Abstract
Background
Phlebotomy is the most commonly treatment used for erythrocytosis and polycythaemia. After the introduction in the medical practice of cell separators, erythrocytapheresis has been also introduced. The aim of the study was to compare the clinical results of the two kinds of treatment.
Patients and methods
We analysed 98 patients affected by different forms of erythrocytosis, divided into three treatment groups: 1) patients undergoing phlebotomy; 2) patients treated only with therapeutic erythrocytapheresis; 3) patients who underwent phlebotomy treatment for a certain period and who were then switched to apheresis treatment. The haematocrit in these patients was maintained at about 45% and they were treated when the haematocrit exceeded the critical threshold of 50%.
Results
The interval between two therapeutic interventions was assumed as indicator. In 80% of the patients treated only with phlebotomy the interval was between 20 days and 2 months, in subjects treated with only erythrocytapheresis the intevals were between 2 and 7 months. In the third group of patients, the switch from phlebotomy to erythrocytapheresis considerably prolonged the interval.
Conclusions
The data showed that erythrocytapheresis was clearly superior to traditional phlebotomy in terms of prolonging the period between one treatment and another, independently of the type of erythrocytosis and of the treatment group.
Keywords: Erythrocytosis, haematocrit, erythrocytapheresis, phlebotomy
Introduction
Erythrocytosis, an increase of the red blood cell count above the threshold value of 6,000,000/μL, with a corresponding rise in the haematocrit (Hct) to over 50%, leads to a increase in the total red blood cell mass. Erythrocytosis is usually secondary to various conditions such as cardiac disorders (particularly congenital), lung diseases [in particular chronic obstructive pulmonary disease (COPD) and emphysema], tumours that produce erythropoietin, renal cysts, smoking, residence at high altitude, post-haematopoietic cell transplant, and endocrine disorders (with adrenocortical dysfunction).
Primary or essential erythrocytosis is rarer, accounting for about 4% of the cases of erythrocytosis. Polycythaemia vera or Vaquez-Osler's disease, which is characterized by increases in the three lines of blood cells (red cells, white cells and platelets) resembles erythrocytosis.
Patients with erythrocytosis have a high risk of severe thromboembolic events, which are directly related to the clear increase in blood viscosity1,2: there is, in fact, a direct correlation between the value of the Hct and the risk of thrombosis3.
An increase of the Hct above 50% leads to slowing of parenchymal blood flow, distension of the microcirculation and tissue hypoxia, because of the decreased oxygen-bearing capacity of the blood. The tissue hypoxia triggers a vicious circle in which there is a progressive increase in the red cell mass and, consequently, further slowing of the blood flow4. In cases secondary to respiratory failure, the clinical symptoms connected to hyperviscosity are already present at Hct values lower than 50%, since there is a particular morphological change in the red blood cells (scleroerythrocytosis), which profoundly alters the deformability of the red blood cells and, therefore, slows blood flow. Local and/or generalised ischaemia as well as clinical symptoms develop in both the arteries and the veins5,6.
From a therapeutic point of view, phlebotomy is the most commonly used, cheapest and practical form of treatment for erythrocytosis and polycythaemia, with the use of specific drugs such as busulphan and hydroxyurea7–9.
Some time ago, cell separators were introduced into medical practice. These instruments enable a particularly sophisticated form of phlebotomy, erythrocytapheresis10, since they allow the removal of a considerable number of red blood cells while maintaining the blood volume, and therefore, the haemodynamic situation unchanged.
Having followed up a certain number of patients with erythrocytosis for some years, we wanted to determine whether the clinical results of traditional phlebotomy treatment could be improved, or at least equalled, by the use of therapeutic erythrocytapheresis (TEA).
Patients and methods
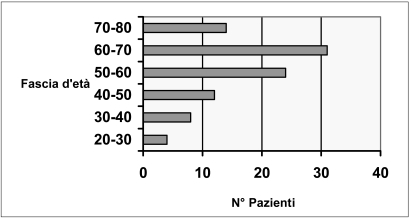
The study population comprised 98 patients, most of whom were male (male to female ratio 8:1), aged between 20 and 80 years old. The age groups most represented were those between 50 and 60 years old (23 patients) and between 60 and 70 years old (31 patients), as illustrated in figure 1.
Figure 1.
Range of ages of the patients treated
Seventy-five subjects had non-specific secondary erythrocytosis (29 had COPD, 26 were heavy smokers, 20 had reduced inspiratory capacity as a consequence of occupational exposure to dusts), 11 had erythrocytosis due to specific causes (four had paraneoplastic forms, four had undergone haematopoietic stem cell transplantation, two had undergone pulmonary lobectomy because of tuberculosis, one had adrenocortical dysfunction with renal cysts), six had stress erythrocytosis (or pseudoerythrocytosis) and, finally, six had polycythaemia rubra vera (that is, the classical Vaquez-Osler's disease). In order to carry out the comparisons, the 98 subjects were divided into three groups: the first group (n = 36) was treated exclusively with phlebotomy; the second group (n = 32), already undergoing treatment with phlebotomy, was changed to TEA once the technique of cell separation had been introduced into our Service; the third group (n = 30) was exclusively treated with the apheresis procedure.
