical mixture of 21 compounds is formed (Scheme 1). All the nucleophiles involved in the substitution of p-fluorine atom of pentafluorophenyl porphyrin derivatives mentioned in this work are free thiol functions. In the case of acetyl-protected thiosugars, the presence of basic diethylamine in the reaction mixture is sufficient to deprotect the acetyl functions to regenerate the parent thiol moieties. Core-centered libraries based on this chemistry have positional isomers (e.g. two copies of a substituent on adjacent versus opposite sides of the porphyrin), such that the statistical reaction of three auxiliaries to the four aryl groups of the porphyrin results in 15 isobaric compounds. Thus isobaric peaks in the mass spectra arise both from isomers and from coincidental m/z overlap at this resolution. Comparison of the ESI-MS spectra of the reaction mixture with that predicted from the simulated spectrum indicates all 15 compounds are present (supporting information).
Scheme 1.
R1 to R11 are the substituents in the libraries examined in this study. The sugars are synthesized as the acetate derivatives and subsequently deprotected by stoichiometric amount of NaOCH3 after formation of the quaternary pyridinium moieties. In the text the derivatives are described according to the numbering of the meso porphyrin positions, 5,10,15,20 e.g. Glu/Glu/Glu/Glu. Note that the Ac protected sugars were used in characterization of the libraries.
Deprotection of the sugars is quantitatively accomplished using an equal molar ratio of NaOMe to the acetate protecting groups. Since ESI-MS does not always give high-quality spectra of the deprotected sugar libraries, MALDI-MS is used. The peaks in MALDI-MS spectra are also consistent with the peaks in the simulated spectra (supporting information). In both cases, Na+ adducts are observed as lower intensity satellites 23 mass units greater than the parent peaks, which is typical for glycosylated compounds. The Na+ adducts account for the small differences between the simulated and experimental spectra.
Using the same strategy, a 55- member porphyrin library, L2, formed from the addition of four different thiols (GluAc, XylAc, PyranAc, SPy, Scheme 1) of which 35 are isobaric. ESI-MS indicate the expected distributions of these core-centered libraries, vide infra (supporting information).
Synthesis of 666-member porphyrin combinatorial library: L3
The successful synthesis of the 21- and 55- member libraries prompted us to construct larger porphyrin libraries with different thio reagents. Since there are numerous studies that correlate the solubility properties such as amphipathicity of PDT agents with what is likely diffusive cell uptake, we chose several sugars and several thio alkanes. Seven different thio reagents were reacted with TPPF20 using similar reaction conditions as noted above but with 2-day reaction times and 40 equivalents of DEA. When seven different thiols are reacted with TPPF20, 406 compounds (210 isobaric) are expected. However under these reaction conditions, substitution of all four fluorines proceeds with 85–90% yields, so there are some members of the library that contain unreacted F groups. Neither the unreacted starting material nor the mono substituted tri-(pentafluorophenyl) compounds were observed in the ESI-MS. A few library members with two 4-fluoro groups were observed with low abundances, and a somewhat greater number and abundance of compounds with a single unreacted 4-fluoro group were found. When the F group is included there are eight possible substituents, yielding a library with 666 members of which there are 330 isobaric species (Scheme 1). The reaction mixture was assayed by ESI-MS by first separating the porphyrinic compounds into various fractions by HPLC and secondly taking the mass spectral profile of each fraction. Neighboring fractions oftentimes contained some of the same compounds and/or isomers, thus confirming the identity of these. Examination of the ESI-MS of all the HPLC fractions allowed identification of almost all of these 330 possible peaks (supporting information). The parent ion peaks of <20 members were not detected in the mass spectra with confidence (those containing 4, 3, or 2 F groups). Some were indicated as the Na+ / K+ or HCOO− adducts, which is consistent with previously observed adducts in the ESI-MS, especially for sugar containing systems.6, 13 There are a few unidentified mass peaks that likely arise from these adducts.
Pentafluorophenyl Corroles
Corroles are porphyrinoid macrocycles missing one of the porphyrin methylene bridges and are similar to the corrins of vitamin B-12 (non-aromatic). Corroles possess 20 π-electrons, out of which 18 are conjugated and hence corrole is an aromatic system like porphyrins. In general, free base corroles are found to have a greater fluorescence quantum yield than porphyrins because they have a direct pyrrole-pyrrole linkage.69, 70 Thus these macrocycles have been proposed as fluorescence sensors and fluorescence imaging agents. Because of the 3− charge of the free base, these compound stabilize high valent metal ions, including some that are suitable for contrast agents such as Ga(III).71 Since the perfluorophenyl groups in triPCF15 are orthogonal to the corrole macrocycle, similar to those on the porphyrin TPPF20, the reactivity of the para fluoro group was expected to be similar.
With triPCF15 corrole a library with two substituents yields six compounds but there are two sets of isomers so there are only four isobaric peaks expected in the mass spectra. Similarly, in the case of three substituents, one expects 18 compounds but 10 unique masses because of the various positional isomers. We find that the substitution chemistry works better for the corrole system using N-methylpyrrolidone (NMP). This indicates a solvent effect on the reactions of the corrole versus porphyrin, which may be a result of differences in the electron withdrawing effects of corroles in comparison to porphyrins and/or the acidity of the corrole pyrrole N may play an important role in these reactions.70
When three equivalents of the thioglucose and thioxylose acetate protected sugars in NMP are stirred overnight in the dark, the ESI-MS of the crude reaction mixture indicates formation of the expected six-member library. However, the attempt to create the 18-member library on a corrole platform using thio pyridine in addition to the protected sugars proved only partially successful. Evaluation of the ESI-MS spectra of the product mixture showed the 17 of the expected 18 compounds and four compounds with the 4-fluoro groups remaining on the corrole systems. Neither an increase in the quantity of the thio derivatives nor increase reaction times (from hrs to days) resulted in complete reactions. The ESI-MS indicates that thio pyridine moiety is less reactive than the thiosugars.
Selection of winning compounds from a solution phase 21-member library using human breast cancer cells
Although there are many potential therapeutic applications of porphyrinoids, at present the primary clinical use is for the photodynamic treatment of macular degeneration and of cancer. For the treatment of cancer, the goal is to kill or eliminate cells by any means that target diseased cells and tissues. There are a variety of cellular processes and components that can be targeted, but since most crucial cellular processes have various repair mechanisms, it may be preferable to target more than one, or even many, parts of the cell to maximize the probability of killing it. Rather than specifically targeting a particular cellular component, or specific cellular process, or evaluating solubility properties, by designing a molecule or a class of molecules and testing them individually for PDT efficacy, we hypothesized that the cells themselves may indicate which molecules bind and are taken up most efficiently. Thus an assay was developed wherein viable human breast cancer cells (MDA-MB-231) were treated with focused solution phase libraries to allow the cells to select the members of the library. Compounds with high affinities and/or uptake into the cells were examined for PDT activities.
After incubation of the cells with library L1 (100 μM in total porphyrin content and 1–5 μM of the individual compounds) for 24 hrs, the cells were rinsed three times with PBS buffer to remove unbound porphyrins and collected. The cells were treated with 2-chloroethanol, sonicated, and the solvents removed. The porphyrins selected by the cancer cells were extracted from the lysates by sonicating in methanol and centrifugation to remove insoluble materials. This was repeated until no porphyrin fluorescence was observed in the methanol. The methanol was concentrated and assayed by MALDI-MS (supporting information). Two major parent ion peaks corresponding to members of the library were identified;55 each can be one of two isomers: (1) Glu/Glu/SPy/SPy, Glu/SPy/Glu/SPy, and (2) Xyl/Glu/SPy/SPy, Xyl/SPy/Glu/SPy. Additionally, two minor peaks were identified; each can be one of two isomers: (3) Glu/Glu/Xyl/SPy, Glu/Xyl/Glu/SPy, and (4) Xyl/Xyl/Glu/SPy, Xyl/Glu/Xyl/SPy. Thus, there are eight potential winning compounds. If one considers the Xyl as a surrogate for Glu (Xyl was used because of the difference in molar mass for mass spectrometry assays) and since most cells do not specifically take up xylose, then these eight possible compounds distill into the three Glu/Glu/SPy/SPy, Glu/SPy/Glu/SPy, and Glu/Glu/Glu/SPy. Since 5,10- porphyrinic isomers are known to have greater affinity to cancer cell membranes and other cellular components than 5,15-isomers5 it is reasonable to eliminate the Glu/SPy/Glu/SPy, but to be sure the identification of the selected isomer was accomplished by cell binding assays of the individual compounds.
