Introduction
Stem cells are immature progenitor cells capable of self-renewal and multilineage differentiation through a process of asymmetric mitosis that leads to two daughter cells, one identical to the stem cell and one capable of differentiation into more mature cells.
Stem cells may be: 1) totipotent, i.e. early embryonic cells (1–3 days from oocyte fertilization), which can give rise to all the embryonic tissues and placenta; 2) pluripotent, i.e. embryonic cells from blastocystis (days 4–14 after oocyte fertilization), which can differentiate only into embryonic tissues belonging to the inner cell mass (ectoderm, mesoderm, and endoderm); or 3) multipotent, i.e. embryonic cells from the 14th day onwards, fetal stem cells, cord blood stem cells, and adult stem cells, which can give rise only to tissues belonging to one embryonic germ layer (ectoderm or mesoderm or endoderm).
Mesenchymal stem cells (MSC) are non-haematopoietic cell precursors initially found in the bone marrow, but actually present in many other tissues. MSC in culture are adherent, proliferating, and capable of multilineage differentiation into several tissues of mesenchymal origin, such as bone marrow stroma, adipose tissue, bone, cartilage, tendon, skeletal muscle, visceral mesoderm, and endothelial cells1–5. Well known and used for bone regeneration for many years, MSC came in the limelight at the end of the 1990s thanks to the evidence that, despite their adult stem cell nature, these cells are capable of pluripotent differentiation, which may be useful for regenerative medicine. In addition, since the beginning of 2000 it has become clear that MSC possess immune regulatory properties that may make them useful in autoimmune diseases.
Mesenchymal stem cells
The presence of MSC of bone marrow origin was formally demonstrated in the second half of the 1970s1, by seeding whole bone marrow samples in culture plastic disks and removing non-adherent cells after some hours. The few adherent “fibroblastic-like” cells formed small cell clusters, defined fibroblast-colony forming units (CFU-F)1, 6. After several culture passages, surviving cells became homogeneous and retained their ability to replicate and form cartilage and bone cells1.
Several studies later confirmed the multipotency of these cells. In the presence of adequate stimuli they differentiate into adipocytes (with formation of cytoplasmic vacuoles containing lipids), osteoblasts (with deposits of hydroxyapatite crystals), chondrocytes (with synthesis of cartilage matrix) and muscle cells (rich in myotubes). This differentiation is detectable through the use of appropriate cell staining and immunochemistry reactions2–5, 7, 8. MSC are also capable of expressing genes of embryonic origin, cell-cell contact molecules, extracellular matrix, such as interstitial type I collagen, fibronectin, type IV collagen and basal membrane laminin. MSC may also secrete cytokines such as interleukin (IL)-7, IL-8, IL-11, stem cell factor (SCF), and stromal-derived-factor-1 (SDF-1) that regulates the homing of haematopoietic stem cells into the bone marrow2–5, 7–11. MSC normally renew the stromal microenvironment necessary for haematopoiesis. Indeed, MSC are capable of supporting in vitro long-term haematopoietic cultures very efficiently12. Patients undergoing allogeneic bone marrow transplantation show a defect oin the stromal cells’ capacity to support the growth of haematopoietic progenitors13; a reduced support to granulocyte-monocyte-colony-forming unit (CFU-GM) formation by bone marrow stroma is well documentable even in patients undergoing autologous and/or chemotherapeutic treatments14. Moreover, co-infusion of MSC and haematopoietic stem cells leads to more rapid haematological recovery after high-dose chemotherapy as compared to haematopoietic stem cell transplant alone15.
MSC are relatively rare in the bone marrow (1/105 mononuclear cells), but they can proliferate very efficiently preserving their stem cell properties in vivo16, 17. The progressive loss of differentiation potential because of senescence generally occurs after about 40 doublings16, 17. MSC may also differentiate in vitro into cells of non-mesodermal origin, such as neurons, skin and gut epithelial cells, hepatocytes and pneumocytes1–5, 18–22, although there is a lack of precision regarding terminology in some papers. MSC are considered different from: (i) multipotent adult progenitor cells (MAPC), which may differentiate in vitro into endothelial, epithelial, and neural cells, as well as cells of mesenchymal origin5, and are probably the common progenitors of haematopoietic and mesenchymal stem cells; (ii) marrow stromal cells or multipotent mesenchymal stromal cells, which possess multilineage differentiation potential restricted only to tissues deriving from mesoderm (fat, bone, cartilage, muscle)23. The discrepancy between terminology and biological features is probably due to variability in methodologies used by different researchers, rather than to the real co-existence of different stem cells of mesenchymal origin, even though a gradient of MSC differentiation potential probably exists, similarly to that for haematopoietic stem cell precursors. Some tissue factors, such as basic fibroblast growth factor (bFGF) or heparin-binding epidermal growth factor (EGF)-like growth factor (HB-EGF), besides enhancing proliferation, may interfere with the differentiation potential of MSC, thus influencing their multipotency24.
