Abstract
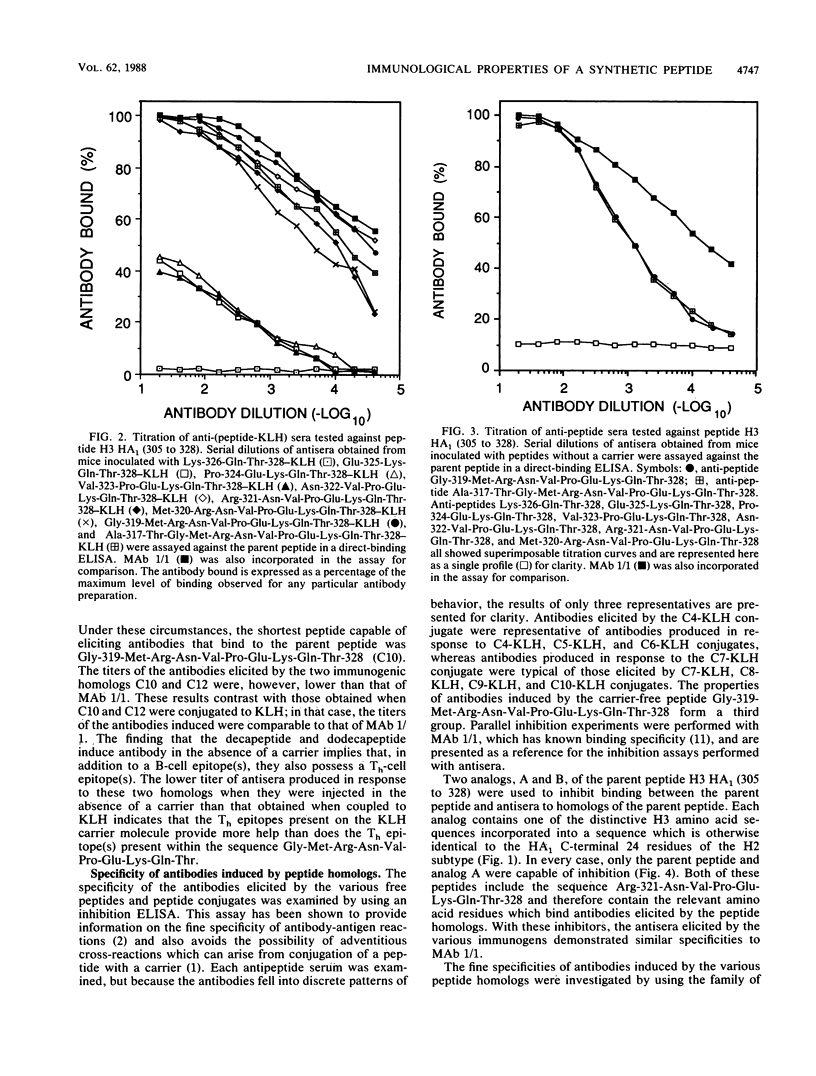
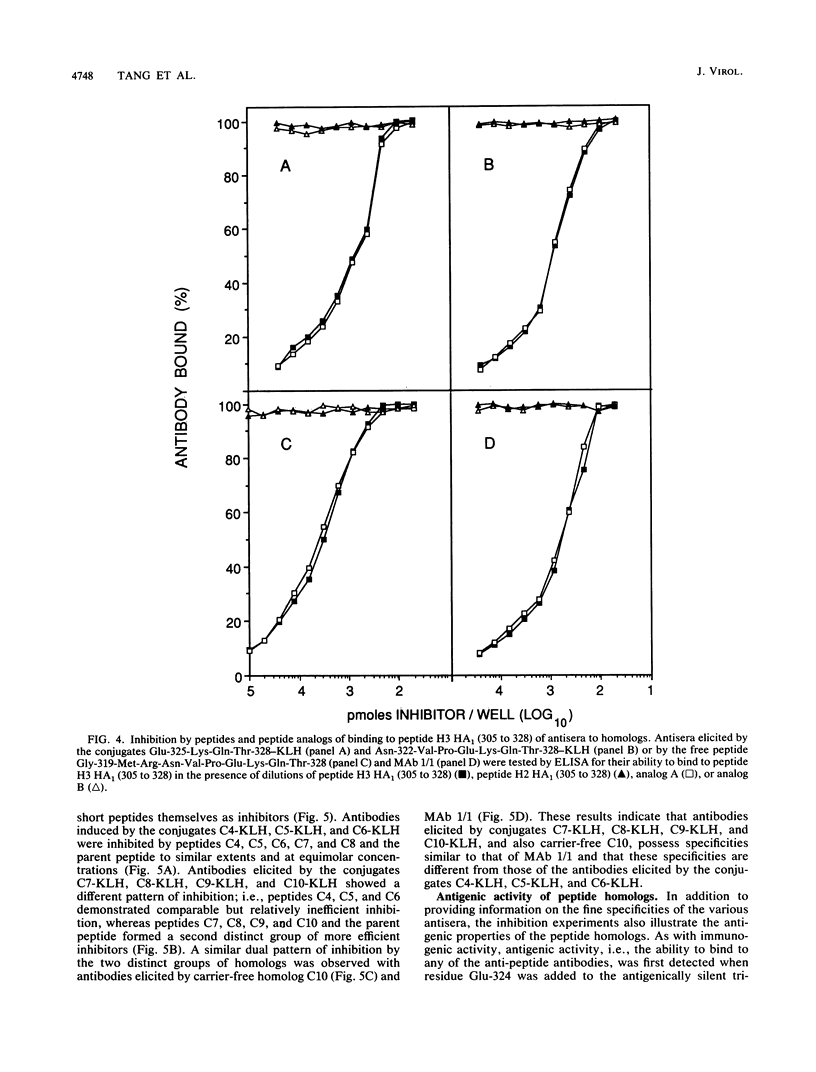
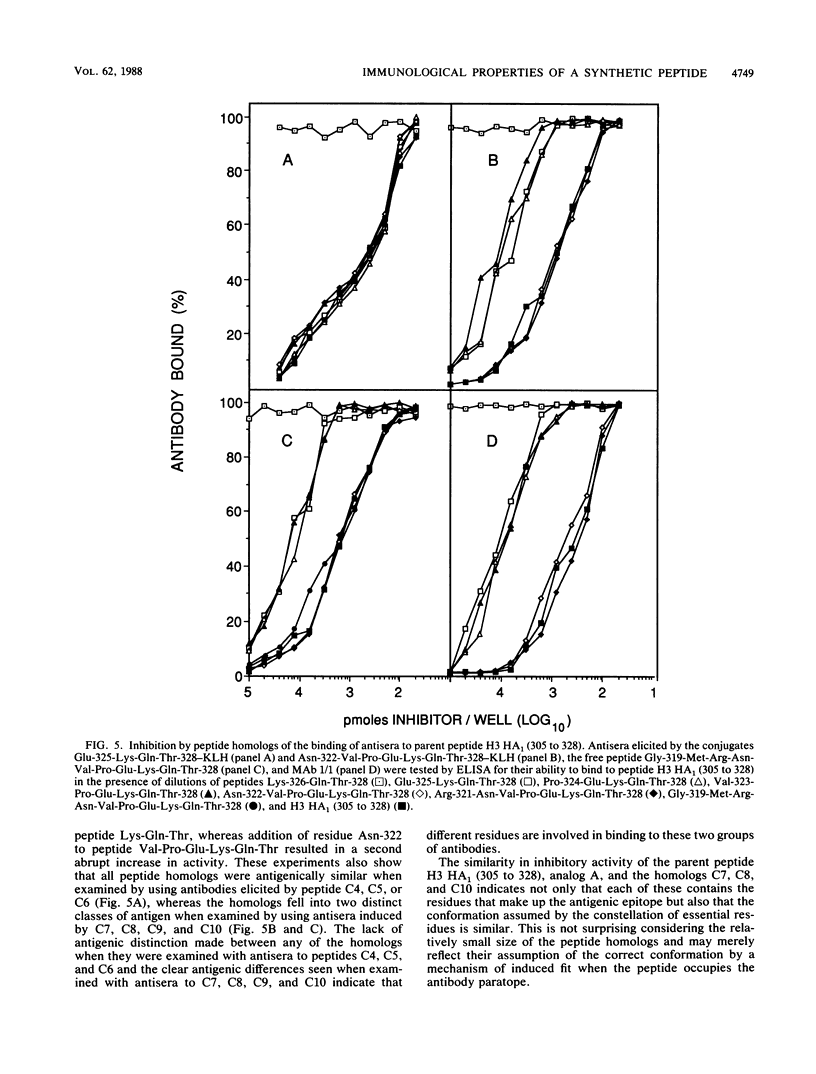
Synthetic peptides of increasing length and corresponding in sequence to the C-terminal end of the HA1 molecule of influenza virus were constructed and examined for their immunogenic and antigenic properties. Peptides containing at least the four C-terminal amino acids, when coupled to keyhole limpet hemocyanin, were capable of eliciting antibody in BALB/c mice that bound to the 24-residue parent peptide H3 HA1 (305 to 328). In the absence of a carrier, the C-terminal decapeptide was the shortest peptide capable of eliciting antibody. The specificity of this antibody was indistinguishable from that of a monoclonal antibody to the parent peptide which recognizes an epitope encompassed by the C-terminal seven residues. All peptides containing at least the C-terminal four residues were able to inhibit completely the binding of this monoclonal antibody to the parent peptide. Taken together, these results indicate that (i) the tetrapeptide is capable of eliciting specific antibody when coupled to a carrier, (ii) this tetrapeptide possesses all of the antigenic information necessary to occupy the