Synthesis of winning compounds and their affinity towards cancer cells
The Glu/Glu/SPy/SPy and Glu/SPy/Glu/SPy and Glu/Glu/Glu/SPy compounds were made by reacting the TPPF20 core with the corresponding stoichiometries of the thiol reagents and separating the compounds by chromatography followed by deprotection of the acetate groups (Table 1). The compounds initially identified from L-1 had polar pyridyl groups, but cationic N-methylpyridinium groups on porphyrins have known affinities for DNA, RNA,72–74 and anionic membranes.75–77 Thus, in addition to the study on winning compounds, it was decided to further this work by investigating compounds bearing the cationic methylpyridinium species (scheme 1, SPy+). The methylation of the pyridine moiety with CH3I is done before deprotection of the sugars.
Table 1.
| Porphyrin Product | Thio reagent | Solvent | Rxn. time | Yield
(%) |
|||
|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | ||||
| R10
R10 |
R10
R10 |
R10
R10 |
R10
R9 |
6-mercaptopurine riboside | DMF | 5 day | 38
30 |
| R11 | R11 | R11 | R11 | 1H,1H,2H,2H-perfluorododecane-1-thiol | ethylacetate:DMF (1:1) | 2 min | 97 |
| R1 | R1 | R4 | R4 | 2,3,4,6-tetra-O-acetyl-glucosyl-thioacetate/4-thiopyridine (1:1)b | DMF | 2 days | 30 |
| R1 | R4 | R1 | R4 | 25 | |||
Reactions are run under an inert atmosphere, 20 equivalents DEA, magnetic stirring.
Remainder of the six possible derivatives: 33%.
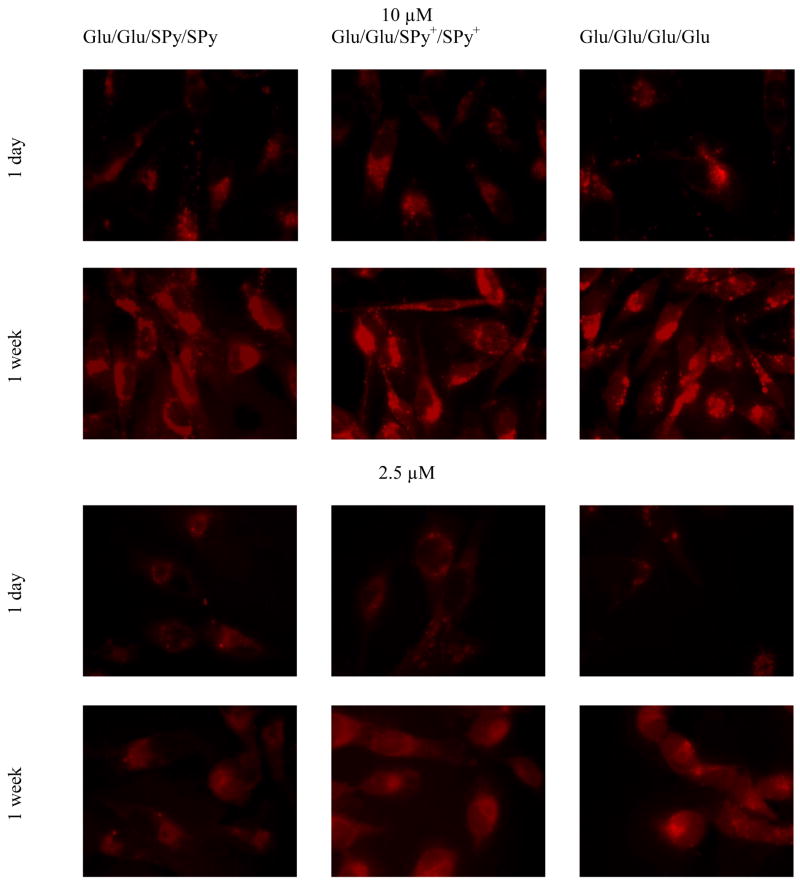
Fluorescence microscopy was used to investigate the affinity of these compounds to human breast cancer MDA-MB-231 cells, and compared to the well-studied TPPF16-Glu4 (Glu/Glu/Glu/Glu) derivative, which was also a member of the library. Figure 1 shows the fluorescence microscopy of MDA-MB-231 cells treated with 2.5 μM and 10 μM of three porphyrin derivatives for 24 hrs under identical conditions. After rinsing the unbound compounds from the cells on the cover slips and fixing, fluorescence images of the cells were taken on the same day and one week later. The observed fluorescence intensity was taken to be proportional to the quantity of porphyrin bound to the cells, and was quantified by an image intensity comparison program, “FLim.”78 When cells are treated with either the uncharged or the cationic 5,10 isomer Glu/Glu/SPy/SPy, Glu/Glu/SPy+/SPy+ or the Glu/Glu/Glu/Glu standard for 24 hrs, the low intensity of the fluorescence observed by fluorescence microscopy seems to indicate that little of these porphyrins is taken up by the cells just after fixing the cells (Figure 1, 1 day). This result is misleading because the same fixed cells examined one week later exhibit a much brighter fluorescence (Figure 1, 1 week), indicating that the porphyrins were indeed taken up by these cells. Since the cells were rinsed to remove unbound porphyrins, no further uptake of porphyrin is possible.
Figure 1.
Assays of porphyrin uptake by MDA-MB-231 cells by comparison of fluorescence intensities. 10 μM and 2.5 μM Glu/Glu/SPy/SPy and the charged derivative Glu/Glu/SPy+/SPy+, are compared to Glu/Glu/Glu/Glu immediately after fixing and one week after fixing the cells. Micrographs were taken under identical conditions and the images are not manipulated. Magnification 60X.
Using the FLim78 program to compare the fluorescence intensity of cells treated with 10 μM of porphyrins at day 1 shows that the charged Glu/Glu/SPy+/SPy+ and Glu/Glu/Glu/Glu have similar intensities and are more intense than the uncharged Glu/Glu/SPy/SPy by a factor of ca. 1.4. The 5,15 isomer, Glu/SPy/Glu/SPy, which was also a possible selection from the 21-member library, shows the poorest uptake (data not shown), so the trans Glu/SPy+/Glu/SPy+ pyridinium derivative was not further evaluated. The 5,15 pyridinium species are known to be poorly absorbed by many cell lines and similar cellular uptake results have been previously reported for 5,10- versus 5,15- isomers.5, 53 Re-examination of the same slides one week later reveals a substantial increase in fluorescence intensity of the 5,10 isomers and that the fluorescence of Glu/Glu/SPy/SPy porphyrin is still significantly less intense than the charged derivative Glu/Glu/SPy+/SPy+ (Figure 1, 1 week). The increased fluorescence intensities indicate that the porphyrins in the cells are initially aggregated (quenching the fluorescence) and that it takes time for them to disaggregate. It is noteworthy that even the Glu/Glu/Glu/Glu derivative, which was shown to be highly active as a PDT agent,55 must be aggregated in the cells to some extent when used at this concentration, which is born out by the UV-Visible spectra (supporting information). This implies that this compound may be more active than initially reported if allowed time to disaggregate.
In order to further discern any differences between the compounds in terms of cell uptake, lower concentrations were also used. The FLim program was used to compare the fluorescence intensity of MDA-MB-231 cells treated with 2.5 μM of the porphyrins as above (Figure 1). Initially a weak fluorescence is observed for all porphyrins tested; however, fluorescence micrographs after one week exhibit a dramatic increase in fluorescence intensity for all porphyrins. E.g. a factor of 49 for the Glu/Glu/Glu/Glu and 41 for the Glu/Glu/SPy+/SPy+ derivatives. Once again, the uncharged Glu/Glu/SPy/SPy shows the poorest uptake into the MDA-MB-231 cells. At this lower concentration the tetraglycosylated compound shows a slightly better cellular uptake than the charged Glu/Glu/SPy+/SPy+ compound.