Some Authors have shown that very small populations of MSC circulate in the peripheral blood25, 26. More recently, MSC have also been detected in tissues other than bone marrow, such as subcutaneous fat (adipose tissue-derived adult stem cells, ADAS)27–29, scalp subcutaneous tissues30, periodontal ligament31, umbilical cord blood32, foetal tissues33–35, as well as lymphoid tissues such as lymph nodes36, and adult human and mouse spleen and thymus37, 38, thus suggesting that a “mesenchymal system” is virtually present in all adult tissues39. In practice, however, only adipose tissue- and cord blood-derived MSC seem to be alternatives to bone marrow-derived MSC for clinical use, although with some differences in terms of CFU-F frequency (higher for adipose tissue-derived MSC, very low for cord blood-derived MSC), immunophenotype (lower expression of CD106 in adipose tissue-derived MSC and of CD90 and CD105 in cord blood-derived MSC), differentiation potential (reduced in cord blood-derived MSC), and gene expression27–29, 40–44.
MSC can be obtained ex vivo from bone marrow samples or from tissues disaggregated into single cell components and resuspended in culture medium. Cells may be seeded in plates or flasks at different concentrations with culture media such as modified Eagle medium (α-MEM) or Dulbecco’s modified Eagle medium (D-MEM), enriched with 5–15% foetal bovine serum and antibiotics, and cultured under appropriate conditions1–5, 24, 37, 38. After a few days, adherent cells form some proliferating clusters with at least 50 cells (CFU-F) that are counted after 10 days and put in relation with the initial seeded cell population to quantify the clonogenic potential of that tissue1, 6, 37, 38. Adherent cell clusters grow very quickly and become confluent, so that cells have to be re-plated periodically for the further expansion. A homogeneous, adherent cell population is generally achieved after 3–5 weeks of culture and keeps proliferating for up to 40 doublings without differentiating spontaneously2–5, 16–19, 24, 37, 38, 44, 45.
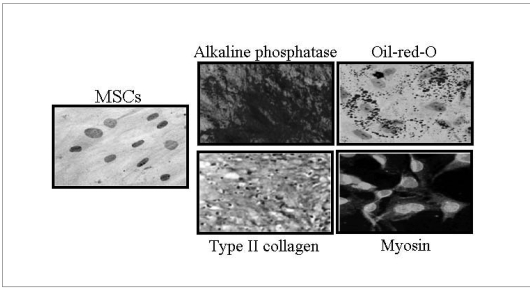
Using specific media, MSC can be induced to differentiate in vitro into different lineages of mesodermal origin, such as adipogenic, osteogenic, chondrogenic, and myogenic lineages2–5, 16–19, 24, 37, 38, 44–46 (Figure 1). Bone marrow MSC normally express low levels of neural markers47. By conditioning MSC with different cytokines, such as bFGF and EGF, some dramatic changes of MSC morphology resembling neural cells may be rapidly achieved together with the strong expression of specific neural markers such as nestin, neurofilaments, MAP-2, β-tubulin and Neu-N. On the other hand, MSC-mature neural cell co-culture as well as MSC injection inside animal brains lead to further cell maturation, with the acquisition of mature glial and neural features and neuronal-like excitability19, 47–54. Bone marrow MSC may be induced to differentiate into neurones by co-culturing them with Schwann cells55. In addition, even more mature neural or astroglial morphology may be obtained by co-culturing neural-primed MSC with astrocytes5, 19, 47–55 or Schwann cells37.
Figure 1.
MSC multilineage differentiation in vitro following culture with specific media. Alkaline phosphatase, Oil-red-O, Type II collagen, and Myosin: staining for osteocyte, adipocyte, condrocyte, and myocyte differentiation, respectively.
Immunophenotype
So far, there are still no specific markers for recognising MSC. MSC may be identified by the lack of expression of haematopoietic (i.e. CD45 and CD34) and endothelial (CD31/PECAM-1) markers, as well as by the expression of combinations of surface molecules such as CD105 (SH2 or endoglin), CD73 (SH3 and SH4), CD106 (VCAM-1), CD44 (hyaluronic acid receptor), CD90 (Thy 1.1), CD29, STRO-1, CD54 (ICAM-1), CD13, CD47, CD146, CD49a, CD164, and CD1662–5, 16–18, 23, 24, 37, 38, 44, 45, 28–35, 56–59. Many other markers may be expressed by MSC, e.g. adhesion molecules, chemokines, cytokine receptors (even of epithelial origin), such as epidermal growth factor receptor (EGFR or HER-1)24, and molecules involved in immune responses (MHC class I and II, CD119/interferon-g-receptor)56, 57. Human MSC expanded in vitro from the bone marrow of patients with haematological neoplasms may heterogeneously express some molecules, such as CD105, CD90, CD184 and HLA-DR, and this feature inversely correlates with bone marrow angiogenesis58. Consequently, it is still difficult to compare precisely the phenotypic pattern of MSC expanded in vitro with that really expressed in vivo in the tissues. Only in vitro and in vivo functional studies in animals may aid the assessment of the MSC nature of these cells.
Immune regulation
MSC possess strong immune regulatory properties that are present in different animal species, although with variable and only partially clarified mechanisms. MSC may suppress immune reactions in vitro and in vivo in a major histocompatibility complex (MHC)-independent manner56, 57, 60.
They inhibit T-cell proliferation in response to polyclonal, non-specific stimuli61, but in a mouse model they can also inhibit antigen-specific immune responses, mediated through both naïve and memory T cells, in a dose-dependent fashion and strictly associated with cell-cell contact60.
The inhibitory properties of MSC affect practically all kinds of immune effector, including CD4+ and CD8+ T cells56, 57, 60–65, B cells56, 66, NK cells56, 67, 68, and monocyte-derived dendritic cells69–72. The MSC interaction determines lymphocyte62 and dendritic cell73 anergy due to early proliferation arrest. Immune regulatory effects are expressed not only by MSC, but also by differentiated cells such as fibroblasts, adipocytes, and osteoblasts61, 74.
