Abstract
Introduction
The considerable increase in the consumption of fresh-frozen plasma (FFP) recorded in 2002 in the Region of Tuscany made it necessary to check the appropriateness of the use of this blood component in all transfusion facilities in the Tuscan network.
Materials and methods
From July 1, 2003 to December 31, 2005, the Regional Blood Transfusion Coordinating Centre carried out an audit on the clinical use of FFP in the 40 structures included in the Tuscan transfusion network. The study had two complementary parts: a review of guidelines on the use of FFP and the involvement of Hospital Transfusion Committees in evaluating the outcome of the audit and in the consequent local policy decisions and in educating clinicians.
Results
The data from all 40 of the regional transfusion structures were analysed. The audit, which was initially retrospective, gradually became prospective. The percentage clinical use of FFP decreased, compared to 2002, in each of the 3 years of the study: a) 2003: − 8.92%; b) 2004: − 2.11%; c) 2005: −1.97%. The inappropriate requests for plasma decreased from 27% to 22.7% of the total. It was possible to classify the inappropriate requests for plasma on the basis of homogeneous, regionally defined criteria. The most frequent inappropriate indication (60.7% of the total) was the use of plasma in the case of haemorrhage in patients with a normal PT and/or PTT or unavailable results. Each hospital revised its own guidelines between 2004 and 2005 and the Hospital Transfusion Committees set up appropriate educational and behavioural interventions.
Conclusions
The capacity of transfusion facilities to make data on the use of blood components available systematically and continuously is an essential feature of clinical governance; systematic clinical auditing increases the level of appropriate behaviours in the transfusion sector, contemporaneously contributing to self-sufficiency in transfusion products, and may direct research towards those clinical settings at greatest risk of inappropriate use of transfusion therapy with FFP.
Keywords: fresh-frozen plasma, transfusion, audit, appropriateness
Introduction
The transfusion of blood components plays a fundamental role in the management of various pathologies and is, sometimes, a life-saving treatment. Blood is, however, an expensive and limited resource; over the last 20 years there has been a progressive increase in demands for this product, mainly as a result of the advances in oncohaematological therapies and the increase in major surgery. The achievement of self-sufficiency in blood and its derivatives, a goal destined to be affected also by changes in the demographic characteristics of the population, is a priority for all health care systems, not only for those in western societies1–6.
In Italy, in the 5 years from 2001–2005, the clinical use of fresh-frozen plasma (FFP) has tended to increase, despite 2% reductions in 2002 and in 2005 compared to the consumption in the respective preceding years, 2001 and 2004. In fact, the consumption of FFP increased from 146,844 L in 2001 to 153,493 L in 20051,7–10.
In 2002, the Tuscany Regional Blood Transfusion Coordinating Centre (RBTCC) for transfusion services found that the use of FFP in the Region clearly contrasted with the national trend; indeed, that year, the use of FFP, reached its peak of 10,625 L (equivalent to about 3L/1,000 residents/year), with a increase of 4% with respect to 2001. Therefore, in 2003, the RBTCC, in the context of a regional benchmarking project lasting several years, that was aimed at continuous improvement of quality and self-sufficiency of the Tuscan transfusion system, introduced a systematic audit of the appropriateness of the clinical use of FFP in the hospitals making up the regional transfusion network.
Inappropriate transfusion therapy with FFP is probably one of the main avoidable risks for patients and it is known that guidelines are not, per se, able to influence clinical practice11,12, because without the help of other instruments, such as auditing and educational interventions, they may not be able to reach a level of diffusion and acceptance such as to be able to direct clinical practice towards the recommended behaviours.
There is, however, evidence showing that clinical auditing is an educational and behavioural strategy that is effective in altering the level of inappropriate transfusion treatment and is able to contribute to self-sufficiency in blood products, to a reduction in health care costs and to the delivery of better health care13.
The aim of this study was to verify the impact of an auditing process on the appropriateness of the clinical use of FFP and to classify the main causes of the inappropriate use of this product within the regional transfusion network.
Materials and methods
The transfusion system of the Region of Tuscany is spread throughout the region and comprises 15 Services of Immunohaematology and Transfusion Medicine (SIMT) and 25 depending on them14; the Tuscan transfusion network is incorporated in regional hospitals, managed by 12 Health Care Companies and by 4 University Hospital Companies, which deliver health care services to a population of about 3,600,000 people.
The study was coordinated by the RBTCC and carried out over a period of 3 years, that is, the second semesters of 2003 and 2004 and throughout 2005; in this period all the regional transfusion structures audited the requests received for FFP and, at the same time, undertook two complementary actions: updating their guidelines and involving the Hospital Transfusion Committees (Comitati Ospedalieri per il Buon Uso del Sangue, COBUS) in the evaluation of the outcome of the audit and in the consequent implications for local policy and education of clinicians.