These results suggest that the porphyrins bind and/or enter the cells as nanoscaled aggregates,79–89 which have significantly quenched fluorescence by well-understood mechanisms including shading, energy transfer, and enhanced vibrational relaxation.9, 90, 91,92 The UV-visible spectra of the compounds of interest in polar organic solvents (e.g. CH3OH) are typical of porphyrin derivatives based on TPPF20; however, the Soret bands of the porphyrins in PBS or cell culture medium without the chromophoric components (no phenol red) are significantly broadened and/or split, which is typical of aggregated porphyrins (supporting information).2, 93 Dynamic light scattering (DLS) of 10 μM of these three compounds (Glu/Glu/Glu/Glu, Glu/Glu/SPy/SPy, Glu/Glu/SPy+/SPy+) in PBS indicate the particles are between 10 nm and 30 nm in diameter. The apparent increase in fluorescence intensity one week after fixing the cells is indicative of the deaggregation of the nanoparticles mediated by the binding of the porphyrins to various cellular components and is kinetically slow. Note that particle size is determined by the complex interplay of intermolecular forces between solute and solvent and that the functional groups are still displayed on the surface of the aggregate.2, 93
Photodynamic activity of selected compounds
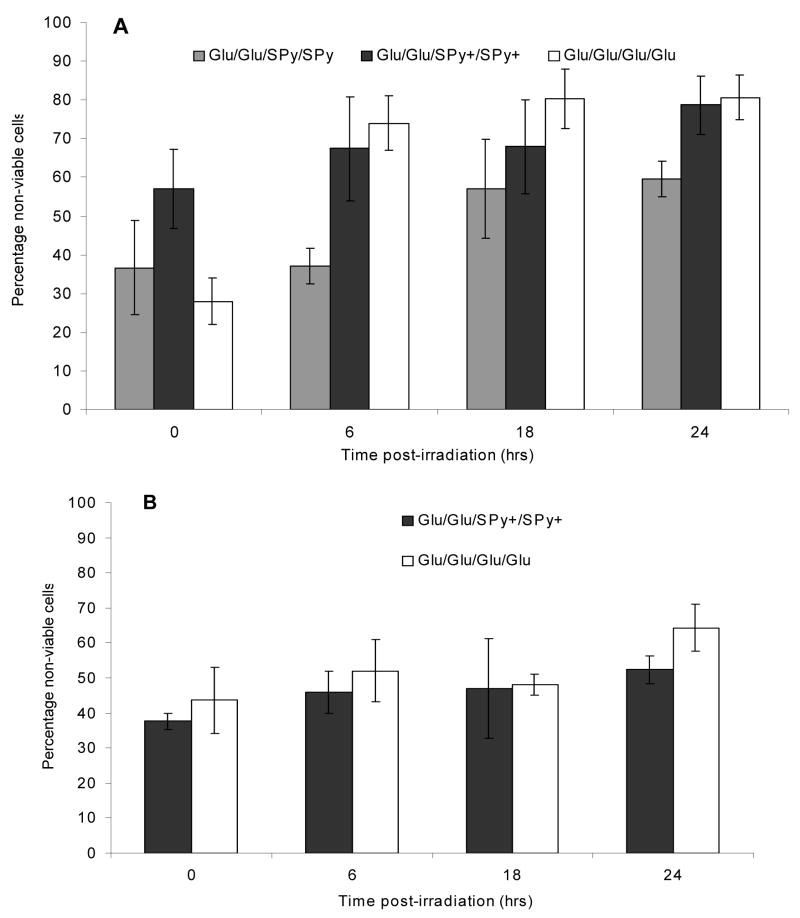
The PDT activity of the winning compounds from L-1 was evaluated using breast cancer cells, MDA-MB-231. Since fluorescence assays indicate that the 5,15- isomers (Glu/SPy/Glu/SPy, Glu/SPy+/Glu/SPy+) do not bind efficiently to these cells they were not evaluated. The uncharged 5,10- isomer Glu/Glu/SPy/SPy and its cationic derivative, Glu/Glu/SPy+/SPy+, were evaluated at 10 μM and 2.5 μM and compared to the Glu/Glu/Glu/Glu compound. After incubation for 20 hrs, the cells were washed 3–5 times to remove unbound porphyrin materials and irradiated for 20 minutes with white light from a 13 Watt fluorescent bulb (0.9 mW/cm2). Cell death was monitored by trypan blue dye staining over a 24 hour period, wherein cells stained blue were counted using a hemacytometer and considered non-viable.
With 10 μM of all three compounds, more than 25% of the cells are necrotic immediately after light irradiation under the specified conditions (Figure 2A). The assay also reveals that the percentage of non-viable cells for Glu/Glu/SPy+/SPy+ and Glu/Glu/Glu/Glu continues to increase with time to >80% after 24 hrs. The cell density is observed to increase, during this time so some of the treated cells are able to divide. The persistent increase in the percentage of non-viable cells over 24 hrs implies a secondary mechanism, i.e. cell death via apoptosis.88, 94–97 The uncharged Glu/Glu/SPy/SPy showed less than 60% cell death over 24 hr and was therefore not evaluated at the lower concentration. One may speculate that the uncharged Glu/Glu/SPy/SPy remains aggregated in the cell and so are poorly fluorescent and have significantly diminished triplet quantum yields.24, 98
Figure 2.
Photodynamic activity of (A) 10 μM Glu/Glu/SPy/SPy and its charged analogue Glu/Glu/SPy+/SPy+ compared to Glu/Glu/Glu/Glu, and (B) 2.5 μM of the Glu/Glu/SPy+/SPy+ and Glu/Glu/Glu/Glu porphyrin derivatives. MDA-MB-231 cell death over a 24 hr period was quantified by trypan blue staining. Cells were irradiated with white light form a 13 W fluorescent bulb for 20 min (0.9 mW/cm2 =10.8 kJ/m2). Results are average of three independent trials ± 1 S.D.
The cationic Glu/Glu/SPy+/SPy+ was evaluated at 2.5 μM and compared to the Glu/Glu/Glu/Glu standard (Figure 2B). At this concentration ca. 40% of the cells are found to be necrotic just after exposure to the light. The percentage of non-viable cells continues to increase to >60% over a 24 hour period. When the cells are treated with this 4-fold lower concentration of Glu/Glu/Glu/Glu, more cells were found to be necrotic immediately following the irradiating light (43% vs. 28%). While for cells treated with Glu/Glu/SPy+/SPy+, there was a decrease in the percentage of necrotic cells just after light exposure (57% vs. 37%). This can be explained by the normalized absorption spectra for varying concentrations of the Glu/Glu/Glu/Glu porphyrin in aqueous solution (PBS), where the half width of the Soret band broadens with increasing concentrations, which is interpreted as indicating aggregation of the chromophores (supporting information).99, 100 Since dye aggregates are less efficient in forming excited state triplets, one expects reduced PDT activity, as observed.
Mixed aryl porphyrin analogues of the porphyrins selected from L-1
The compounds selected from L1 and studied above have the pyridinium moiety attached to the tetrafluorophenyl group on the macrocycle. Thus these compounds have different hydrophobic properties and polarities than porphyrins bearing the pyridinium directly on the meso position that we53 and other groups24, 50 have studied. Secondly, the molecular dynamics of the pyridinium-S-tetrafluorophenyl bonds have an impact on the molecular properties of these compounds. Thus the molecular properties of Glu/Glu/SPy/SPy and Glu/Glu/SPy+/SPy+ that dictate intermolecular interactions, especially the formation of aggregates in aqueous solutions, may be significantly different than compounds wherein the pyridinium moiety is directly attached to the porphyrin. The reduced dynamics and increased charge density per molecular volume may play a role in cell uptake.
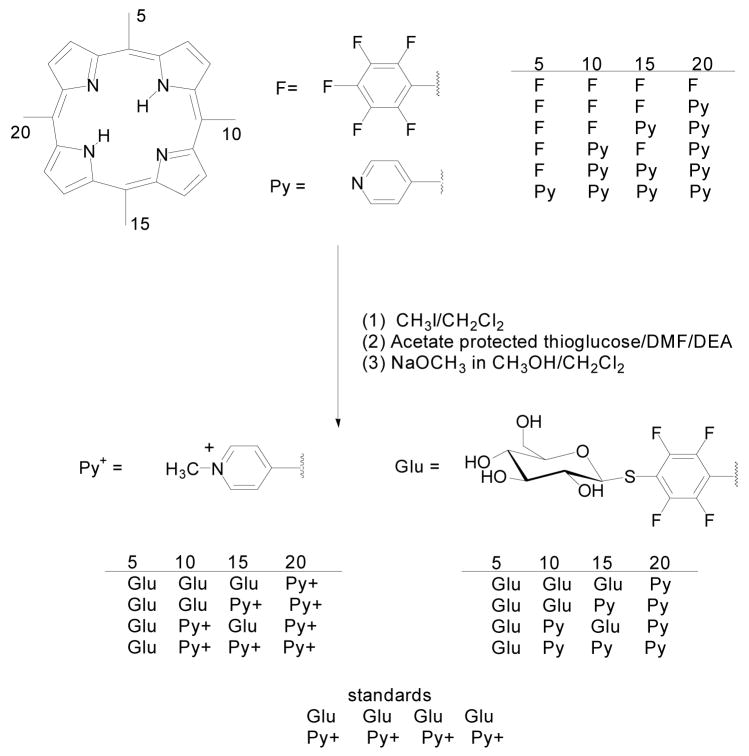
To investigate these differences in hydrophobicity and dynamics on aggregation and cell uptake, we made analogues of the selected compounds wherein the pyridinium is directly attached to the porphyrin. The reaction of pyrrole with pentafluorobenzaldehyde and 4-pyridinecarboxaldehyde (2:1:1 equivalents) results in a statistical mixture of six porphyrins that are readily separated by flash chromatography.101 This facile, scalable reaction affords three of the desired porphyrin cores wherein the pentafluorophenyl and pyridyl groups can be separated and quantitatively modified (Scheme 3). The coupling of thiols to the pentafluorophenyl groups of these mixed aryl porphyrins proceeds with similar yields as TPPF20.