In vivo, MSC prolong the survival of MHC-incompatible skin transplants in baboons63; in humans they lower the risk of graft-versus-host disease (GvHD) when transplanted together with haematopoietic stem cells75; they cure the symptoms of grade IV GvHD, refractory to immunosuppressive therapy76; and, in mice, they improve the clinical features of experimentally induced autoimmune encephalomyelitis77.
Various mechanisms are involved in MSC immune regulatory properties, including the release of soluble factors and cell-cell contact56, 57, 60–72. Unlike in the mouse model60, in humans the inhibitory effect of MSC persists even in the absence of cell-cell contact56, 65, 78, 79. Among various soluble factors, transforming growth factor-b1, hepatocyte growth factor61, 67, prostaglandin E2, vascular endothelial growth factor67, 72, and indoleamine 2,3-dioxygenase38, 56, 64 have been shown to play a role in MSC-mediated immune regulation. Even interferon-gamma, which is a main activation molecule for immune responses, induces MSC immune regulatory effects towards CD4+ and CD8+ T cells, NK cells, and B cells56.
The expansion of CD4+CD25+ (Foxp3+) regulatory T cells in the target cell population has been shown by some Authors72, although this evidence is still controversial56, 60. The existence of many different mechanisms demonstrates a redundancy of the inhibitory function of MSC, suggesting its relevance also in vivo.
Mesenchymal stem cells for clinical use
MSC for clinical use must be collected and expanded ex vivo in dedicated facilities, with filtered laminar flow of environmental air and controlled access (‘stem cell factory’), in compliance with Good Manufacturing Practice (GMP) rules, which are normally used for industrial production of intravenously-administered drugs. These rules are absolute sterility, specific reagents without autologous proteins and growth factors not authorised for clinical use, and numerous microbiological, virological, immunological, immunophenotypic and functional quality controls to guarantee that the cell product that will be used in vivo is safe, qualitatively corresponds to the requirements imposed by law, and possibly effective. Each phase of the ex vivo cell production must be standardised and traceable, from sample collection (bone marrow, adipose tissue, cord blood, etc.), to cell seeding and culture (even by using closed culture systems to prevent any kind of contamination), to adherent cell splitting, harvest, qualitative characterisation, cryopreservation, and in vivo inoculation80. Obviously, the place where cell production is carried out is pivotal. In the facilities dedicated to cell manipulation the ‘class cascade’, i.e. the presence of different areas compartimentalised according to the GMP rules, is fundamental: the laboratories must have a very low air contamination by particles (class B), contain sterile woods with virtually no particle air contamination (class A, suitable for cell manipulation), and an access filter-zone confined in class B, where the wearing of disposable clothes and access are controlled. Access to the laboratories is obtained through confined areas with higher particle air contamination (class C), which are reached through a wearing room (class D), which, in turn, is connected to the external part of the ‘stem cell factory’ and has similar particle air contamination. Thus, there is always a one-way access to the laboratories for cell manipulation, from the areas with higher particle air contamination to the virtually sterile areas; in addition, disposable clothes and accurate disinfection are used to prevent any risk to the cell product. Particle contamination below the maximum values approved for each area is achieved through the maintenance of air pressure gradients (about 15 Pa) between the highest and the lowest class area, and through specific systems of air filtering, recycling, and vertical fluxes (for more details see: European cGMP - Annex 1: Manufacture of Sterile Medicinal Products).
Regenerative medicine
Bone regeneration
MSC have been used in several animal models to repair major segmental bone defects81, 82. In a mouse model of osteogenesis imperfecta, a congenital disease of mesenchymal tissues characterised by defective bone formation, bone marrow MSC were infused into irradiated mice, with formation of normally functioning bone and cartilage tissues deriving from the transplanted cells83. Three months after their infusion into children with osteogenesis imperfecta, MSC caused an increase of the osteoblastic component, formation of new laminar bone, a general improvement in the total mineral content, reduction in the frequency of pathological fractures, and measurable body growth84.
MSC seeding onto natural or synthetic biomaterials represents the most effective way to induce regeneration and repair of bone, cartilage or tendon tissues85–87. In particular, non-porous, biologically inert materials, such as ceramic and titanium, have been replaced by porous biomaterials, which are reabsorbable and osteoconductive, such as hydroxyapatite and tricalcium phosphate88, 89. Some biodegradable polymers, such as poly-L-lactide (PLA) and poly-L-lactide-co-glycolide (PLGA)90 are also effective. This approach has been successfully used in vivo for the resolution of critical segmental bone defects in which spontaneous local regeneration does not occur and which are unresponsive to the implantation of osteoconductive devices alone91. Local implantation of porous biomaterials covered with autologous bone marrow MSC represents the most effective approach to repairing bone defects92, such as avulsed phalanx93 and wide mandibular defects94.
Cartilage regeneration
Up to a few years ago, the only approach to cure joint cartilage defects consisted in the local injection of autologous, in vitro-expanded, chondrocyte suspensions, described for the first time in 199495. More recently, bone marrow MSC have been used in vivo to repair partial or complete cartilage or meniscus defects in animal models, exploiting several types of biomatrices, especially hyaluronate, as the support96–100. In these animal models there has been evidence of meniscus regeneration, reduction in subchondral bone remodelling, less joint cartilage degeneration, and reduced formation of osteophytes as compared with controls treated with hyaluronate only; all these effects were produced without signs of inflammation, thus confirming, in vivo, the immune regulatory effect of MSC99. Similar results have been obtained using autologous MSC seeded in a gelatinous matrix of type I collagen or hyaluronate and calcium phosphate, and applied to major osteochondral defects of the knee joint100, 101.