The audit was conducted by manual review of transfusion requests by medical staff of the SIMT and the Transfusion Sections. Each transfusion structure was asked for the following data for each year: a) method of carrying out the appropriateness audit (retrospective or prospective) of the FFP transfusion requests received; b) total number of requests for FFP received; c) number of FFP requests audited; d) classification of the requests, on the basis of literature data and specific hospital guidelines, into: 1) appropriate; 2) inappropriate and, from 2004, 3) into a grey zone, with the aim of dividing the definitely inappropriate requests from those that were potentially inappropriate; e) classification of the inappropriate requests, from July 2004, according to the following, predefined, homogeneous regional criteria: 1) haemorrhage in a surgical context with PT and/or PTT not available; 2) haemorrhage in a non-surgical context with PT and/or PTT not available; 3) haemorrhage with PT and/or PTT normal; 4) prophylaxis of haemorrhagic events with PT and/or PTT normal or not performed; 5) prophylaxis of haemorrhagic events in the presence of altered PT and/or PTT; 6) hypoalbuminaemia and/or nutritional purposes; 7) requests based on predefined formulas; 8) concomitance of more than one of the preceding causes of inappropriateness; 9) other causes.
The data collected by the SIMT and the Transfusion Sections were subsequently sent at regular intervals to the RBTCC, which processed them, and reported them together with information on the regional production and consumption of FFP from 2002 to 2005; furthermore, the clinical use of FFP from 2002 to 2005 was also compared with the consumption of red blood cells (RBCs), a parameter used by the RBTCC as a regional indicator of the intensity of health care activities.
The statistical analysis (Student's t test) was carried out with GraphPad InStat software (version 3.00, GraphPad Software, San Diego, CA – USA).
Results
Data were received from all 40 regional transfusion structures and analysed. Each hospital revised its own guidelines between 2004 and 2005, also in the light of the guidelines published in July 2004 in the British Journal of Haematology by the British Committee for Standards in Haematology15.
The indications, for which the clinical use of FFP is considered appropriate, are reported below:
- correction of a congenital deficiency of a clotting factor for which the specific factor concentrate is lacking;
- acquired deficiency of multiple clotting factors, when PT and aPTT are > 1.5 normal levels, in the presence of an ongoing haemorrhage or severe risk of haemorrhage;
- use as a fluid replacement in the apheretic treatment of thrombotic microangiopathies;
- reconstitution of whole blood for exchange transfusions;
- hereditary angioedema due to C1 esterase deficiency, in the absence of a specific plasma-derivative.
In the same period, the COBUS of all the structures involved in the study evaluated the results of the audits carried out and involved clinicians in a shared review of the guidelines on transfusion therapy with FFP and in their diffusion within the hospitals.
The overall data for the 3 years examined on the way the auditing was carried out showed that, on average, 56% of the transfusion requests underwent systematic, prospective auditing.
The mean percentage of prospective audits of the requests increased in a statistically significant manner from 30% in 2003 to 65% in 2004 (p = 0.0001) and to 72% in 2005 (p < 0.0001); the increase between 2004 and 2005 was not statistically significant (p = 0.26) (Table I).
Table I.
Region of Tuscany: trend in the percentage of prospective audits of Fresh Frozen Plasma (FFP) requests from 2003 to 2005
|
Prospective audits of FFP requests in the period 2003–2005 (%) | ||||
|---|---|---|---|---|
| 2003 | 2004 | 2005 | 2003–2005 | |
| Mean | 30 | 65 | 72 | 56 |
| Standard deviation | 16 | 36 | 33 | 34 |
| Median | 37 | 77 | 85 | 45 |
| Range | 0–45 | 0–100 | 0–100 | 0–100 |
| 95% Confidence Interval | 20.3 – 39.7 | 43.2 – 86.6 | 52.2 – 91.7 | 45 – 67 |
| p | ||||
| 2003 vs 2004 | 2003 vs 2005 | 2004 vs 2005 | ||
| 0.0001 | < 0.0001 | 0.26 | ||
The total percentage of requests subjected to appropriateness auditing increased from 39.2%, in 2003, to 99.1%, in 2005; that of the inappropriate requests changed from 27% in 2003 to 26.7% in 2004, and to 22.7% in 2005; furthermore, in the period of 18 months between July 2004 and December 2005, 5.6% of the requests checked (900/15,996) were classified in the grey zone (Table II).
Table II.
Region of Tuscany: classification of the transfusion requests for fresh-frozen plasma (FFP) received and audited from 2003 to 2005
| FFP requests | 2003 (6 months) | 2004 (6 months) | 2005 (12 months) | 2004–2005 (18 months) |
|---|---|---|---|---|
| Received | 7,841 | 5,978 | 11,099 | 17,077 |
| Audited | 3,074 | 4,997 | 10,999 | 15,996 |
| Audited % | 39.2 | 83.5 | 99.1 | 93.7 |
| Appropriate | 2,244 | 3,263 | 7,999 | 11,262 |
| Appropriate % | 7 3 | 65.3 | 72.7 | 70.4 |
| Not appropriate | 830 | 1,335 | 2,499 | 3,834 |
| Not appropriate % | 2 7 | 26,7 | 22,7 | 2 4 |
| Grey zone | 0 | 399 | 501 | 900 |
| Grey zone % | 0 | 8 | 4,6 | 5,6 |
In the 24 months of the study, the Tuscan transfusion structures received a total of 24,918 requests for FFP and audited 19,070 (76.5%); 13,506 (70.8%) of the requests were considered appropriate, 4,664 (24.5%) inappropriate and 900 (4.7% of the total in the 3 years) fell in the grey zone.