Scheme 3.
Mixed aryl porphyrin derivatives with the pyridyl and pyridinium moieties directly attached to the macrocycle.
Uptake by Breast Cancer Cells
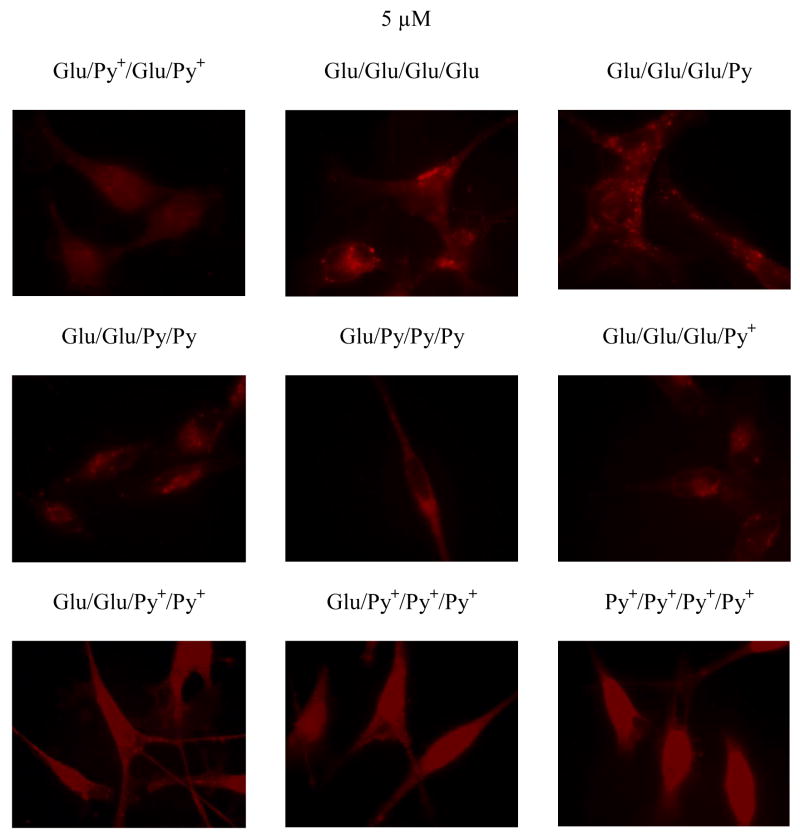
Each of the analogues of the selected compounds from the combinatorial library (Scheme 3) were incubated with MDA-MB-231 cells at 5 μM for 20 hrs. Following this period, the cells were rinsed with PBS to remove unbound porphyrin and fixed with 4% paraformaldehyde. Using fluorescence microscopy, the fluorescence intensity and uptake/binding of each isomer was evaluated. For cells treated with, Glu/Glu/Glu/Py, Py+/Py+/Py+/Glu, Glu/Glu/Py+/Py+ and the standards Py+/Py+/Py+/Py+, Glu/Glu/Glu/Glu the fluorescence micrographs yield relative intensities of 1:1.2:1.4:1.2:1.4, respectively (Figure 3). The fluorescence intensities of cells treated with Glu/Py+/Glu/Py+, Glu/Glu/Py/Py, Glu/Py/Py/Py, and Glu/Glu/Glu/Py+ are negligible. Thus the Glu/Glu/Glu/Glu and Glu/Glu/Py+/Py+ derivatives are the best binders to this cell line. Consistent with previous reports,5 this study also shows that cationic species bind better than uncharged species.
Figure 3.
Different affinities of pyridinium functionalized glycosylated porphyrins toward human breast cancer MDA-MB-231 cells assayed by fluorescence images and compared with the tetraglucose porphyrin (5 μM incubated with cells followed by rinsing and fixing the cells). For cells treated with 5 μM Glu/Glu/Glu/Py, Glu/Py+/Py+/Py+, Py+/Py+/Py+/Py+, Glu/Glu/Py+/Py+, and Glu/Glu/Glu/Glu the fluorescence intensity ratios are 1:1.2:1.2:1.4:1.4 respectively. The fluorescence intensities of cells treated with 5 μM Glu/Py+/Glu/Py+, Glu/Glu/Py/Py, Glu/Py/Py/Py, and Glu/Glu/Glu/Py+ are significantly less. Magnification 60X.
Optical properties
For the amphipathic porphyrins in aqueous solvents there is a broadening of the Soret bands near 418 nm and red or blue shifts of Q bands compared to the spectra in methanol or to compounds freely soluble in water such as the Py+/Py+/Py+/Py+ porphyrin. The UV-vis data suggests significant aggregation of especially the uncharged mixed aryl porphyrins, yet some degree of aggregation is indicated for the other macrocycles as well. Despite the peripheral hydrophilic moieties, the porphyrin core remains hydrophobic and pi-stacking is still possible. Additionally, hydrogen bonding and electrostatic interactions are weaker, but not eliminated in water. Since all of these intermolecular forces are in equilibrium, the relative concentrations largely dictate the degree of aggregation, e.g. as observed for the Glu/Glu/Glu/Glu compound.
The triplet quantum yield for TPP is 80% ± 10%, and for TPPF20 is >80%.102 The fluorescence quantum yields of the mixed aryl derivatives and other derivatives in methanol and PBS were evaluated. These calculations suggest that the Glu/Glu/Glu/Glu, with a reduced fluorescence quantum yield of ΦF = 7% in methanol and PBS, has a concomitantly greater triplet quantum yield than the parent TPPF20. This effect is probably due to both the electronic differences and of replacing the fluorides with the sulfur atoms. The importance of triplet quantum yield is that it is the triplet state that sensitizes the formation of singlet oxygen, which as mentioned before, is the dominant pathway to cellular necrosis or induction of apoptosis. Thus the quantum yields of singlet oxygen (Φox) are expected to be greater compared to nonfluorinated porphyrin derivatives such as tetraphenylporphyrin. Although the fluorescence quantum yield of these porphyrins is 6–10% in methanol and 3–8% in PBS, the large extinction coefficient (~2×105 M−1cm−1) and extended integration times permits the observation of ca. 100 nM concentrations by fluorescence microscopy. Note that the major porphyrin derivatives studied in this research have similar fluorescence quantum yields in both methanol and PBS media; therefore the observed relative intensities should be proportional to the quantities of porphyrins inside the cells. The exceptions to this are the macrocycles with neutral, pyridyl groups, which may be obfuscated by a small degree of aggregation that is not clearly visible in the UV-visible spectra.
Conclusion
A simple, one-step reaction to functionalize the TPPF20 porphyrin platform with different primary thio reagents results in solution phase combinatorial libraries with 21, 55, and 666 members. These porphyrin libraries bearing carbohydrate, polar pyridyl, and alkane moieties were synthesized and characterized by mass spectrometry. A proof-of-principle method based on mass spectral identification of winning compounds selected by human breast cancer MDA-MB-231 cells indicates that two pyridyl and two sugar motifs are important for binding. Synthesis and evaluation of the two possible isomers containing two pyridyls and two glucosyl moieties reveals that the isomer with the sugars on one side and the polar groups on the other side of the macrocycle binds this cell line better than the arrangement with the sugars on opposite sides. In addition, although nucleophilic thio and amine reagents can substitute and react with the pentafluorophenyl corrole, the reactivity of these nucleophiles are not uniform towards the triPCF15 system and thus at present the corrole is not as efficient as a core for the formation of solution phase combinatorial libraries as the TPPF20. An unexpected finding is that several of these compounds form stable 10–30 nm nanoparticles which are readily taken up by the cells. This last observation may afford a mechanism to deliver porphyrinoids across the blood/brain barrier. Aggregations is a complex equilibrium phenomena that depends on the intermolecular interactions between the porphyrins (so varies with molecular structure), the conditions (which vary dramatically from solution to the specific location in the cell), and dynamics (e.g. the role of cellular processes). The issue of aggregation is an important consideration in drug efficacy and will be the subject of a future report.
Experimental Methods
General
UV-visible spectra were recorded with a Cary spectrophotometer system. Absorption maxima are given in nanometers (solutions in methanol, unless otherwise indicated). Electrospray ionization mass spectra (ESI-MS) were recorded on an Agilent 1100 LC/MSD equipped with a diode array detector. Results are given as m/z+ H+ (relative intensity). 1H NMR was run on a Bruker Avance 300MHz or 500MHz spectrometer. Chemical shifts are reported in parts per million (ppm). Baker-flex (J. T. Baker) silica gel IB2 pre-coated sheets (2.5cm × 7.5cm) were used for analytical thin layer chromatography (TLC) and silica gel 60Å supplied by Sorbent Technologies, Inc. was used for column chromatography. Basic alumina (J. T. Baker) was also used. Preparative TLC was carried out using EM Science (Merck) silica gel 60Å. All solvents (Fischer Scientific HPLC grade) were used without further purification. 5,10,15-tris-pentafluorophenylcorrole (triPCF15) was purchased from Frontier Scientific. 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin (TPPF20) was either purchased from Aldrich or Frontier Scientific.
