Abstract
Glycated polymers have already been widely employed for cell transfection studies as cell possess specific lectins. However, up to now, these glycated polymers have barely been investigated for their cell adhesive properties, save macrophages. In this work, we use polyelectrolyte multilayer films made of poly(L-lysine) and poly(L-glutamic) acid as polymeric substrates to investigate the role of sugar molecules, e.g. mannose and lactose, on the adhesion of primary cells as compared to that of a tumor cell line. The glycated polymeric films were compared to ungrafted and chemically cross-linked films, which are known to present opposite adhesive properties. A differential adhesion could be evidenced on mannose grafted films: primary chondrocytes adhere and proliferate well on these films whereas chondrosarcoma cells do not grow well. Although present, the effect of lactose on cell adhesion was much less important. This adhesion, mediated by glycated polymers, appears to be specific. These results show that it is possible to use glycated polyelectrolytes not only as non viral vectors but also as cell adhesive substrates.
Introduction
Surface modification of materials using polyelectrolyte multilayers (PEM) to improve biocompatibility and to develop adhesive or anti-adhesive coatings has become very attractive 1-3. The layer-by-layer technique was introduced approximately ten years ago by Decher and al. 4,5. It takes benefit from its simplicity and versatility to become more widely employed in biomedical applications 6-8. The control of the chemical composition of the films and of their physico-chemical properties is particularly important as they can affect their biological activity. For example, it has already been shown that surface charge, multilayer pH9, film hydrophobicity or hydrophilicity and swellability10 can affect cellular adhesion. Polysaccharide films, for example, have been shown to exhibit antimicrobial properties11 and can be used as reservoirs of bioactive molecules such as anti-tumor drugs 12. An approach for changing film properties resides in the modification of their surface chemistries. It is widely recognized that specific adhesive peptides can favor specific cell types and their receptors. The most investigated sequence is probably the RGD motif due to its natural presence in a large number of matrix proteins such as collagen and fibronectin 13. A variety of RGD containing peptides, either linear or cyclic have already been grafted to different biomaterials surfaces and tested in term of cell behavior 14,15. In the PEM films, such strategy has been employed by grafting the tripeptide motif to polyacrylic acid 16 or a 15 amino acids peptide containing the RGD motif (CGPKGDRGDAGPKGA) to poly(L-glutamic) acid 17. The positive effect of the peptide on cell adhesion was demonstrated for murine fibroblasts 16 and for primary osteoblasts 17. However, it has to be noted that film mechanical properties were also found to play a great role in cell adhesion on PEM films 18,19. It was indeed shown that film stiffness had a stronger influence on cell proliferation than did chemical modification by the adhesive peptide 17.
Beside the known adhesive peptides, another class of molecules has gained lots of attention within the past years especially in the domain of cell transfection. This class is composed of all the sugars (eg mannose, lactose, galactose….), which are common components of the lectins that are present at the cell surface and are involved in cell interactions 20. Glycated proteins are also present on the cell surface and play a great role in metastatic processes, such as cancer and inflammation 21. So far, glycated polyelectrolytes and more especially glycated cationic polymers have been mostly used as non viral vectors for cell transfection and internalization studies (like endocytosis and phagocytosis) in different cell types such as epithelial cells, liver cells, dendritic cells and macrophages 22. The role of carbohydrates as molecular determinants in self/non-self recognition has been recognized for many years and a family of proteins known as the C-type lectins is playing a great role in carbohydrate recognition within the immune system. The mannose receptor family is composed of four type I transmembrane proteins (mannose receptor, M-type phospholipase A2 receptor, DEC-205 and Endo 180) 23. Of these receptors, only the mannose receptor and Endo 180 have confirmed monosaccharide binding activity 24. Recently, Howard and Isacke have shown that Endo180 is present in vivo in cartilage and is localized in vitro on the chondrocyte surface 25.
In terms of biomaterial applications, it is important to investigate both cell lineage and primary cells adhesion. For instance, to improve the biocompatibility of implanted prostheses following a chirurgical ablation 26, it may be of interest to develop surfaces that promote the re-colonization by primary cells but not by tumor cells. Co-culture of primary cells may also be of interest for cell-cell interaction studies and tissue engineering applications 27. In addition, whereas the grafting of specific adhesive peptides such as the RGD motif has already been widely investigated 14, there are only few studies dealing with adhesive properties of carbohydrate modified surfaces. In a recent work, Donati et al. investigated the behavior of chondrocytes cultured in a lactose modified chitosan hydrogel 28 and observed that they tend to form aggregates and produce higher levels of matrix proteins. Due to the large number of glycoproteins present at the cell surface, these molecules may be an alternative way to favor cell interaction with a substrate. In addition, the cost and difficulty of grafting sugar molecules are less than that of grafting a synthetic peptide.
In this work, the aim was to evaluate whether it is possible to modulate the adhesion of primary chondrocyte and chondrosarcoma cells on polyelectrolyte multilayers by the presence of a glycated polyelectrolyte layer at the film's surface. Experiments were performed on poly(L-lysine)/poly(L-glutamic) acid (PLL/PGA) multilayers with either lactose or mannose molecules grafted to the PGA. The choice of PGA instead of PLL for the grafting relies on previous adhesion studies of (PLL/PGA) films which showed increased cell viability on PGA compared to PLL29,30. Glycated polyelectrolyte multilayers were compared to cross-linked PEM, which are also known to change cell adhesive properties 31.