Other types of matrix, based on synthetic polymers such as PLA and PLGA102, or the addition of factors such as recombinant human bone morphogenetic protein-2103, 104, improve the effectiveness of treatment with MSC. The combined approach of MSC, bioactive matrices, and osteoconductive growth factors is most effective for treating joint cartilage defects103, 104. Autologous bone marrow MSC have also been used for the treatment of patients with osteoarthritis, exploiting the immune regulatory effect of these cells: arthroscopic and histological improvements have been recorded, although a significant clinical recovery, as compared with controls, has not been observed105.
Regeneration of tendon, skeletal muscle, and myocardium
The use of MSC to induce tendon repair has been investigated in animal models and humans102, 106. Autologous MSC, dispersed in type I collagen gel, can produce about 20% recovery of tendon functions, although in a dose-independent way and with heterotopic bone formation in about 30% of cases107. A similar approach led to a 37% improvement of the biomechanicaal properties, tissue architecture and functions of Achilles’ tendons as compared to those of normal controls at 12 months after transplantation108. Some exogenous growth/differentiation factors (GDF), such as GDF-5, GDF-6 and GDF-7, further improve such results109, as does the use of biomaterials based on PLGA instead of collagen gel110. Mechanical stimulation of fibres improves the repair mechanisms111.
MSC have been used to restore the structure and functions of skeletal muscles, in cases of muscle dystrophy or other congenital myopathies. The inoculation of human adult MSC into mdx mice (an animal model of Duchenne’s muscle dystrophy) led to the formation of myofibres and long term-acting satellite cells, the restoration of dystrophin expression in the sarcolemma and the production of several muscle growth factors112, even by using human bone marrow MSC with the entire sequence of dystrophin113. These effects are potentially useful in human Duchenne’s muscle dystrophy, but so far there is no clear evidence of de novo muscle regeneration and clinical improvement mediated by MSC.
Several studies have shown that MSC have a cardiomyogenic potential after myocardial infarction114–118. In a randomised clinical study carried out in 69 patients and based on the intracoronary infusion of autologous bone marrow MSC, left ventricular perfusion and heart contractile function improved remarkably after 3 months119. However, there was very little formation of new cardiomyocytes derived from the transplanted MSC120: it is, therefore, believed that the observed cardiac functional improvement observed is due to other mechanisms, such as the release of soluble trophic factors with a paracrine effect and the stimulation of residual cardiac stem cells121.
Neural tissue regeneration
Systemically infused bone marrow MSC colonise virtually all organs, where these cells survive only in the presence of local proliferation122, 123. MSC do not normally seem to pass through the blood-brain barrier: they can survive, migrate, and differentiate into neural-glial cells after in utero intraventricular injection inside foetal rat brains51. Functional recovery has been shown following in vivo transplantation of these cells inside the lesion in animal models of Parkinson’s disease, hypoxic-ischaemic neural damage and retinal injury124. So far, however, there have been no significant clinical studies unequivocally showing that MSC possess neural regenerative activity in humans.
Gene therapy
MSC may be engineered with genes coding for molecules that are missing in genetic or acquired defects or with therapeutic activity, such as erythropoietin, insulin or coagulation factors; however, preliminary results need to be confirmed with in vivo studies to assess whether the correction of the deficiency is long-lasting5, 125.
Immune-modulatory therapy
Acute graft-versus-host disease
MSC can inhibit the immune responses against minor histocompatibility antigens such as HY60, 82, they can prevent the occurrence of GvHD if co-transplanted with haaematopoietic stem cells75, and they can completely modulate grade IV GvHD refractory to immunosuppressive drugs76. Similar results have been obtained with adipose tissue-derived MSC126. On this background, various clinical trials with autologous or allogeneic MSC are currently in progress, evaluating the effect of these cells on preventing GvHD in MHC-unrelated transplants and treating severe acute GvHD, which is associated with a high mortality due to infectious complications, especially if intestinal mucosa is involved. These trials are based on the collection and expansion of MSC obtained from the same donor of the haematopoietic stem cells.
Autoimmunity
Allogeneic bone marrow MSC may inhibit T- and B-cell proliferation and functions in the BXSB mouse, which is an animal model of human systemic erythematous lupus127. MSC-based therapeutic approaches for collagen disorders refractory to conventional immunosuppressive agents are currently under examination105, 128, 129. On the other hand, MSC infusion is clearly associated with a lower incidence and improved clinical features in experimental autoimmune encephalomyelitis, an animal model of human multiple sclerosis77, similarly to what can be achieved with neural stem cells130. Transplantation of MSC could play an important role in inflammatory diseases of the central nervous system, especially if they were able to migrate through the blood-brain barrier, thus coupling their regenerative potential and immune regulatory effects77, 131.
Anti-cancer cell therapy
It has been shown that MSC may support and amplify the proliferation of solid tumours both in vitro and in vivo, by favouring cancer dissemination and proliferating inside the tumour as fibroblasts of the vascular-stromal axis38, 132, 133. This property must always be considered when large numbers of MSC are infused systemically, even for regenerative purposes. However, MSC transfection with genes coding for molecules with antiproliferative activity, such as interferon-beta, not only inhibits neoplastic growth in vitro, but also lowers cancer development in vivo132. Similar results have been obtained with gliomas134. Therefore, cell therapy with MSC engineered to produce anti-proliferative molecules could be an efficient strategy for specific anti-cancer treatments with few side effects.