Synthesis
The porphyrins bearing different meso aryl groups (5,10-bis-pentafluorophenyl-15,20-di-pyridin-4-yl-porphyrin (F/F/Py/Py), 5,15-bis-pentafluorophenyl-10,20-di-pyridin-4-yl-porphyrin (F/Py/F/Py) and 5-pentafluorophenyl-10,15,20-tri-pyridin-4-yl-porphyrin (F/Py/Py/Py)) were synthesized by the typical mixed aldehyde condensation method101 with pyrrole, pyridyl-4-carboxaldehyde, and pentafluorobenzaldehyde in propionic acid. Synthesis of 2,3,4,6-tetra-O-acetyl-glucosylthioacetate has been reported earlier.6
Synthetic methods to couple thio reagents to TPPF20 and the mixed aryl porphyrins
Generally, a mixture of porphyrin, thio reagent, and diethyl amine were stirred in DMF in the dark under nitrogen at room temperature. After evaporation of the solvent (50°C and reduced pressure), the crude product was purified by silica gel flash column chromatography using increasingly polar eluents (chloroform/methanol or dichloromethane/methanol). The major fraction was collected and subjected to one more column chromatography.
5,10,15,20-tetrakis[4-(6′-thio-purine riboside)-2,3,5,6-tetrafluorophenyl]porphyrin (Ribo/Ribo/Ribo/Ribo)
A mixture of TPPF20 (10 mg, 10.2 μmol), 6-mercaptopurine riboside (93.3 mg, 328.6 μmol) and diethyl amine (40 μL, 388 μmol) were stirred in DMF (3 mL), for 5 days. Flash chromatography used a chloroform/methanol solvent mixture, 80:20 v/v. The major fraction was collected and subjected to a second column, first using 90:10 v/v chloroform/methanol to remove partially reacted porphyrins and then with 80:20 v/v of the same solvents to isolate the product. Yield: 7.8 mg, 38%; UV-Vis (CHCl3/CH3OH): 415, 507, 540(s), 583 and 641. ESI-MS: 2053 (M+Na+)
5,10,15,20-tetrakis[4-(1′-thio-1′H,1′H,2′H,2′H-perfluorododecyl)-2,3,5,6-tetrafluorophenyl]porphyrin (C12F21/C12F21/C12F21/C12F21)
1H,1H,2H,2H-perfluorododecane-1-thiol (65 mg, 112 μmol) was dissolved in 3 mL ethyl acetate/DMF (2:1 v/v) and DEA (20 μL, 194 μmol) was added. TPPF20 (11.5 mg, 11.8 μmol), dissolved in a minimum amount of DMF (~1 mL) was added to this solution and N2 was bubbled through this solution for 2–3 minutes. The product precipitates and was collected by filtration, redissolved in hot acetone, and purified by silica gel chromatography using hexane/acetone (9:1 v/v) initially followed by hexane/acetone (3:1 v/v). The yield was 36.8 mg, 97%. UV-Vis (acetone): 411, 503, 536(s), 581 and 644. MALDI-MS: 3215 (MH+).
5,10,15-tris-[4-(1′-thio-2′,3′,4′,6′-O-acetyl-glucosyl)-2,3,5,6-tetrafluorophenyl]-20- (pyridin-4-yl)porphyrin (GluAc/GluAc/GluAc/Py)
A mixture of F/F/F/Py (20 mg, 22.5μmol), 2,3,4,6-tetra-O-acetyl-glucosylthioacetate (55 mg, 135 μmol) and diethyl amine (46 μL, 450 μmol) were stirred in a mixture of DMF, chloroform and methanol (4:4:1 v/v, 3mL). The product was purified by flash column chromatography using dichloromethane/methanol, (98:2 v/v) mixture. Yield 36 mg, 83%. NMR 1H NMR (500MHz, CDCl3): δ = 9.12 (d, J = 2.54 Hz, 2H), 8.99 (m, 8H), 8.22 (d, J = 4.7 Hz, 2H), 5.41 (t, 3H), 5.24 (m, 9H), 4.34 (s, 6H), 3.92 (m, 3H), 2.25 (m, 9H), 2.10 (m, 27H) and −2.82 (s, 2H). UV-Vis (CHCl3): 415, 511, 543, 586 and 642. ESI-MS: 1918 (MH+) and 1940 (M + Na+).
5,10-bis-[4-(1′-thio-2′,3′,4′,6′-O-acetyl-glucosyl)-2,3,5,6-tetrafluorophenyl]-15,20-di-(pyridin-4-yl)porphyrin (GluAc/GluAc/Py/Py)
A mixture of F/F/Py/Py (20 mg, 25.1 μmol), 2,3,4,6-tetra-O-acetyl-glucosylthioacetate (41 mg, 100.9 μmol) and diethyl amine (51 μL, 494 μmol) were stirred in a solvent mixture of DMF, chloroform and methanol (4: 4:1 v/v, 3 mL), for 24h. The crude product was purified by flash column chromatography using dichloromethane/methanol, 97:3 v/v. Yield 30.5 mg, 82%. 1H NMR (500MHz, CDCl3): δ = 9.12 (d, J = 5.1 Hz, 4H), 8.96 (m, 8H), 8.22 (d, J = 5.1 Hz, 4H), 5.40 (t, 2H), 5.23 (m, 6H), 4.33 (s, 4H), 3.92 (m, 2H), 2.25 (m, 6H), 2.10 (m, 18H) and −2.81 (s, 2H). UV-Vis (CHCl3): 416, 511, 543, 586. ESI-MS: 1485 (MH+)
5,15-bis-[4-(1′-thio-2′,3′,4′,6′-O-acetyl-glucosyl)-2,3,5,6-tetrafluorophenyl]-10,20-di-(pyridin-4-yl)porphyrin (GluAc/Py/GluAc/Py)
F/Py/F/Py (20 mg, 25.1 μmol), 2,3,4,6-tetra-O-acetyl-glucosylthioacetate (41 mg, 100.9 μmol and diethyl amine (51 μL, 494 μmol) were stirred in DMF/chloroform/methanol (4:4:1 v/v, 3 mL) for 24h. The eluent used for column chromatography was dichloromethane/methanol, 97:3 v/v. Yield 31 mg, 84%. 1H NMR (500MHz, CDCl3): δ = 9.15 (d, J = 5.2 Hz, 4H), 8.98 (m, 8H), 8.31 (s, 4H), 5.39 (t, 2H), 5.25 (m, 6H), 4.33 (s, 4H), 3.92 (m, 2H), 2.22 (m, 6H), 2.10 (m,18H) and −2.81 (s, 2H). UV-Vis (CHCl3): 415, 509, 542, 584 and 641. ESI-MS: 1485 (MH+) and 1507 (M+Na+).
5--[4-(1′-thio-2′,3′,4′,6′-O-acetyl-glucosyl)-2,3,5,6-tetrafluorophenyl]-10,15,20-tri- (pyridin-4-yl)porphyrin (GluAc/Py/Py/Py)
A mixture of F/Py/Py/Py (20 mg, 28.2μmol), 2,3,4,6-tetra-O-acetyl-glucosylthioacetate (23 mg, 56.4 μmol and diethyl amine (58 μL, 564 μmol) were stirred in a mixture of DMF, chloroform and methanol (4:4:1 v/v, 3mL). The eluent for column chromatography was dichloromethane/methanol, (97:3 v/v) mixture. Yield 27 mg, 90%. 1H NMR (500MHz, CDCl3): δ = 9.11 (d, J 4.8 Hz, 6H), 8.94 (m, 8H), 8.21 (d, J = 5.3 Hz, 6H), 5.39 (t, 1H), 5.23 (m, 3H), 4.33 (s, 2H), 3.90 (m, 1H), 2.25 (m, 3H), 2.11 (m, 9H) and −2.85 (s, 2H). UV-Vis (CHCl3): 415, 511, 543, 586 and 642. ESI-MS: 1052 (MH+).
Synthesis of 21-member porphyrin library
A mixture of TPPF20 (30 mg, 30.8 μmol), 2,3,4,6-tetra-O-acetylglucosylthioacetate (17.1 mg, 42.1 μmol), 2,3,4-tri-O-acetyl-xylosylthioacetate (14.1 mg, 42.1 μmol), 4-mercaptopyridine (4.7 mg, 42.1 μmol) and diethylamine (63.6 μL, 616 μmol) were stirred in DMF in the dark under nitrogen at room temperature for 24 hrs. Solvent was evaporated and the library was subjected to ESI-MS without purification. The library (5 mg, 2.87 μmol) was stirred with sodium methoxide (53.5 μL of 0.5 M in CH3OH, 2.87 μmol) in dry CH3OH/CH2Cl2 (10 mL, 9:1) for 1 hour and followed by neutralization with a pH 7.2 ammonium acetate buffer. A large excess of NaOCH3 should be avoided as it partially cleaves the porphyrin-saccharide conjugate. The solvents were evaporated and the library was subjected to MALDI-MS.