The analysis of the percentage of requests for FFP, which were audited by the individual transfusion structures (data not shown), revealed increases that were statistically significant for the comparison between 2003 and 2004 (p = 0.03) and between 2003 and 2005 (p = 0.02), but not for the comparison between 2004 and 2005 (p = 0.2).
The trends in the percentages of appropriate, not appropriate and grey zone requests, analysed for the individual hospitals for the period under consideration, did not show statistically significant changes (data not shown), although there was a general tendency to a reduction in the total percentage of inappropriate and grey zone requests for FFP.
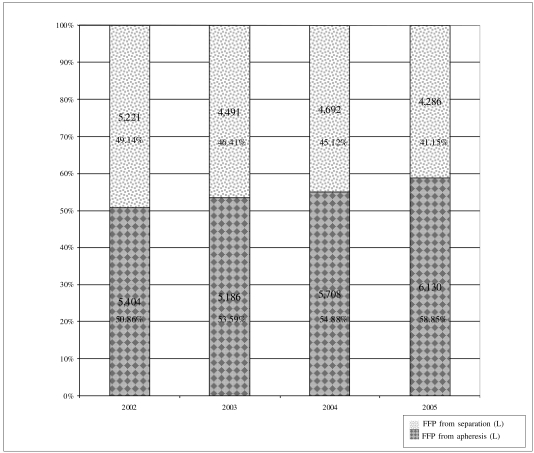
Compared with the level of use in 2002, the clinical use of FFP (in litres) decreased in each of the 3 years of the study: a) 2003: − 8.92%; b) 2004: − 2.11%; c) 2005: − 1.97%; similarly, there was a reduction in the litres of FFP derived from separation that were used clinically: a) 2003: −13.99%, b) 2004: − 10.13%, c) 2005: − 17.91%, and a steady increase in the percentage of FFP from apheresis used clinically (Table III) (Figure 1).
Table III.
Region of Tuscany: consumption of fresh-frozen plasma (FFP) for clinical use from 2002 to 2005 and production of the FFP in the same period.
| 2002 | 2003 | 2004 | 2005 | ||
|---|---|---|---|---|---|
| FFP produced (L) | 57,400 | 59,036 | 62,626 | 65,979 | |
| Difference in production compared to 2002 (%) | + 2.85 | + 9.1 | + 14.95 | ||
| FFP distributed for clinical use (L) | from apheresis | 5,404 | 5,186 | 5,708 | 6,130 |
| from separation | 5,221 | 4,491 | 4,692 | 4,286 | |
| Total | 10,625 | 9,677 | 10,400 | 10,416 | |
| Difference in the distribution of FFP for clinical use compared to 2002 (%) | from apheresis | − 4.35 | + 5.62 | + 13.43 | |
| from separation | − 13.99 | − 10.13 | − 17.91 | ||
| Total | − 8.92 | − 2.11 | − 1.97 |
Figure 1.
Region of Tuscany: fresh-frozen plasma (FFP) from apheresis and from separation used for clinical purposes from 2002 to 2005. The values are expressed in litres and as a percentage of the total.
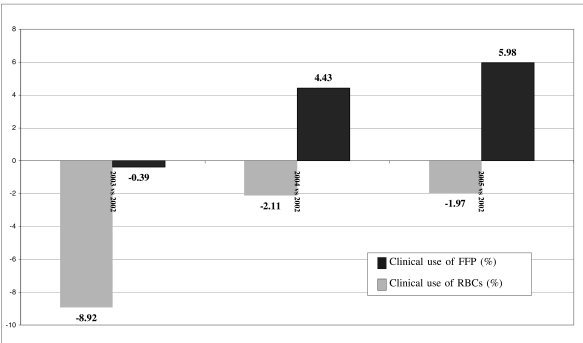
The year in which the clinical use of FFP was lowest was 2003; there were then moderate increases in 2004 and 2005, although the levels of 2002 were not reached. The increased use of FFP (in litres) from 2003 to 2005 was, nevertheless, percentage-wise, less than that expected on the basis of the increased consumption of RBCs in the same period. RBC use is an indicator of the intensity of health care services recorded by the RBTCC throughout the region; in fact, the consumption of RBC units was 145,498 units in 2002, decreased in 2003 to 144,921 units (−0.39 %) then rose in 2004 to 151,947 units (+ 4.43 %) and in 2005 to 154,080 units (+ 5.98 %) (Figure 2).
Figure 2.