The phlebotomies were carried out using the traditional technique, removing 450 mL of whole blood on each occasion. In order not to cause volume imbalances, the bloodletting was conducted slowly (over about 10 minutes) and physiological saline was administered in compensation; the volume administered was usually equivalent to that of the blood removed.
The TEA procedures were carried out using an MCS-plus (Haemonetics Italia srl, Lainate, MI, Italy) discontinuous flow cell separator: the procedures were divided into two or three cycles, enabling 450–500 g of concentrated red blood cells to be removed in about 35 minutes and for the patient's own plasma to be returned together with 500 mL (or more, in elderly subjects) of physiological saline as fluid compensation. The instrument has software which, based on the initial Hct, body weight and sex of the subject, precisely determines the blood volume and Hct at the end of the procedure. This enables removal of a personalised number of red blood cells and automatic isovolaemic compensation.
The periods between two consecutive treatments were dictated by the Hct: patients underwent phlebotomy or erythrocytapheresis (depending on their treatment group) when the Hct rose to 50±1%. The aim was to bring the Hct to below 45% and maintain it at about this value for as long as possible.
Full blood counts were performed 48 h after the treatment and systematically repeated every 15–20 days in order to follow the trend in Hct over time. When the Hct reached about 50% or above, a new therapeutic procedure was performed.
Besides being prescribed specific drugs, the patients were advised to increase their intake of fluids, stop smoking, limit food intake and avoid or reduce their use of alcohol.
We also evaluated the cost of the procedures, taking into account the cost of the apheresis kit (about 60 •), that of the phlebotomy bag (about 16 •), and the costs of human resources, based on the time to perform the procedures.
Results
Not being able to present the clinical results for each patient treated, we have assumed that the period between one therapeutic intervention and other is a valid indicator of the results obtained with the three different types of treatment. In fact, as described previously, the interval is dictated (when not imposed) by the increase in Hct towards values considered dangerous for the patient.
For ease of presentation and clarity of interpretation, the results are reported in tables I and II: the intervals between treatments are reported separately for the three groups (only phlebotomy; only TEA; first phlebotomy and then TEA).
Table I.
Difference in the time interval between treatments in patients in two treatment groups: one treated only with phlebotomy, the other treated exclusively with TEA
| Time interval | Group I: 36 patients. Phlebotomy only | Group II: 30 patients. TEA only |
|---|---|---|
| 20 days | 9 | 0 |
| 1–1.5 months | 12 | 0 |
| 2 months | 8 | 4 |
| 3 months | 4 | 5 |
| 4 months | 3 | 4 |
| 5 months | 0 | 6 |
| 6 months | 0 | 7 |
| 7 months | 0 | 4 |
| Mean | 51.66 | 139 |
| Median | 40 | 150 |
| Standard deviation | 29.80 | 49.50 |
p<0.001
Table II.
Changes in the time interval between treatments in patients belonging to the same group: this group of patients was treated first with only phlebotomy, then with only TEA
| Time interval | Group III: 32 patients treated first with only phlebotomy then with only TEA | |
|---|---|---|
| 20 days | 8 | 0 |
| 1–1.5 months | 10 | 0 |
| 2 months | 6 | 5 |
| 3 months | 5 | 6 |
| 4 months | 3 | 5 |
| 5 months | 0 | 7 |
| 6 months | 0 | 7 |
| 7 months | 0 | 2 |
| Mean | 54.06 | 130.31 |
| Median | 40 | 135 |
| Standard deviation | 31.60 | 46.73 |
p<0.001
As expected, most patients undergoing only phlebotomy (more precisely, 29 of 36; 80%) required repeated treatment after a fairly short period of between 20 days and 2 months, while only 20% (7 of 36) were able to have treatment at longer intervals (3 or 4 months).
In most of patients, the Hct fell by 3–3.5 percentage points and then often rose sharply, such that in some cases the Hct had returned to the starting level already by the 20 day control. This pattern was not, however, uniform since in the same patients there were periods in which the Hct remained fairly stable for 2 or 3 months and other periods in which the rise of the Hct towards high values was slow. No explanation for this behaviour was found.
In contrast, TEA was associated with much longer intervals (from 2 to 7 months) between treatments. As can be seen from table I, the majority of the patients treated with TEA (21 out of 30; 70%) enjoyed significant periods between treatments (from 4 to 7 months, p<0.001).
This same result was confirmed in the third group: patients who had changed from traditional phlebotomy therapy to TEA had considerably longer intervals between therapeutic procedures (table II, p<0.001).
In most of the patients undergoing TEA, the decrease in Hct from the threshold value could be even more than 8 percentage points and the subsequent increase was usually slow and progressive.
The apheresis treatment did not cause any adverse events of particular significance, although it should be noted that perioral paraesthesia developed during the course of four TEA procedures; these episodes were promptly treated by the health care staff.
The negative aspect of TEA is its cost. A global improvement in treatment is accompanied by a higher financial outlay for both materials and use of human resources. Overall, a TEA procedure costs about three times more than a traditional phlebotomy session.