Synthesis of 666-member porphyrin library
R= Glu, Xyl, SPy, SPen, SHex, SOct, SBrBzyl, F: A mixture of TPPF20 (15.4 mg, 15.8 μmol), 2,3,4,6-tetra-O-acetyl-glucosylthioacetate (6.36 mg, 15.8 μmol), 2,3,4-tri-O-acetyl-xylosylthioacetate (5.28 mg, 15.8 μmol), 4-mercaptopyridine (1.76 mg, 15.8 μmol), thiopentane (1.65 mg, 15.8 μmol), thiohexane (1.87 mg, 15.8 μmol), thiooctane (2.31 mg, 15.8 μmol) 4-bromo-phenylmethanethiol (3.20 mg, 15.8 μmol) and diethyl amine (65 μL, 632 μmol) were stirred in DMF (4 mL), for 1 day in the dark under nitrogen at room temperature. After evaporation of the solvent at reduced pressure at 50°C, the crude product was subjected to ESI-MS.
Synthesis of winning compounds
5,15-di-[4-(1′-thio-2′,3′,4′,6′-O-acetyl-glucosyl)-2′,3′,5′,6′-tetrafluorophenyl]-10,20-di-[4-(4′-thiopyridy)l-2,3,5,6-tetrafluorophenyl]porphyrin (GluAc/SPy/GluAc/SPy) and 5,10-di-[4-(1′-thio-2′,3′,4′,6′-O-acetyl-glucosyl)-2′,3′,5′,6′-tetrafluorophenyl]-15,20-di-[4(-4′-thiopyridy)l-2,3,5,6-tetrafluorophenyl]porphyrin (GluAc/GluAc/SPy/SPy)
A mixture of TPPF20 (30 mg, 30.8 μmol), 2,3,4,6-tetra-O-acetyl-glucosylthioacetate (25.0 mg, 61.6 μmol), 4-mercaptopyridine (6.8 mg, 61.6 μmol) and diethylamine (63.6 μL, 616 μmol) were stirred in DMF in the dark under nitrogen at room temperature for 24 hrs. The resulting mixture was subjected to a flash silica gel column using 0 – 3% gradient of methanol in methylene chloride as eluent, followed by preparatory thin layer chromatography using 5% methanol in methylene chloride as eluent to obtain the acetate protected glucose 5,15-glycosyl and 5,10-glycosyl TPPF16 isomers with yields of 6.9% and 10.0%, respectively. 5,15-glycosyl isomer: 1H-NMR (500MHz, CDCl3 ): δ = 9.00 (m, 8H), 8.63 (s (br), 4H), 7.41 (s (br)), 5.39 (t, , 2H), 5.24 (m, 6H), 4.34 (m, 4H), 3.93 (m, 2H), 2.25 (m, 6H), 2.10 (m, 18H), −2.82 (s, 2H). UV-Vis (CH2Cl2) λmax, nm: 415, 508, 542, 584, 638. ESI-MS: 1845 (MH+). 5,-10-glycosyl isomer: 1H-NMR (500MHz, CDCl3 ): δ = 9.04 (m, 8H), 8.68 (d, J = 3.88Hz, 4H), 7.40 (d, J = 5.93, 4H), 5.40 (t, 2H), 5.24 (m, 6H), 4.34 (m, 4H), 3.93 (m, 2H), 2.25 (m, 6H), 2.10 (m, 18H), −2.79 (s, 2H). UV-Vis (CH2Cl2) λmax, nm: 415, 508, 542, 584, 638. ESI-MS: 1845 (MH+)
Quaternization and deprotection of porphyrins having pyridyl and glucose functions
The formation of the quaternary pyridinium groups on the porphyrins was accomplished by stirring a slight excess of CH3I in CH2Cl2 containing minimum amount of CH3OH at 40°C overnight. After evaporation of the solvent under reduced pressure, the porphyrins were dissolved in a 1:1 CH2Cl2/CH3OH mixture and passed through column packed with prewashed Amberlite IRA-900 ion exchange resin to exchange the I− for Cl−. The cationic porphyrins were then purified by precipitation from hexanes.
Deprotection of the acetate protected thioglucose was carried out by treating the compounds with stoichiometric amount NaOCH3 per acetate group at room temperature in a 9:1 v/v solution of methanol/methylene chloride for 1 hour. The solution was then neutralized to a pH of 7.2 with an ammonium acetate buffer and purified by precipitation using hexane and/or diethyl ether. The mass spectral details of all the cationic as well as deprotected glucose porphyrins are given in supporting information.
Cell culture
Cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM), 10% bovine calf serum, 1% antimycotic at 37°C and in 5% CO2 atmosphere.6 For experiments, 2x105 cells/mL were seeded in cell culture plates and then allowed to grow for 24 hrs.
Extraction of 21-member library from human breast cancer (MDA-MB-231) cells
The 21-member library (6.74 mM) dissolved in 144 μL CH3OH was added to 10 mL culture medium in a 100 mm cell culture dishes to make a final concentration of 100 μM in total porphyrin concentration (1–5 μM per member). 24 hrs later, the cells were rinsed with medium to wash off any unbound porphyrins and collected in an Eppendorf tube with a rubber policeman in 0.5 mL distilled water. The cells from two of these were used in the following steps. 1.5 mL 2-chloroethanol was added to the mixture and sonicated for 1.5 minute with pulses on ice. Solvents were evaporated and 300 μL of CH3OH was added. The mixture was centrifuged and the supernatant was collected. The solvent was evaporated again and 100 μL of CH3OH was added. The mixture was centrifuged again; the supernatant was collected and submitted for MALDI-MS analysis.
Fluorescence imaging of cells
Cells were plated onto cover slips in cell culture dishes. Porphyrins (dissolved in methanol) were added to the cultures to a final concentration of 2 to 10 μM. Twenty-four hrs later cells were washed twice with PBS (136 mM NaCl, 2.6 mM KCl, 1.4 mM KH2PO4, 4.2 mM Na2HPO4) and fixed in 4% paraformaldehyde solution in PBS for 20 min at room temperature. The cells were then washed with PBS 3–5 times. The cover slips were mounted in Dako fluorescent mounting medium, put onto slides, air dried, and then visualized using a Nikon Optiphot 2 fluorescence microscope where images were captured as high quality TIFF files. (Excitation: 505–565 nm and emission: 565–685 nm). For comparison and to record cell morphology, images were also captured as JPEG images using a phase contrast light microscope. For each set of experiments, cells were cultured and the fluorescence images were taken under identical culture and microscopic conditions. Quantitative comparisons of fluorescence intensities of the fluorescence micrographs were calculated by the FLim program.78
Supplementary Material
Procedures for all cell assays, fluorescence microscopy, representative HPLC/ESI-MS, MALDI of cell extract, fluorescence quantum yield and 1H NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.
Scheme 2.
The reactions to form the trisubstituted corroles require more equivalents of the thiol reagent and longer reaction times, and result in lower yields, e.g. the triglycosylated compound. The same procedures do not yield the expected statistical mixture of corrole libraries when more than one thiol reagent is used. These experiments reveal that the para F group on the central aryl moiety is somewhat less reactive than those on the opposing aryl moieties.
Acknowledgments
The authors acknowledge support from the National Institutes of Health (NIH)-SCORE program (GM60654) the PSC-CUNY fund and National Science Foundation (CHE-0554703). Support for infrastructure and instrumentation in the sciences at Hunter College comes from the National Institutes of Health, including the RCMI program (RR-03037), the National Science Foundation, and the City University of New York. Dr. Nathan Stevens (Columbia University) is thanked for coding FLim.
Footnotes
Dedicated to the memory of R. Bruce Merrifield, friend and colleague.