Region of Tuscany: comparison between the changes in percentage consumption of fresh-frozen plasma (FFP) and red blood cells (RBC) in the years 2003–2005 compared to in 2002
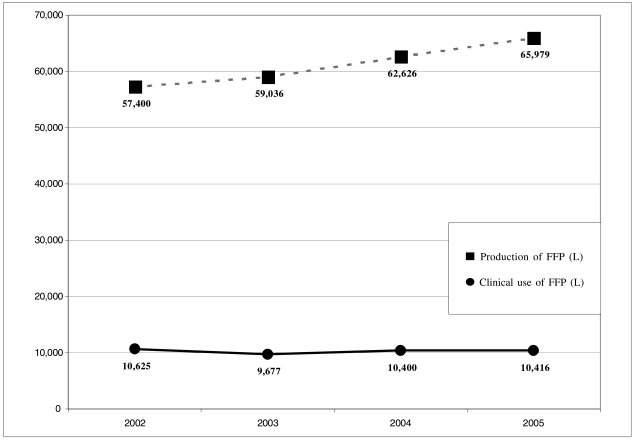
The regional production of plasma changed from 57,400 L in 2002 to 65,979 L in 2005 (Table III) (Figure 3). The relationship (in litres) between FFP used clinically and the total amount of FFP produced showed a tendency to decrease with respect to that in 2002: a) 2002: 18.51%; b) 2003: 16.39%; c) 2004: 16.6%; and d) 2005: 15.78%.
Figure 3.
Region of Tuscany: production of fresh-frozen plasma (FFP) and clinical use of FFP from 2002 in litres
In order to classify the types of inappropriate requests according to predefined criteria established by the Region, an analysis was conducted on 15,996 requests (93.7% of the total 17,077 received) for FFP during 18 consecutive months from July 1, 2004 to December 31, 2005; 3,834 (24%) of the requests were considered inappropriate, 11,262 (70.4%) appropriate and 900 (5.6%) fell in the grey zone (Table II).
Table IV reports the types of inappropriate requests and their proportional incidence in decreasing order.
Table IV.
Region of Tuscany: causes of inappropriate use of fresh-frozen plasma as revealed by the audit of requests from July 1, 2004 to December 31, 2005
| Cause of inappropriate use | Number of requests | % |
|---|---|---|
| Haemorrhage in a surgical setting with PT and/or PTT not available | 1,281 | 33.4 |
| Haemorrhage in non-surgical setting with PT and/or PTT not available | 572 | 14.9 |
| Haemorrhage with PT and/or PTT normal | 475 | 12.4 |
| Prophylaxis against bleeding with PT and/or PTT normal or not carried out | 470 | 12.3 |
| Prophylaxis against bleeding with PT and/or PTT altered | 436 | 11.4 |
| Hypoalbuminaemia and/or nutritional purposes | 212 | 5.5 |
| Concomitance of more than one inappropriateness | 199 | 5.2 |
| Other causes | 9 7 | 2.5 |
| Requests based on a predefined formula | 9 2 | 2.4 |
| Total | 3,834 | 100 |
The analysis of the types of inappropriate requests recorded by the individual hospitals (data not shown) revealed the following: (i) there were 2,328 inappropriate requests for FFP (60.7% of all inappropriate requests) for the treatment of haemorrhagic events in patients with PT and/or PTT values unavailable or normal): 38.7% of these requests (n=901) came from three University hospitals; (ii) 1,853 requests (48.3% of the total) were defined inappropriate because they were made without the results of screening coagulation tests: 59.2% of these (n=758) were from the same three University hospitals; (iii) 906 of the inappropriate requests (23.7% of the total) were made for prophylaxis of haemorrhagic events (with PT and/or PTT not available, normal or altered): 29.6% of these requests (n=268) were made by a single University hospital; 3) treatment of hypoalbuminaemia and/or nutritional purposes were the reasons for 212 inappropriate requests for FFP (5.5% of the total): 56.6% of these requests (n=120) came from one University hospital.
Discussion
The systematic review of 19,070 requests for FFP, equivalent to 76.5% of all requests for the period 2003–2005, showed that the inappropriate use of FFP in Tuscany decreased from 27% in 2003 to 22.7%, in 2005. The mean reduction over the whole 3-year period was 24.5%; this result, if transferred into daily clinical practice, implies that of every four requests for FFP, one is inappropriate. The percentage of inappropriate clinical use of FFP recorded in this regional study is however, in the lower range (10–74%) reported in previous studies on the same subject carried out between 1986 and 2006, although these were conducted in heterogeneous health care systems, including some non-western countries6,16–37. The percentage reduction in inappropriate use (4.3%) was detectable, although relatively small; it is compatible with results obtained in other studies carried out in health care systems comparable with the Italian system. In these studies, prospective auditing was combined with corrective actions (such as improving blood component request form) and educational interventions aimed at improving clinical practice17,29. The decrease in inappropriate requests for FFP during the course of the study was attributed to various factors, probably acting together: a) the increase in audits of appropriateness (+ 44.3% in 2004 and + 15.6% in 2005) and, in particular, the continuous increase of prospective auditing from 2003, even if this did not reach statistical significance in 2005; b) the interventions carried out between 2004 and 2005, such as the divulgation of the updated guidelines within the hospitals and the education of clinicians promoted by the COBUS. In fact, the COBUS are able to influence the appropriateness of the use of blood components significantly38. The results of the present study confirm already existing evidence on the capacity of behavioural interventions to modify the level of inappropriate transfusion therapy13. Moreover, this regional study seems to indicate that auditing and educational interventions, particularly if combined with a review of the guidelines on transfusion therapy, can enhance their diffusion and acceptance, improving the appropriateness of blood product use. The analysis of the regional consumption of plasma, during the period of the study, confirmed the increase of appropriateness of FFP therapy. Other indices of the more appropriate use of FFP are the considerable reduction in the clinical use of plasma obtained by separation in favour of that from apheresis (with consequent less exposure of recipients to donors) and the lesser percentage increase in FFP use compared to that of RBC use. The increased use of RBCs in the same period reflects the considerable intensification of health care, that occurred in the Region from 2003 onwards, particularly in transplant medicine39. This could explain why, in the same period that prospective auditing increased significantly, there was also a moderate increase in the consumption of FFP, although this never reached the levels of 2002.