Conclusions
Since the end of the 1990s a large amount of data concerning MSC biology and differentiation/immune regulatory potential has been published, even though many of these data still remain contradictory. MSC have some advantages in terms of availability, expandability, transplantability, and capability of immune regulation, without the ethical implications associated with the use of embryonic stem cells. Pre-clinical studies in animals have shown that a therapeutic approach involving MSC is feasible in different fields of tissue regenerative medicine and immune-modulating cell therapy, although many potential clinical applications remain to be confirmed.
References
- 1.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hemopoietic organs. Exp Hematol. 1976;4:267–74. [PubMed] [Google Scholar]
- 2.Prockop DJ. Marrow stromal cells as stem cells for nonhemopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Smith JR, Pochampally R, Perry A, et al. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 2004;22:823–31. doi: 10.1634/stemcells.22-5-823. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 6.Castro-Malaspina H, Gay Re, Resnick G, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. [PubMed] [Google Scholar]
- 7.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 8.Colter DC, Class R, DiGirolamo CM, et al. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. PNAS. 2000;97:3212–8. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva WA, Jr, Covas DT, Panepucci RA, et al. The profile of gene expression of human marrow mesenchymal stem cells. Stem Cells. 2003;21:661–9. doi: 10.1634/stemcells.21-6-661. [DOI] [PubMed] [Google Scholar]
- 10.Tremain N, Korkko J, Ibberson D, et al. MicroSAGE analysis of 2,353 expressed genes in a single cell-derived colony of undifferentiated human mesenchymal stem cells reveals mRNAs of multiple cell lineages. Stem Cells. 2001;19:408–18. doi: 10.1634/stemcells.19-5-408. [DOI] [PubMed] [Google Scholar]
- 11.Bleul CC, Fuhlbrigge RC, Casasnovas JM, et al. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–9. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadereit S, Deeds LS, Haynesworth SE, et al. Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34+/CD38− early progenitors cultured over human MSCs as a feeder layer. Stem Cells. 2002;20:573–82. doi: 10.1634/stemcells.20-6-573. [DOI] [PubMed] [Google Scholar]
- 13.Banfi A, Bianchi G, Galotto M, et al. Bone marrow stromal damage after chemo/radiotherapy: occurrence, consequences, and possibilities of treatment. Leuk Lymphoma. 2001;42:863–70. doi: 10.3109/10428190109097705. [DOI] [PubMed] [Google Scholar]
- 14.Carlo-Stella C, Tabilio A, Regazzi E, et al. Effect of chemotherapy for acute myelogeneous leucemia on hemopoietic and fibroblast marrow progenitors. Bone Marrow Transplant. 1997;20:465–71. doi: 10.1038/sj.bmt.1700916. [DOI] [PubMed] [Google Scholar]
- 15.Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–16. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 16.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32:414–25. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Dennis JE, Charbord P. Origin and differentiation of human and murine stroma. Stem Cells. 2002;20:205–14. doi: 10.1634/stemcells.20-3-205. [DOI] [PubMed] [Google Scholar]
- 18.Reyes M, Lund T, Lenvik T, et al. Purification and ex-vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–25. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 19.Woodbury D, Schwarz EJ, Prockop DJ, et al. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–70. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Sato Y, Araki H, Kato J, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–63. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 21.Kotton DN, Ma BY, Cardoso WV, et al. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–8. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 22.Timper K, Seboek D, Eberhardt M, et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135–40. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 23.Horwitz EM, Le Blanc K, Dominici M, et al. The International Society for Cellular Therapy. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–5. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 24.Krampera M, Pasini A, Rigo A, et al. HB-EGF/HER-1 signalling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multi-lineage differentiation. Blood. 2005;106:59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]
- 25.Roufosse CA, Direkze NC, Otto WR, et al. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36:585–97. doi: 10.1016/j.biocel.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Bianchi DW. Fetal cells in the mother: from genetic diagnosis to diseases associated with fetal cell microchimerism. Eur J Obstet Gynecol Reprod Biol. 2000;92:103–8. doi: 10.1016/s0301-2115(00)00432-2. [DOI] [PubMed] [Google Scholar]
- 27.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 28.Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–24. doi: 10.1159/000080341. [DOI] [PubMed] [Google Scholar]
- 29.Katz AJ, Tholpady A, Tholpady SS, et al. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23:412–23. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 30.Shih DT, Lee DC, Chen SC, et al. Isolation and characterization of neurogenic mesenchymal stem cells in human scalp tissue. Stem Cells. 2005;23:1012–20. doi: 10.1634/stemcells.2004-0125. [DOI] [PubMed] [Google Scholar]
- 31.Trubiani O, Di Primio R, Traini T, et al. Morphological and cytofluorimetric analysis of adult mesenchymal stem cells expanded ex vivo from periodontal ligament. Int J Immunopathol Pharmacol. 2005;18:213–21. doi: 10.1177/039463200501800204. [DOI] [PubMed] [Google Scholar]
- 32.Erices A, Conget P, Minguel JJ. Mesenchymal progenitor cells in human umbelical cord blood. Br J Haematol. 2000;109:235–42. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 33.Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396–402. doi: 10.1182/blood.v98.8.2396. [DOI] [PubMed] [Google Scholar]