Discussion and conclusions
From the data presented, it seems clear that TEA is superior to the usual phlebotomy treatment produced a clear decrease in the Hct and in maintaining the level below the dangerous threshold of 50% for a longer time. This was expected, given that TEA enables removal of a greater number of red blood cells without exposing the patient to the feared risk of volaemic imbalances11,12 and is an efficient, safe and well-tolerated method for treating erythrocytoses and polycythaemia13–16.
In particular, the data obtained are valid and stable over the long-term (on average, 4–5 months). Furthermore, from a subjective point of view, the patient reports a sense of well-being that is maintained for an appreciable period after the apheresis. The main problem of TEA remains its cost, particularly nowadays, when the resources available for health care are ever more limited and continuously curtailed. Its wide-scale use could, however, reduce costs considerably.
In any case, from a clinical point of view, taking into consideration the lower risk of thromboembolism, the better perfusion (and consequently better oxygenation) of the tissues, in particular of the brain and heart, and the clear decrease in blood viscosity, it may be concluded that the cost-effectiveness balance is sufficiently positive.
It is, however, essential to design protocols to optimise the level of Hct in relation to the patient's type of erythroctyosis. It is equally important to involve other specialists (haematologists, experts in coagulation and vascular disorders) in order to obtain synergism from the different treatments, thus improving the patient's quality of life.
Acknowledgements
We thank the nursing staff of the SIMT of Crotone whose precious work contributed to the realisation of this study.
References
- 1.Thomas DJ, Marshall J, Ross-Russel RW, et al. Effect of haematocrit on cerebral blood-flow in man. Lancet. 1977;ii:941–3. doi: 10.1016/s0140-6736(77)90885-6. [DOI] [PubMed] [Google Scholar]
- 2.Pearson TC, Wetherley-Mein G. Vascular occlusive episodes and venous haematocrit in primary proliferative polycythaemia. Lancet. 1978;ii:1219–22. doi: 10.1016/s0140-6736(78)92098-6. [DOI] [PubMed] [Google Scholar]
- 3.Williams WJ, Bentler E, Erslev AJ, Rundles RW. Hematology. New York, NY: Mc Graw-Hill-Book Company; 1977. [Google Scholar]
- 4.Di Lollo F, Di Lollo S. Poliglobulie croniche dell'adulto e danni vascolari. Rec Progr Med. 1985;76:135–8. [PubMed] [Google Scholar]
- 5.Breda R, Ciappi G. Atti 78° Congr. Soc. It. Med. Int. Pozzi; Roma: 1977. Il Salasso. [Google Scholar]
- 6.Doll DC, Greenberg BR. Cerebral thrombosis in smokers' polycythemia. Ann Int Med. 1985;102:786–7. doi: 10.7326/0003-4819-102-6-786. [DOI] [PubMed] [Google Scholar]
- 7.Berk PD, Golberg JD, Donovan PB, et al. Therapeutic recommendations in Polycythemia Vera Group protocols. Semin Hematol. 1986;23:132–43. [PubMed] [Google Scholar]
- 8.Gruppo Italiano Studio Policitemia. Polycythemia vera: the natural history of 1213 patients followed 20 years. Ann Int Med. 1995;123:656–64. doi: 10.7326/0003-4819-123-9-199511010-00003. [DOI] [PubMed] [Google Scholar]
- 9.Tefferi A. Current management of polycythemia vera [review] Leuk Lymphoma. 2002;43:1–7. doi: 10.1080/10428190210200. [DOI] [PubMed] [Google Scholar]
- 10.Geddo E, Rossi L, Cerboni D, et al. Introduzione ai principi generali dell'emodiluzione. La Trasf del Sangue. 1985;30:101–4. [Google Scholar]
- 11.Mannini L, Graziani G, Bartoli V, et al. Emodiluizione nella policitemia vera e nell'eritrocitosi: esperienza clinica e di laboratorio. Boll Agg Soc It Emaferesi. 1987;3 (suppl):29–32. [Google Scholar]
- 12.Valbonesi M, Bruni R. Clinical application of therapeutic erythrocytapheresis (TEA) Trasf Scienc. 2000;22:183–94. doi: 10.1016/s0955-3886(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 13.Kaboth U, Rumpf KW, Kiersch T, et al. Advantages of isovolemic large-volume erythrocytapheresis as a rapidly effective and long-lasting treatment modality for red blood cell depletion in patients with polycythemia vera. Ther Apher. 1997;1:131–4. doi: 10.1111/j.1744-9987.1997.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 14.Staack D, Stange K, Schnurstein K. Appropriate application of erythroapheresis in treating various internal medical conditions. Transfus Science. 1999;21:240–1. [Google Scholar]
- 15.Migliaccio M, D'Agostino MP, D'Angiolino A, et al. Ruolo dell'eritrocitoaferesi nella terapia della poliglobulia secondaria. La Trasf del Sangue. 1998;43 (suppl):77. [Google Scholar]
- 16.Cabibbo S, Bonomo P. Eritroaferesi ed eritroexchange con separatore cellulare. Abstracts 2°incontro SIMTI – Abruzzo; Montesilvano. 16–17 May 2003. [Google Scholar]