The possibility of classifying requests in a grey zone, introduced in the audit from 2004, enabled 900 potentially inappropriate requests to be picked up in the following 18 months.
These requests were separated from the definitely inappropriate requests, thus reducing the "bias" of mistaken classification and undoubtedly contributing to reducing the percentage of inappropriate requests40,41. Furthermore, the use of the grey zone was attributed the capacity to increase the robustness of the studies on the inappropriateness of transfusion therapy, precisely because of the separation that this zone enables between potentially and truly inappropriate transfusions42.
The analysis of about 16,000 requests made in 18 consecutive months, showing that 24% of these were inappropriate, enabled the main causes of the inappropriateness to be determined and grouped in predefined classes. The highest percentage of inappropriate requests (60.7%) was for the treatment of haemorrhagic events, both in medical and surgical settings, without information on baseline haemostatic parameters (PT and PTT) or in the presence of normal values for these parameters. It, therefore, appears that there are situations in which the therapeutic choice seems to be dictated predominantly by the clinical evaluation, independently of laboratory information, since the data were missing completely in 48.3% of the cases. This last figure probably underestimates the real percentage of requests made in the absence of laboratory data, because it does not take into account the 12.3% of inappropriate requests for the prophylaxis of haemorrhagic events in which the absence of PT and/or PTT was combined with normal results for these parameters.
Thus, the lack of laboratory data we found seems to be greater than the 34.1% reported by other Authors16; this difference is probably due to the different design of the study mentioned, which also evaluated the laboratory data of patients transfused with FFP, with the aid of a specific database. Both results were, however, encouraging in the light of the data from the Sanguis study in 1994, which found that PT data were missing in 84% of the transfusion recipients of FFP in 43 European hospitals43.
In second place as a cause of inappropriate use of FFP, was prophylaxis of bleeding (23.7% of inappropriate requests). Transfusion therapy with FFP to correct coagulation test abnormalities before carrying out invasive procedures is a common clinical practice, even if there are no "evidence-based" guidelines on this issue. Haemostasis is a complex process of interactions between pro-coagulant molecules, platelets, natural anticoagulants, the fibrinolytic system and the endothelium. The screening tests of haemostasis prior to surgery are usually PT/INR and PTT; these laboratory tests have been developed to identify causes of bleeding in patients with a demonstrated haemorrhagic diathesis (high pre-test probability) and not to evaluate haemostasis in patients with a negative history for haemorrhage, nor has it been demonstrated that they are able to do so. The transfusion of FFP before an invasive procedure, in order to correct mild to moderate changes in coagulation tests, is not, therefore, able to correct the anomaly or to reduce the perceived haemorrhagic risk44–54.
The use of FFP to correct hypoproteinaemia or for nutritional purposes is greater in developing countries, but it is difficult to find a justification for this practice in the Italian health care system19.
The present study also reveals that inappropriate use of FFP is more common in University hospitals than in smaller, peripheral hospitals, as already reported in the literature18; this could be due to the greater complexity of cases treated in the University hospitals and the greater amount of blood components distributed by these hospitals, both factors which could limit the possibility of carrying out audits and adhering to guidelines.
Possible limitations of this study are: a) that the audit did not use information technology, which, with a specific database, would have enabled a whole set of diagnostic, clinical and laboratory data to be processed and compared at national and international levels16,41,55–57; b) the definition of inappropriateness did not take into account the dose of FFP used and, therefore, the possible administration of subtherapeutic doses19,41,46,58,59; c) the comparison between the years was not homogeneous, because the data for 2005 were not divided by semester, but referred to the whole calendar year.
The capacity of transfusion structures to make data available systematically and continuously on the use of blood components is an essential feature of clinical governance60; systematic clinical auditing, particularly if performed at a large scale such as regionally, thus enabling the acquisition of a substantial quantity of data, increases the degree of appropriate behaviours in the field of transfusion therapy, contributes to self-sufficiency, and can direct clinical research towards those sectors in which inappropriate treatment is greatest. Transfusion medicine is, in fact, a transversal medical discipline in which there are few adequately structured trials that provide evidence on the indications and efficacy of transfusion therapy. Appropriately designed clinical trials could modify some decisions, so that they are made on "evidence-based" criteria61. This is what is happening in the USA, where a multicentre clinical trial should provide evidence on which to decide on the clinical use of FFP in patients with liver disease and INR values of 1.3 – 2, who are candidates for invasive procedures61; this multicentre study was triggered by a retrospective audit53, followed by a prospective audit48, conducted in the same hospital, and a systematic review of the literature on the subject49.