References
- 1.Garno JC, Xu C, Bazzan G, Batteas JD, Drain CM. In: ACS Symposium series: Metal-Containing and Metallo-Supramolecular Polymers and Materials. Schubert US, Newcome GR, Manners I, editors. Vol. 928. American Chemical Society; Washington, DC: 2006. pp. 168–183. [Google Scholar]
- 2.Drain CM, Smeureanu G, Patel S, Gong X, Garno J, Arijeloye J. New J Chem. 2006;12:1834–1843. [Google Scholar]
- 3.Drain CM, Goldberg I, Sylvain I, Falber A. Top Curr Chem. 2005;245:55–88. [Google Scholar]
- 4.Drain CM, Chen X. Self-Assembled Porphyrinic Nanoarchitectures. In: Nalwa HS, editor. Encyclopedia of Nanoscience & Nanotechnology. Vol. 9. American Scientific Press; New York: 2004. pp. 593–616. [Google Scholar]
- 5.Chen X, Drain CM. Drug Design Reviews - Online. 2004;1(3):215–234. [Google Scholar]
- 6.Chen X, Hui L, Foster DA, Drain CM. Biochemistry. 2004;43(34):10918–10929. doi: 10.1021/bi049272v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirohara S, Obata M, Ogata S, Ohtsuki C, Higashida S, Ogura S, Okura I, Takenaka M, Ono H, Sugai Y. J Photochem Photobiol, B. 2005;78(1):7–15. doi: 10.1016/j.jphotobiol.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Drain CM, Kirmaier C, Medforth CJ, Nurco DJ, Smith KM, Holten D. J Phys Chem. 1996;100(29):11984–11993. [Google Scholar]
- 9.Drain CM, Gentemann S, Roberts JA, Nelson NY, Medforth CJ, Jia S, Simpson MC, Smith KM, Fajer J, Shelnutt JA, Holten D. J Am Chem Soc. 1998;120(15):3781–3791. [Google Scholar]
- 10.Retsek JL, Drain CM, Kirmaier C, Nurco DJ, Medforth CJ, Smith KM, Sazanovich IV, Chirvony VS, Fajer J, Holten D. J Am Chem Soc. 2003;125:9787–9800. doi: 10.1021/ja020611m. [DOI] [PubMed] [Google Scholar]
- 11.Linton B, Hamilton AD. Chem Rev. 1997;97:1669–1680. doi: 10.1021/cr960375w. [DOI] [PubMed] [Google Scholar]
- 12.Samaroo D, Soll CE, Todaro LJ, Drain CM. Org Lett. 2006;8(22):4985–4988. doi: 10.1021/ol060946z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasetto P, Chen X, Drain CM, Franck RW. Chem Commun. 2001:81–82. [Google Scholar]
- 14.Lahiri J, Fate GD, Ungashe SB, Groves JT. J Am Chem Soc. 1996;118:2347–2358. [Google Scholar]
- 15.Hunt JA, Lee J, Groves JT. Chem Biol. 1997;4(11):845–858. doi: 10.1016/s1074-5521(97)90117-4. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Hunt JA, Groves JT. J Am Chem Soc. 1998;120(30):7493–7501. [Google Scholar]
- 17.Eldred SE, Stone DA, Gellman SH, Stahl SS. J Am Chem Soc. 2003;125(12):3422–3423. doi: 10.1021/ja028242h. [DOI] [PubMed] [Google Scholar]
- 18.Breslow R, Fang Z. Tetrahedron Lett. 2002;43:5197–5200. [Google Scholar]
- 19.Merlau ML, Mejia MdP, Nguyen ST, Hupp JT. Angew Chem Int Ed. 2001;40(22):4239–4242. doi: 10.1002/1521-3773(20011119)40:22<4239::AID-ANIE4239>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Purrello R, Gurrieri S, Lauceri R. Coord Chem Rev. 1999;190–192:683–706. [Google Scholar]
- 21.Rakow NA, Suslick KS. Nature. 2000;406(6797):710–713. doi: 10.1038/35021028. [DOI] [PubMed] [Google Scholar]
- 22.Okura I. J Porphyrins Phthalocyanines. 2002;6:268–270. [Google Scholar]
- 23.Zhang C, Suslick KS. J Am Chem Soc. 2005;127:11548–11549. doi: 10.1021/ja052606z. [DOI] [PubMed] [Google Scholar]
- 24.Sternberg ED, Bruckner C, Dolphin D. Tetrahedron. 1998;54:4151–4202. [Google Scholar]
- 25.MacDonald IJ, Dougherty TJ. J Porphyrins Phthalocyanines. 2001;5(2):105–129. [Google Scholar]
- 26.Dougherty TJ. Photochem Photobiol. 1993;58(6):895–900. doi: 10.1111/j.1751-1097.1993.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 27.Lambrechts SAG, Aalders MCG, Demidova TN, Hasan T, Hamblin MR. Photochem Photobiol Sci. 2005;4(7):503–509. doi: 10.1039/b502125a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamblin MR, Hasan T. Photochem Photobiol Sci. 2004;3(5):436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maiya BG, Hariprasad G, Giribabu L. Resonance. 2000;5(8):13–21. [Google Scholar]
- 30.Maiya BG. Resonance. 2000;5(6):15–29. [Google Scholar]
- 31.Maiya BG. Resonance. 2000;5(4):6–18. [Google Scholar]
- 32.Nyman ES, Hynninen PH. J Photochem Photobiol B: Biol. 2004;73(1–2):1–28. doi: 10.1016/j.jphotobiol.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Pandey RK. J Porphyrins Phthalocyanines. 2000;4(4):368–373. [Google Scholar]
- 34.Pandey RK, Zheng G. Porphyrins as photosensitizers in photodynamic therapy. In: Kadish KM, Smith KM, Guilard R, editors. The Porphyrin Handbook. Vol. 6. Academic Press; San Diego: 2000. pp. 157–230. [Chapter 43] [Google Scholar]
- 35.Pandey SK, Gryshuk AL, Graham A, Ohkubo K, Fukuzumi S, Dobhal MP, Zheng G, Ou Z, Zhan R, Kadish KM, Oseroff A, Ramaprasad S, Pandey RK. Tet. 2003;59(50):10059–10073. [Google Scholar]
- 36.Acra A, Ayoub G. J Water Supply: Res Technol - AQUA. 1997;46(4):218–223. [Google Scholar]
- 37.Maisch T, Szeimies RM, Abels C, Jori G. Photochem Photobiol Sci. 2004;3(10):907–917. doi: 10.1039/b407622b. [DOI] [PubMed] [Google Scholar]
- 38.Tome JPC, Neves MGPMS, Tome AC, Cavaleiro JAS, Soncin M, Magaraggia M, Ferro S, Jori G. J Med Chem. 2004;47(26):6649–6652. doi: 10.1021/jm040802v. [DOI] [PubMed] [Google Scholar]
- 39.Wainwright M. Curr Med Chem. 2002;9(1):127–143. doi: 10.2174/0929867023371355. [DOI] [PubMed] [Google Scholar]
- 40.Orenstein A, Klein D, Kopolovic J, Winkler E, Malik Z, Keller N, Nitzan Y. FEMS Immun Med Microbiol. 1998;19:307–314. doi: 10.1111/j.1574-695X.1997.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 41.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Photochem Photobiol. 1992;55(1):89–96. doi: 10.1111/j.1751-1097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 42.Gardner PR, Nguyen DDH, White CW. Arch Biochem Biophys. 1996;325(1):20–28. doi: 10.1006/abbi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 43.Batinic-Haberle I, Liochev SI, Spasojevic I, Fridovich I. Arch Biochem Biophys. 1997;343(2):225–233. doi: 10.1006/abbi.1997.0157. [DOI] [PubMed] [Google Scholar]
- 44.Caughey WS, Raymond LD, Horiuchi M, Caughey B. Proc Natl Acad Sci U S A. 1998;95(21):12117–12122. doi: 10.1073/pnas.95.21.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Priola SA, Caughey B, Caughey WS. Curr Opin Microbiol. 1999;2(5):563–566. doi: 10.1016/s1369-5274(99)00020-x. [DOI] [PubMed] [Google Scholar]
- 46.Priola SA, Raines A, Caughey WS. Science. 2000;287:1503–1504. doi: 10.1126/science.287.5457.1503. [DOI] [PubMed] [Google Scholar]
- 47.Kocisko DA, Engel AL, Harbuck K, Arnold KM, Olsen EA, Raymond LD, Vilette D, Caughey B. Neurosci Lett. 2005;388(2):106–111. doi: 10.1016/j.neulet.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 48.Kocisko DA, Caughey WS, Race RE, Roper G, Caughey B, Morrey JD. Antimicrob Agents Chemother. 2006;50(2):759–761. doi: 10.1128/AAC.50.2.759-761.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider R, Schmitt F, Frochot C, Fort Y, Lourette N, Guillemin F, Muller JF, Barberi-Heyob M. Bioorg Med Chem. 2005;13:2799–2808. doi: 10.1016/j.bmc.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 50.Bonnett R. Chem Soc Rev. 1995:19–32. [Google Scholar]
- 51.Liu MO, Tai C-h, Sain M-z, Hu AT, Chou F-i. J Photochem Photobiol, A. 2004;165:131–136. [Google Scholar]