- 34.Panepucci RA, Siufi JL, Silva WA, Jr, et al. Comparison of gene expression of umbilical cord vein and bone marrow-derived mesenchymal stem cells. Stem Cells. 2004;22:1263–78. doi: 10.1634/stemcells.2004-0024. [DOI] [PubMed] [Google Scholar]
- 35.In’t Anker PS, Scherjon SA, Kleijburg-van der Keur C, et al. Isolation of mesenchymal stem cells of fetal and maternal origin from human placenta. Stem Cells. 2004;22:1338–45. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 36.Ame-Thomas P, Maby-El Hajjami H, Monvoisin C, et al. Human mesenchymal stem cells isolated from bone marrow and lymphoid organs support tumor B-cell growth: role of stromal cells in follicular lymphoma pathogenesis. Blood. 2007;109:693–702. doi: 10.1182/blood-2006-05-020800. [DOI] [PubMed] [Google Scholar]
- 37.Krampera M, Marconi S, Pasini A, et al. Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone. 2007;40:382–90. doi: 10.1016/j.bone.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Krampera M, Sartoris S, Cosmi L, et al. Immune regulation by mesenchymal stem cells derived from adult spleen and thymus. Stem Cells Develop. 2007 doi: 10.1089/scd.2007.0024. in press. [DOI] [PubMed] [Google Scholar]
- 39.Meirelles LdS, et al. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 40.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 41.Bieback K, Kern S, Kluter H, et al. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625–34. doi: 10.1634/stemcells.22-4-625. [DOI] [PubMed] [Google Scholar]
- 42.Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells - basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–27. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 43.Cetrulo CL., Jr Cord-blood mesenchymal stem cells and tissue engineering. Stem Cell Rev. 2006;2:163–8. doi: 10.1007/s12015-006-0023-x. [DOI] [PubMed] [Google Scholar]
- 44.Majumdar MK, Thiede MA, Mosca JD. Phenotipic and functional comparison of cultures of bone marrow-derived mesenchymal stem cells (MSC) and stromal cells. J Cell Physiol. 1998;166:585–92. doi: 10.1002/(SICI)1097-4652(199807)176:1<57::AID-JCP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 45.Zohar R, Sodek J, McCulloch CAG. Characterization of stromal progenitor cells enriched by flow cytometry. Blood. 1997;90:3471–81. [PubMed] [Google Scholar]
- 46.Kadivar M, Khatami S, Mortazavi Y, et al. In vitro cardiomyogenic potential of human vein-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2006;340:639–47. doi: 10.1016/j.bbrc.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 47.Tondreau T, Lagneaux L, Dejeneffe M, et al. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72:319–26. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- 48.Brazelton TR, Rossi FM, Keshet GI, et al. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–9. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 49.Mezey E, Chandross KJ, Harta G, et al. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–82. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 50.Bonilla S, Silva A, Valdes L, et al. Functional neural stem cells derived from adult bone marrow. Neuroscience. 2005;133:85–5. doi: 10.1016/j.neuroscience.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 51.Munoz-Elias G, Woodbury D, Black IB. Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor functions. Stem Cells. 2003;21:437–48. doi: 10.1634/stemcells.21-4-437. [DOI] [PubMed] [Google Scholar]
- 52.Long X, Olszewski M, Huang W, et al. Neural cell differentiation in vitro from adult human bone marrow mesenchymal stem cells. Stem Cells Dev. 2005;14:65–9. doi: 10.1089/scd.2005.14.65. [DOI] [PubMed] [Google Scholar]
- 53.Jori FP, Napolitano MA, Melone MA, et al. Molecular pathways involved in neural in vitro differentiation of marrow stromal stem cells. J Cell Biochem. 2005;94:645–55. doi: 10.1002/jcb.20315. [DOI] [PubMed] [Google Scholar]
- 54.Wislet-Gendebien S, Hans G, Leprince P, et al. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23:392–402. doi: 10.1634/stemcells.2004-0149. [DOI] [PubMed] [Google Scholar]
- 55.Zurita M, Vaquero J, Oya S, et al. Schwann cells induce neuronal differentiation of bone marrow stromal cells. Neuroreport. 2005;16:505–8. doi: 10.1097/00001756-200504040-00017. [DOI] [PubMed] [Google Scholar]
- 56.Krampera M, Cosmi L, Angeli R, et al. Role of the IFN-γ in the immunomodulatory activity of human mesenchymal stem cells. Stem cells. 2005;24:386–98. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 57.Le Blanc K, Tammik L, Sundberg B, et al. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 58.Campioni D, Moretti S, Ferrari L, et al. Immunophenotypic heterogeneity of bone marrow-derived mesenchymal stromal cells from patients with hematological disorders: correlation with bone marrow microenviroment. Haematologica. 2006;91:364–8. [PubMed] [Google Scholar]
- 59.Bianco P, Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–8. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naïve and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 61.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 62.Glennie S, Soeiro I, Dyson PJ, et al. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–7. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 63.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 64.Meisel R, Zibert A, Laryea M, et al. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 65.Rasmusson I, Ringden O, Sundberg B, et al. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–13. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 66.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 67.Sotiropoulou PA, Perez SA, Gritzapis AD, et al. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 68.Spaggiari GM, Capobianco A, Becchetti S, et al. Mesenchymal stem cell (MSC)/natural killer (NK) cell interactions: evidence that activated NK cells are capable of killing MSC while MSC can inhibit IL-2-induced NK cell proliferation. Blood. 