Footnotes
Presented in part at the 39th SIMTI National Congress of Transfusion Medicine (Paestum, SA, Italy, October, 4–7, 2006) and the 59th Meeting of the AABB (Miami Beach, FL, USA, October 21–24, 2006).
References
- 1.Catalano L, Abbonizio F, Giampaolo A, et al. Registro nazionale e regionale del sangue e del plasma. Rapporto 2005. Istituto Superiore di Sanità. Available at: http://www.iss.it/binary/publ/cont/06-30.1163668346.pdf.
- 2.Sullivan MT, Cotten R, Read EJ, et al. Blood collection and transfusion in the United States in 2001. Transfusion. 2007;47:385–94. doi: 10.1111/j.1537-2995.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 3.Greinacher A, Fendrich K, Alpen U, et al. Impact of demographic changes on the blood supply: Mecklenburg-West Pomerania as a model region for Europe. Transfusion. 2007;47:395–401. doi: 10.1111/j.1537-2995.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- 4.Vamvakas EC. Epidemiology of blood transfusion and forecasts of demand for blood. In: Vamvakas EC, editor. Evidence-Based Practice of Transfusion Medicine. Bethesda: American Association of Blood Banks; 2001. pp. 177–99. [Google Scholar]
- 5.Currie CJ, Patel TC, McEvan P, et al. Evaluation of the future supply and demand for blood products in the United Kingdom National Health Service. Transfus Med. 2004;14:19–24. doi: 10.1111/j.0958-7578.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- 6.Yeh CJ, Wu CF, Hsu WT, et al. Transfusion audit of fresh-frozen plasma in southern Taiwan. Vox Sang. 2006;91:270–4. doi: 10.1111/j.1423-0410.2006.00819.x. [DOI] [PubMed] [Google Scholar]
- 7.Catalano L, Abbonizio F, Hassan JH. Registro nazionale e regionale del sangue e del plasma. Rapporto 2001. Istituto Superiore di Sanità. Available at: http://www.iss.it/binary/publ/publi/0315.1109149613.pdf.
- 8.Catalano L, Abbonizio F, Giampaolo A, et al. Registro nazionale e regionale del sangue e del plasma. Rapporto 2002. Istituto Superiore di Sanità. Available at: http://www.iss.it/binary/publ/publi/0341.1109236509.pdf.
- 9.Catalano L, Abbonizio F, Giampaolo A, et al. Registro nazionale e regionale del sangue e del plasma. Rapporto 2003. Istituto Superiore di Sanità. Available at: http://www.iss.it/binary/publ2/cont/04-36.1140439702.pdf.
- 10.Catalano L, Abbonizio F, Giampaolo A, et al. Registro nazionale e regionale del sangue e del plasma. Rapporto 2004. Istituto Superiore di Sanità. Available at: http://www.iss.it/binary/publ/cont/05-45.1141828723.pdf.
- 11.Lomas J, Anderson GM, Domnick-Pierre K, et al. Do practice guidelines guide practice? The effect of a consensus statement on the practice of physicians. N Engl J Med. 1989;321:1306–11. doi: 10.1056/NEJM198911093211906. [DOI] [PubMed] [Google Scholar]
- 12.McClelland B. Effective use of blood components. In: Murphy MF, Pamphilon DH, editors. Practical Transfusion Medicine. Oxford: Blackwell Science; 2001. pp. 65–76. [Google Scholar]
- 13.Wilson K, MacDougall L, Ferguson D, et al. The effectiveness of interventions to reduce physician's levels of inappropriate transfusion: what can be learned from a systematic review of the literature. Transfusion. 2002;42:1224–9. doi: 10.1046/j.1537-2995.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 14.Picinini V, Abbonizio F, Catalano L, et al. Mappa delle strutture trasfusionali esistenti sul territorio nazionale (aggiornamento 2005) Istituto Superiore di Sanità. Available at: http://www.iss.it/binary/publ/cont/0394_9303_2006_I_06-S1.1145354064.pdf.
- 15.O'Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 16.Palo R, Capraro L, Hovilehto S, et al. Population-based audit of fresh frozen plasma transfusion practices. Transfusion. 2006;46:1921–5. doi: 10.1111/j.1537-2995.2006.00998.x. [DOI] [PubMed] [Google Scholar]
- 17.Hui CH, Williams I, Davis K. Clinical audit of the use of fresh frozen plasma and platelets in a tertiary teaching hospital and the impact of a new transfusion request form. Intern Med J. 2004;35:283–8. doi: 10.1111/j.1445-5994.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 18.Soutar RL, Jobanputra S, Tait RC. A two-phase audit of fresh frozen plasma: a regional approach [letter] Transfus Med. 2004;14:75–6. doi: 10.1111/j.0958-7578.2004.00480.x. [DOI] [PubMed] [Google Scholar]
- 19.Kakkar N, Kaurt R, Dhanoa J. Improvement in fresh frozen plasma transfusion practice: results of an outcome audit. Transfus Med. 2004;14:231–5. doi: 10.1111/j.0958-7578.2004.00505.x. [DOI] [PubMed] [Google Scholar]
- 20.Schofield WN, Rubin GL, Dean MG. Appropriateness of platelet, fresh frozen plasma and cryoprecipitate transfusion in New South Wales public hospitals. Med J Aust. 2003;178:117–21. doi: 10.5694/j.1326-5377.2003.tb05101.x. [DOI] [PubMed] [Google Scholar]
- 21.Chng WJ, Tan MK, Kuperan P. An audit of fresh frozen plasma usage in an acute general hospital in Singapore. Singapore Med J. 2003;44:574–8. [PubMed] [Google Scholar]
- 22.Stainsby D, Burrowes-King V. Audits of the appropriate use of fresh frozen plasma. Blood Matters. 2002;10:7–9. Available at: http://www.blood.co.uk/hospitals/library/pdf/bm10.pdf.