- 52.Henderson BW, Dougherty TJ. Photochem Photobiol. 1992;55(1):145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 53.Drain CM, Gong X, Ruta V, Soll CE, Chicoineau PF. J Comb Chem. 1999;1:286–290. doi: 10.1021/cc990011s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berlin K, Jain RK, Richert C. Biotechnol Bioeng Comb Chem. 1998;61(2):107–118. doi: 10.1002/(sici)1097-0290(199821)61:2<107::aid-bit5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 55.Chen X. Ph.D. Dissertation. The Graduate School and University Center of the City of New York; New York: 2004. [Google Scholar]
- 56.An H, Cook PD. Chem Rev. 2000;100(9):3311–3340. doi: 10.1021/cr990014r. [DOI] [PubMed] [Google Scholar]
- 57.Carell T, Wintner EA, Rebek J, Jr, Sutherland AJ. Angew Chem Int Ed. 1994;33(20):2061–2064. [Google Scholar]
- 58.An H, Cummins LL, Griffey RH, Bharadwaj R, Haly BD, Fraser AS, Wilson-Lingardo L, Risen LM, Wyatt JR, Cook PD. J Am Chem Soc. 1997;119(16):3696–3708. [Google Scholar]
- 59.An H, Haly BD, Fraser AS, Guinosso CJ, Cook PD. J Org Chem. 1997;62(15):5156–5164. [Google Scholar]
- 60.An H, Haly BD, Cook PD. J Med Chem. 1998;41:706–716. doi: 10.1021/jm970598u. [DOI] [PubMed] [Google Scholar]
- 61.Gayo LM. Biotechnol Bioeng Comb Chem. 1998;61(2):95–106. [PubMed] [Google Scholar]
- 62.Andrus MB, Turner TM, Sauna ZE, Ambudkar SV. J Org Chem. 2000;65(16):4973–4983. doi: 10.1021/jo000453m. [DOI] [PubMed] [Google Scholar]
- 63.Boger DL, Lee JK. J Org Chem. 2000;65(19):5996–6000. doi: 10.1021/jo000382r. [DOI] [PubMed] [Google Scholar]
- 64.Liu G, Fan Y, Carlson JR, Zhao ZG, Lam KS. J Comb Chem. 2000;2:467–474. doi: 10.1021/cc000016l. [DOI] [PubMed] [Google Scholar]
- 65.Martin SW, Romine JL, Chen L, Mattson G, Antal-Zimanyi IA, Poindexter GS. J Comb Chem. 2004;6:35–37. doi: 10.1021/cc034018s. [DOI] [PubMed] [Google Scholar]
- 66.Neuville L, Zhu J. Tetrahedron Lett. 1997;38(23):4091–4094. [Google Scholar]
- 67.Battioni P, Brigaud O, Desvaux H, Mansuy D, Traylor TG. Tetrahedron Lett. 1991;32(25):2893–2896. [Google Scholar]
- 68.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40(11):2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 69.Simkhovich L, Galili N, Saltsman I, Goldberg I, Gross Z. Inorg Chem. 2000;39:2704–2705. doi: 10.1021/ic991342c. [DOI] [PubMed] [Google Scholar]
- 70.Mahammed A, Weaver JJ, Gray HB, Abdelas M, Gross Z. Tetrahedron Lett. 2003;44:2077–2079. [Google Scholar]
- 71.Bendix J, Dmochowski IJ, Gray HB, Mahammed A, Simkhovich L, Gross Z. Angew Chem Int Ed. 2000;39:4048–4051. doi: 10.1002/1521-3773(20001117)39:22<4048::aid-anie4048>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 72.Pasternack RF, Gibbs EJ, Villafranca JJ. Biochemistry. 1983;22:2406–2414. doi: 10.1021/bi00279a016. [DOI] [PubMed] [Google Scholar]
- 73.Pasternack RF, Garrity P, Ehrlich B, Davis CB, Gibbs EJ, Orloff G, Giartosio A, Turano C. Nucleic Acids Res. 1986;14(14):5919–5931. doi: 10.1093/nar/14.14.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pasternack RF, Bustamante C, Collings PJ, Giannetto A, Gibbs EJ. J Am Chem Soc. 1993;115:5393–5399. [Google Scholar]
- 75.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Antimicrob Agents Chemother. 2000;44(3):522–527. doi: 10.1128/aac.44.3.522-527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drain CM, Mauzerall D. Biophys J. 1992;63:1556–1563. doi: 10.1016/S0006-3495(92)81739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mauzerall D, Drain CM. Biophys J. 1992;63:1544–1555. doi: 10.1016/S0006-3495(92)81738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stevens NFLim. http://casilab10.sci.ccny.cuny.edu/~nathan/projects/FLim/index.html.
- 79.Ahmad K. Lancet Oncol. 2002;3(8):451. doi: 10.1016/s1470-2045(02)00700-3. [DOI] [PubMed] [Google Scholar]
- 80.Brigger I, Dubernet C, Couvreur P. Adv Drug Delivery Rev. 2002;54:631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 81.Fu J, Li X, Ng DKP, Wu C. Langmuir. 2002;18(10):3843–3847. [Google Scholar]
- 82.Konan YN, Berton M, Gurny R, Allemann E. Eur J Pharm Sci. 2003;18(3–4):241–249. doi: 10.1016/s0928-0987(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 83.Roy I, Ohulchanskyy TY, Pudavar HE, Bergey EJ, Oseroff AR, Morgan J, Dougherty TJ, Prasad PN. J Am Chem Soc. 2003;125:7860–7865. doi: 10.1021/ja0343095. [DOI] [PubMed] [Google Scholar]
- 84.Yan F, Kopelman R. Photochem Photobiol. 2003;78(6):587–591. doi: 10.1562/0031-8655(2003)078<0587:teomis>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 85.Vargas A, Pegaz B, Debefve E, Konan-Kouakou Y, Lange N, Ballini JP, van den Bergh H, Gurny R, Delie F. Int J Pharm. 2004;286(1–2):131–145. doi: 10.1016/j.ijpharm.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 86.Pegaz B, Debefve E, Borle F, Ballini JP, van de Bergh H, Konan-Kouakou YN. J Photochem Photobiol, B. 2005;80(1):19–27. doi: 10.1016/j.jphotobiol.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 87.Chithrani BD, Ghazani AA, Chan WCW. Nano Lett. 2006;6(4):662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 88.Wieder ME, Hone DC, Cook MJ, Handsley MM, Gavrilovic J, Russell DA. Photochem Photobiol Sci. 2006;5(8):727–734. doi: 10.1039/b602830f. [DOI] [PubMed] [Google Scholar]
- 89.McCarthy JR, Perez JM, Bruckner C, Weissleder R. Nano Lett. 2005;5(12):2552–2556. doi: 10.1021/nl0519229. [DOI] [PubMed] [Google Scholar]
- 90.Harriman A, Hosie RJ. J Photochem. 1981;15:163–167. [Google Scholar]
- 91.Kuszaj S, Kaszycki P, Wasylewski Z. Chem Phys Lipids. 1996;83(2):153–160. doi: 10.1016/0009-3084(96)02605-9. [DOI] [PubMed] [Google Scholar]
- 92.Akins DL, Ozcelik S, Zhu HR, Guo C. J Phys Chem. 1996;100(34):14390–14396. [Google Scholar]
- 93.Gong X, Milic T, Xu C, Batteas JD, Drain CM. J Am Chem Soc. 2002;124(48):14290–14291. doi: 10.1021/ja027405z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng TI, Chang CJ, Guo MJ, Wang YH, Yu JS, Wu HY, Jou MJ. Ann N Y Acad Sci. 2005;1042(1):419–428. doi: 10.1196/annals.1338.035. [DOI] [PubMed] [Google Scholar]
- 95.Schulze-Bergkamen H, Krammer PH. Semin Oncol. 2004;31(1):90–119. doi: 10.1053/j.seminoncol.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 96.Kanduc D, Mittelman A, Serpico R, Sinigaglia E, Sinha AA, Natale C, Santacroce R, Corcia MGD, Lucchese A, Dini L, Pani P, Santacroce S, Simone S, Bucci R, Farber E. Int J Oncol. 2002;21:165–170. [PubMed] [Google Scholar]
- 97.Granville DJ, Hunt DWC. Curr Opin Drug Discovery Dev. 2000;3(2):232–243. [PubMed] [Google Scholar]
- 98.Calzavara-Pinton PG, Venturini M, Sala R. J Photochem Photobiol, B. 2005;78(1):1–6. doi: 10.1016/j.jphotobiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 99.Kasha M, Rawls HR, El-Bayoumi MA. Pure Appl Chem. 1965;11(34):371–392. [Google Scholar]
- 100.Hunter CA, Sanders JKM. J Am Chem Soc. 1990;112:5525–5534. [Google Scholar]
- 101.Drain CM, Lehn JM. Chem Commun. 1994:2313–2315. (correction 1995, p503) [Google Scholar]
- 102.Yang SI, Seth J, Strachan JP, Gentlemann S, Kim D, Holten D, Lindsey JS, Bocian DF. J Porphyrins Phthalocyanines. 1999;3(2):117–147. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Procedures for all cell assays, fluorescence microscopy, representative HPLC/ESI-MS, MALDI of cell extract, fluorescence quantum yield and 1H NMR spectra. This material is available free of charge via the Internet at http://pubs.acs.org.