2006;107:1484–90. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 69.Zhang W, Ge W, Li C, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263–71. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 70.Jiang XX, Zhang Y, Liu B, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–6. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 71.Beyth S, Borovsky Z, Mevorach D, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–9. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 72.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2004;105:1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 73.Ramasamy K, Fazekasova H, Lam EW, et al. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–6. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 74.Gotherstrom C, Ringden O, Tammik C, et al. Immunological properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239–45. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 75.Ringden O, Labopin M, Bacigalupo A, et al. Transplantation of peripheral blood stem cells as compared with bone marrow from HLA-identical siblings in adult patients with acute myeloid leukemia and acute lymphoblastic leukemia. J Clin Oncol. 2002;20:4655–64. doi: 10.1200/JCO.2002.12.049. [DOI] [PubMed] [Google Scholar]
- 76.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. The Lancet. 2004;363:1439–41. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 77.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T cell anergy. Blood. 2005;106:1755–61. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 78.Tse WT, Pendleton JD, Beyer WM, et al. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in trasplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 79.Rasmusson I, Ringden O, Sundberg B, et al. Mesenchymal stem cell inhibit lymphocytes proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 80.European Commission – Health & Consumer Protection Directorate-General. Technical requirements for the coding, processing, preservation, storage, and distribution of human tissues and cells. Directive 2004/23/EC [Google Scholar]
- 81.Kon E, Muraglia A, Corsi A, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49:328–37. doi: 10.1002/(sici)1097-4636(20000305)49:3<328::aid-jbm5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 82.Petite H, Viateau V, Bensaid W, et al. Tissue-engineered bone regeneration. Nat Biotechnol. 2000;18:959–63. doi: 10.1038/79449. [DOI] [PubMed] [Google Scholar]
- 83.Pereira RF, Halford KW, O’Hara MD, et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. PNAS. 1995;92:4857–61. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Horwitz EM, Prockop DJ, Fitzpatrick LA, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–13. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 85.Kadiyala S, Young RG, Thiede MA, et al. Culture expanded canine mesenchymal stem cells possess osteochondrogenic potential in vivo and in vitro. Cell Transplant. 1997;6:125–34. doi: 10.1177/096368979700600206. [DOI] [PubMed] [Google Scholar]
- 86.Richards M, Huibregtse BA, Caplan AI, et al. Marrow-derived progenitor cell injections enhance new bone formation during distraction. J Orthop Res. 1999;17:900–8. doi: 10.1002/jor.1100170615. [DOI] [PubMed] [Google Scholar]
- 87.Cancedda R, Dozin B, Giannoni P, et al. Tissue engineering and cell therapy of cartilage and bone. Matrix Biol. 2003;22:81–91. doi: 10.1016/s0945-053x(03)00012-x. [DOI] [PubMed] [Google Scholar]
- 88.Rose FR, Oreffo RO. Bone tissue engineering: hope vs hype. Biochem Biophys Res Commun. 2002;292:1–7. doi: 10.1006/bbrc.2002.6519. [DOI] [PubMed] [Google Scholar]
- 89.Vats A, Tolley NS, Polak JM, et al. Scaffolds and biomaterials for tissue engineering: a review of clinical applications. Clin Otolaringol. 2003;28:165–72. doi: 10.1046/j.1365-2273.2003.00686.x. [DOI] [PubMed] [Google Scholar]
- 90.El-Amin SF, Attawia M, Lu HH, et al. Integrin expression by human osteoblasts cultured on degradable polymeric materials applicable for tissue engineered bone. J Orthop Res. 2002;20:20–8. doi: 10.1016/S0736-0266(01)00062-6. [DOI] [PubMed] [Google Scholar]
- 91.Bruder SP, Kurth AA, Shea M, et al. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155–62. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 92.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–6. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 93.Vacanti CA, Bonassar LJ, Vacanti MP, Shufflebarger J. Replacement of an avulsed phalanx with tissue-engineered bone. N Engl J Med. 2001;344:1511–4. doi: 10.1056/NEJM200105173442004. [DOI] [PubMed] [Google Scholar]
- 94.Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364:766–70. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 95.Brittberg M, Lindahl A, Nillson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 96.Jorgensen C, Noel D, Apparailly F, et al. Stem cells for repair of cartilage and bone: the next challenge in osteoarthritis and rheumatoid arthritis. Ann Rheum Dis. 2001;60:305–9. doi: 10.1136/ard.60.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schultz O, Sittinger M, Haeupl T, et al. Emerging strategies of bone and joint repair. Arthritis Res. 2000;2:433–6. doi: 10.1186/ar123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caplan AI, Elyaderani M, Mochizuki Y, et al. Principles of cartilage repair and regeneration. Clin Orthop. 1997;342:254–69. [PubMed] [Google Scholar]
- 99.Murphy JM, Kavalkovitch KW, Fink D, et al. Regeneration of meniscal tissue and protection of articulat cartilage by injection of mesenchymal stem cells. Osteoarthritis Cartilage. 2000;8(suppl B):S25. [Google Scholar]
- 100.Wakitani S, Goto T, Pineda SJ, et al. Mesenchymal cell-based repair of large, full thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–92. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 101.Gao J, Dennis JE, Solchaga LA, et al. Repair of osteochondral defect with tissue engineered two-phases composite material of injectable calcium phosphate and hyaluronan derivative. Tissue Engineering. 2002;8:827–37. doi: 10.1089/10763270260424187. [DOI] [PubMed] [Google Scholar]