- 23.Luk C, Eckert KM, Barr RM, et al. Prospective audit of the use of fresh-frozen plasma, based on Canadian Medical Association transfusion guidelines [letter] Can Med Assoc J. 2002;166:1539–40. [PMC free article] [PubMed] [Google Scholar]
- 24.Prathiba R, Jayaranee S, Ramesh JC, et al. An audit of fresh frozen plasma usage in a tertiary referral centre in a developing country. Malays J Pathol. 2001;23:41–6. [PubMed] [Google Scholar]
- 25.Beloeil H, Brosseau M, Benhamou D. Transfusion of fresh frozen plasma (FFP): audit of prescriptions. Ann Fr Anesth Reanim. 2001;20:686–92. doi: 10.1016/s0750-7658(01)00462-2. [DOI] [PubMed] [Google Scholar]
- 26.Hameedullah, Khan FA, Kamal RS. Improvement of intraoperative fresh frozen plasma transfusion practice -impact of medical audits and provider education. J Pak Med Assoc. 2000;50:253–6. [PubMed] [Google Scholar]
- 27.Marti-Carvajal AJ, Munoz-Navarro SR, Pena-Marti GE, et al. An audit of appropriate use of blood products in adult patients in a Venezuelan general university hospital. Int J Qual Health Care. 1999;11:391–5. doi: 10.1093/intqhc/11.5.391. [DOI] [PubMed] [Google Scholar]
- 28.Jones HP, Jones J, Napier JA, et al. Clinical use of FFP: results of a retrospective process and audit outcome. Transfus Med. 1998;8:37–41. doi: 10.1046/j.1365-3148.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 29.Tuckfield A, Haeusler MN, Grigg AP, et al. Reduction of inappropriate use of blood products by prospective monitoring of transfusion request forms. Med J Aust. 1997;167:473–6. doi: 10.5694/j.1326-5377.1997.tb126674.x. [DOI] [PubMed] [Google Scholar]
- 30.Marconi M, Almini D, Pizzi MN, et al. Quality assurance of clinical transfusion practice by implementation of the privilege of blood prescription and computerized prospective audit of blood requests. Transfus Med. 1996;6:11–9. doi: 10.1046/j.1365-3148.1996.d01-48.x. [DOI] [PubMed] [Google Scholar]
- 31.Cheng G, Wong HF, Chan A, et al. The effects of a self educating blood component request form and enforcements of transfusion guidelines on FFP and platelet usage, Queen Mary Hospital, Hong Kong. British Committee for Standards in Hematology (BCSH) Clin Lab Haematol. 1996;18:83–7. doi: 10.1046/j.1365-2257.1996.00153.x. [DOI] [PubMed] [Google Scholar]
- 32.Schots J, Steenssens L. Blood usage review in a Belgian university hospital. Int J Qual Health Care. 1994;6:41–5. doi: 10.1093/intqhc/6.1.41. [DOI] [PubMed] [Google Scholar]
- 33.Thomson A, Contreras M, Knowles S. Blood component treatment: a retrospective audit in five major London hospitals. J Clin Pathol. 1991;44:734–7. doi: 10.1136/jcp.44.9.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnette RE, Fish DJ, Eisenstaedt RS. Modification of fresh-frozen plasma transfusion practices through educational intervention. Transfusion. 1990;30:253–7. doi: 10.1046/j.1537-2995.1990.30390194348.x. [DOI] [PubMed] [Google Scholar]
- 35.Brien WF, Butler RJ, Inwood MJ. An audit of blood component therapy in a Canadian general teaching hospital. CMAJ. 1989;140:812–5. [PMC free article] [PubMed] [Google Scholar]
- 36.Mozes B, Epstein M, Ben-Bassat I, et al. Evaluation of the appropriateness of blood and blood product transfusion using preset criteria. Transfusion. 1989;29:473–6. doi: 10.1046/j.1537-2995.1989.29689318442.x. [DOI] [PubMed] [Google Scholar]
- 37.Blumberg N, Laczin J, McMican A, et al. A critical survey of fresh-frozen plasma use. Transfusion. 1986;26:511–3. doi: 10.1046/j.1537-2995.1986.26687043615.x. [DOI] [PubMed] [Google Scholar]
- 38.Hayes SL, Torella F. The role of hospital transfusion committees in blood product conservation. Transfus Med Rev. 2004;18:93–104. doi: 10.1016/j.tmrv.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Organizzazione Toscana Trapianti (OTT) Attività di donazione e trapianto di organi, tessuti e cellule nella Regione Toscana. OTT Report 2005. Available at: http://www.salute.toscana.it/sst/ott/pdf/report_ott_2005.pdf.