paratope of a monoclonal antibody elicited by the longer parent peptide, and (iii) the decapeptide contains all of the information necessary to elicit a specific immune response and therefore carries an epitope recognized by T cells as well as one recognized by B cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briand J. P., Muller S., Van Regenmortel M. H. Synthetic peptides as antigens: pitfalls of conjugation methods. J Immunol Methods. 1985 Apr 8;78(1):59–69. doi: 10.1016/0022-1759(85)90329-1. [DOI] [PubMed] [Google Scholar]
- Brown L. E., Murray J. M., Anders E. M., Tang X. L., White D. O., Tregear G. W., Jackson D. C. Genetic control and fine specificity of the immune response to a synthetic peptide of influenza virus hemagglutinin. J Virol. 1988 May;62(5):1746–1752. doi: 10.1128/jvi.62.5.1746-1752.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. C., Poumbourios P., White D. O. Simultaneous binding of two monoclonal antibodies to epitopes separated in sequence by only three amino acid residues. Mol Immunol. 1988 May;25(5):465–471. doi: 10.1016/0161-5890(88)90166-6. [DOI] [PubMed] [Google Scholar]
- Jackson D. C., Tang X. L., Brown L. E., Murray J. M., White D. O., Tregear G. W. Antigenic determinants of influenza virus hemagglutinin. XII. the epitopes of a synthetic peptide representing the C-terminus of HA1. Virology. 1986 Dec;155(2):625–632. doi: 10.1016/0042-6822(86)90222-9. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Green N. Analysis of the antigen specificity of influenza haemagglutinin-immune human T lymphocyte clones: identification of an immunodominant region for T cells. Immunology. 1983 Dec;50(4):659–666. [PMC free article] [PubMed] [Google Scholar]
- Nestorowicz A., Tregear G. W., Southwell C. N., Martyn J., Murray J. M., White D. O., Jackson D. C. Antibodies elicited by influenza virus hemagglutinin fail to bind to synthetic peptides representing putative antigenic sites. Mol Immunol. 1985 Feb;22(2):145–154. doi: 10.1016/s0161-5890(85)80008-0. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Taylor W. R. A sequence pattern common to T cell epitopes. EMBO J. 1988 Jan;7(1):93–100. doi: 10.1002/j.1460-2075.1988.tb02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoofs P. G., Geysen H. M., Jackson D. C., Brown L. E., Tang X. L., White D. O. Epitopes of an influenza viral peptide recognized by antibody at single amino acid resolution. J Immunol. 1988 Jan 15;140(2):611–616. [PubMed] [Google Scholar]