- 102.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cell and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–5. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beuningen HM, van Glansbeek HL, Kraan PM, van der, et al. Differential effects of local application BMP-2 or TGF-(beta) on both articular cartilage composition and osteophyte formation. Osteoarthritis Cartilage. 1998;6:306–17. doi: 10.1053/joca.1998.0129. [DOI] [PubMed] [Google Scholar]
- 104.Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–52. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 105.Wakitani S, Imoto K, Yamamoto T, et al. Human autologous culture expanded bone marrow mesenhymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199–206. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 106.Awad HA, Butler DL, Boivin GP, et al. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 1999;5:267–77. doi: 10.1089/ten.1999.5.267. [DOI] [PubMed] [Google Scholar]
- 107.Awad HA, Boivin GP, Dressler MR, et al. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003;21:420–31. doi: 10.1016/S0736-0266(02)00163-8. [DOI] [PubMed] [Google Scholar]
- 108.Young RG, Butler DL, Weber W, et al. Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res. 1998;16:406–13. doi: 10.1002/jor.1100160403. [DOI] [PubMed] [Google Scholar]
- 109.Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–30. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ouyang HW, Goh JC, Mo XM, et al. The efficacy of bone marrow stromal cell seeded knitted PLGA fiber scaffold for Achilles tendon repair. Ann N Y Acad Sci. 2002;961:126–9. doi: 10.1111/j.1749-6632.2002.tb03064.x. [DOI] [PubMed] [Google Scholar]
- 111.Juncosa-Melvin N, Boivin GP, Galloway MT, et al. Effects of cell to collagen ratio in mesenchymal stem cell-seeded. Implants on tendon repair biomechanics and histology. Tissue Eng. 2005;11:448–57. doi: 10.1089/ten.2005.11.448. [DOI] [PubMed] [Google Scholar]
- 112.De Bari C, Dell’Accio F, Vandenabeele F, et al. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160:909–18. doi: 10.1083/jcb.200212064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Goncalves MA, de Vries AA, Holkers M, et al. Human mesenchymal stem cells ectopically expressing full-length dystrophin can complement Duchenne muscular dystrophy myotubes by cell fusion. Hum Mol Genet. 2006;15(2):213–21. doi: 10.1093/hmg/ddi438. [DOI] [PubMed] [Google Scholar]
- 114.Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–30. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 115.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 116.Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 117.Mathur A, Martin JF. Stem cells and repair of the heart. Lancet. 2004;364:183–92. doi: 10.1016/S0140-6736(04)16632-4. [DOI] [PubMed] [Google Scholar]
- 118.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 119.Chen SL, Fang WW, Ye F, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–5. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 120.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–65. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 121.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat Clin Pract Cardiovas Med. 2007;4:S21–S26. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 122.Devine SM, Bartholomew AM, Mahmud N, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29:244–55. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 123.Devine SM, Cobbs J, Jennings M, et al. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 124.Corti S, Locatelli F, Strazzer S, et al. Neuronal generation from somatic stem cells: current knowledge and perspectives on the treatment of acquired and degenerative central nervous system disorders. Curr Gene Ther. 2003;3:247–72. doi: 10.2174/1566523034578375. [DOI] [PubMed] [Google Scholar]
- 125.Bartholomew A, Patil S, Mackay A, et al. Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo. Hum Gene Ther. 2001;12:1527–41. doi: 10.1089/10430340152480258. [DOI] [PubMed] [Google Scholar]
- 126.Yanez R, Lamina ML, Garcia Castro J, et al. Adipose tissue derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–91. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 127.Deng W, Han Q, Liao L, et al. Effects of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA Cell Biol. 2005;24:458–63. doi: 10.1089/dna.2005.24.458. [DOI] [PubMed] [Google Scholar]
- 128.El-Badri NS, Maheshwari A, Sanberg PR. Mesenchymal stem cells in autoimmune disease. Stem Cells Dev. 2004;13:463–72. doi: 10.1089/scd.2004.13.463. [DOI] [PubMed] [Google Scholar]
- 129.Jorgensen C, Djouad F, Fritz V, et al. Mesenchymal stem cells and rheumatoid arthritis. Joint Bone Spine. 2003;70:483–5. doi: 10.1016/j.jbspin.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 130.Pluchino S, Quattrini A, Brambilla E, et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–94. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- 131.Uccelli A, Zappia E, Benvenuto F, et al. Stem cells in inflammatory demyelinating disorders: a dual role for immunosuppression and neuroprotection. Expert Opin Biol Ther. 2006;6:17–22. doi: 10.1517/14712598.6.1.17. [DOI] [PubMed] [Google Scholar]
- 132.Studeny M, Marini FC, Champlin RE, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-β delivery into tumours. Cancer Res. 2002;62:3603–8. [PubMed] [Google Scholar]
- 133.Ramasamy R, Lam EW-F, Soeiro I, et al. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2006;21:304–10. doi: 10.1038/sj.leu.2404489. [DOI] [PubMed] [Google Scholar]
- 134.Nakamizo A, Marini F, Amano T, et al. Human Bone Marrow-Derived Mesenchymal Stem Cells in the Treatment of Gliomas. Cancer Res. 2005;65:3307–18. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]