- 40.Hasley PB, Lave J, Kapoor WN. The necessary and the unnecessary transfusion: a critical review of reported appropriateness rates and criteria for red cell transfusions. Transfusion. 1994;34:110–5. doi: 10.1046/j.1537-2995.1994.34294143936.x. [DOI] [PubMed] [Google Scholar]
- 41.Rotschild JM, McGurk S, Honour M, et al. Assessment of education and computerized decision support interventions for improving transfusion practice. Transfusion. 2007;47:228–39. doi: 10.1111/j.1537-2995.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 42.Kanter MH. The transfusion audit as a tool to improve transfusion practice: a critical appraisal. Transfus Sci. 1998;19:69–81. doi: 10.1016/s0955-3886(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 43.Use of blood products for elective surgery in 43 European hospitals. The Sanguis Study Group. Transfus Med. 1994;4:251–68. [PubMed] [Google Scholar]
- 44.Holand L, Sarode R. Should plasma be transfused prophylactically before invasive procedures? Curr Opin Hematol. 2006;13:447–51. doi: 10.1097/01.moh.0000245688.47333.b6. [DOI] [PubMed] [Google Scholar]
- 45.Gajic O, Dzik WH, Toy P. Fresh frozen plasma and platelet transfusion for non bleeding patients in the intensive care unit: benefit or harm? Crit Care Med. 2006;34 (5 Suppl):S170–3. doi: 10.1097/01.CCM.0000214288.88308.26. [DOI] [PubMed] [Google Scholar]
- 46.Holland LL, Brooks JP. Toward rational fresh frozen plasma transfusion. Am J Clin Pathol. 2006;126:133–9. doi: 10.1309/NQXH-UG7H-ND78-LFFK. [DOI] [PubMed] [Google Scholar]
- 47.Triulzi DJ. The art of plasma transfusion therapy [editorial] Transfusion. 2006;46:1268–70. doi: 10.1111/j.1537-2995.2006.00923.x. [DOI] [PubMed] [Google Scholar]
- 48.Adel-Wahab OI, Healy B, Dzick WH. Effect of fresh frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion. 2006;46:1279–85. doi: 10.1111/j.1537-2995.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 49.Segal JB, Dzick WH Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413–25. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 50.Holland LL, Foster TM, Marlar RA, et al. Fresh frozen plasma is ineffective for correcting minimally elevated international normalized ratios [letter] Transfusion. 2005;45:1234–5. doi: 10.1111/j.1537-2995.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 51.Dara SI, Rana R, Afessa B, et al. Fresh frozen plasma transfusion in critically ill medical patients with coagulopathy. Crit Care Med. 2005;33:2667–71. doi: 10.1097/01.ccm.0000186745.53059.f0. [DOI] [PubMed] [Google Scholar]
- 52.Wallis JP, Dzik S. Is fresh frozen plasma overtransfused in the United States? [letter] Transfusion. 2004;44:1674–5. doi: 10.1111/j.0041-1132.2004.00427.x. [DOI] [PubMed] [Google Scholar]
- 53.Dzick W, Rao A. Why do physicians request fresh frozen plasma? [letter] Transfusion. 2004;44:1393–4. doi: 10.1111/j.0041-1132.2004.00422.x. [DOI] [PubMed] [Google Scholar]
- 54.Stanworth SJ, Brunskill SJ, Hyde CJ, et al. Is fresh frozen plasma clinically effective? A systematic review of randomised controlled trials. Br J Haematol. 2004;126:139–52. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]
- 55.Zimmermann M, Büscher M, Linhardt C, et al. A survey of blood component use in a German university hospital. Transfusion. 1997;37:1075–83. doi: 10.1046/j.1537-2995.1997.371098016449.x. [DOI] [PubMed] [Google Scholar]
- 56.Titlestad K, Kristensen T, Jorgensen J, et al. Monitoring transfusion practice – a computerized procedure. Transfus Med. 2002;12:25–34. doi: 10.1046/j.1365-3148.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- 57.Titlestad K, Georgsen J, Jorgensen J, et al. Monitoring transfusion practices at two university hospitals. Vox Sang. 2001;80:40–7. doi: 10.1046/j.1423-0410.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 58.Santagostino E, Mancuso ME, Morfini M, et al. Solvent/detergent plasma for prevention of bleeding in recessively inherited coagulation disorders: dosing, pharmacokinetics and clinical efficacy. Haematologica. 2006;91:634–9. [PubMed] [Google Scholar]
- 59.Chowdhury P, Saayman UP, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol. 2004;125:69–73. doi: 10.1111/j.1365-2141.2004.04868.x. [DOI] [PubMed] [Google Scholar]
- 60.Casati G. Clinical governance: an ethical appeal or a management programme? [editorial] Blood Transfus. 2006;4:251–64. [Google Scholar]
- 61.Dzick WH. The NHLBI Clinical Trials Network in transfusion medicine and hemostasis: an overview. J Clin Apher. 2006;21:57–9. doi: 10.1002/jca.20092. [DOI] [PubMed] [Google Scholar]