Experimental
Polyelectrolyte solutions and film architectures
The preparation of poly(L-lysine) (PLL, 60kDa, Sigma), poly(L-glutamic acid) (PGA, 40 kDa, Sigma, as determined by multi-angle laser light scattering) solutions and the buildup of (PLL/PGA)6 films (where 6 corresponds to the number of layer pairs) was previously described 17. PLL and PGA were dissolved at 1 mg/mL in a Hepes-saline buffer (25mM Hepes, 0.137M NaCl) at pH=7.4. During film buildup, all the rinsing steps were performed in the Hepes-saline buffer. Four different types of films, prepared in Hepes saline buffer, were investigated: (PLL/PGA)6 (native), cross-linked (PLL/PGA)6 films (noted ∼CL), (PLL/PGA)5-PLL/PGA-mannose (noted ∼mannose), (PLL/PGA)5-PLL/PGA-lactose (noted ∼lactose). The film cross-linking was performed with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) in the presence of N-Hydroxysulfo-succinimide (sulfo-NHS) (both purchased from Sigma-Aldrich). EDC and Sulfo-NHS were dissolved in 0.15 M NaCl (pH=5) respectively at 200 mM and 50 mM while 500 μL of the EDC/NHS solution was added onto PEM-coated slides. After 18 hours at 4°C, the slides were washed with the solution containing 0.15 M NaCl.
Synthesis of the PGA-(PEG)3-β-lactose 10% conjugate and of the PGA-(PEG)3-α-mannose 10% conjugate
The synthesis of PGA-(PEG)3-β-lactose (PEG is polyethyleneglycol) was performed according to the protocol described by Elbert et al. 32. In total, 20 mg (0.134 mmol of acid group) of PGA, 14.13 mg (0.026 mmol of amino group) of β-lactose-(PEG)3-NH2 and 5 mg of N-hydroxysulfosuccinimide (NHS) were dissolved in 1.5 mL of 20mM Hepes buffer at pH=6.5. 5 mg of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) (0.026 mmol) was then dissolved in the mixture under stirring. The reaction was allowed to proceed for 4 hours at room temperature. The reacted mixture was then dialyzed twice (Spectra/Por, cut off at MW 3500) for 2×12 hours in a NaCl solution (145 mM, pH=6.5) and 2×12 hours in deionized water. The product was dried by lyophilization and stored at -20°C. PGA-(PEG)3-β-lactose 1H NMR (300 MHz, D2O, reference t-BuOH 1.28ppm) : 7.40-7.47 (m, 1H HPGA), 5.03-5.21 (m, HPGA), 4.50-4.07 (m, 1H HPGA, 1.33H H β-lactose), 3.57-4.16 (m, 1.8H, HCH2O), 3.3-3.48 (m, 0.2H, HNHCH2), 2.85-2.99 (m, 0.2H, HSCH2), 1.94-2.31 (m, 4H, HCH2-PGA), 2.85-2.99 (m, 0.2H, HSCH2). By 1H NMR, the area of the signal at 4.50-4.07 ppm gives the effective degree of modification which is found to be 11.1%.
The same procedure was followed for the PGA-α-mannose grafting. In total, 30 mg (0.1987 mmol of acid group) of PGA, 7.4 mg (0.0199 mmol of amino group, in order to obtain a grafting ratio of 10%) of α-mannose-(PEG)3-NH2. PGA-(PEG)3-α-mannose 1H NMR (300 MHz, D2O, reference t-BuOH 1.28ppm) :7.40-7.47 (m, 1H HPGA), 5.38 (s, 0.1H, HHα-mannose), 5.03-5.21 (m, HPGA), 4.32-4.42 (m, 1H, HCH-PGA), 3.57-4.16 (m, 1.8H, HCH2O), 3.30-3.48 (m, 0.2H, HNHCH2), 2.85-2.99 (m, 0.2H, HSCH2), 1.94-2.31 (m, 4H, HCH2-PGA), 2.85-2.99 (m, 0.2H, HSCH2). By 1H NMR, comparing the integration signal at 5.38 ppm (HHα-mannose) with that at 4.32-4.42 ppm (CH of glutamic acid), the affective degree of modification was found to be 10%.
The PGA-mannose and PGA-lactose were dissolved at 0.5 mg/mL in the same buffer as PLL and PGA.
Film characterization by quartz crystal microbalance (QCM) and atomic force microscopy (AFM)
The (PLL/PGA) film buildup was followed by in situ quartz crystal microbalance (QCM-Dissipation, Qsense, Sweden) 33,34. The quartz crystal was excited at its fundamental frequency (about 5 MHz) as well as at the third, fifth and seventh overtones (corresponding respectively to 15, 25 and 35 MHz). Changes in the resonance frequencies, δf, and in the vibration relaxation after the excitation is stopped were measured at the four frequencies.
The films were imaged in contact mode in air with the Nanoscope IV from Veeco (Santa Barbara, CA, USA) 17. Deflection and height mode images were scanned simultaneously at a fixed scan rate (2 Hz) with a resolution of 512×512 pixels. The mean roughness of the films was measured over 5μm×5μm areas. It was calculated according to: where zij is the height of a given pixel, zmean is the average height of the pixels, and Nx and Ny are the number of pixels in the x and y directions. Force spectroscopy measurements were performed as previously described on a custom made AFM in order to determine the Young's moduli of the films 35. As the (PLL/PGA)6 films are very thin, they were deposited on a PEM precursor film composed of (PLL/HA)24 film acting as a spacer between the (PLL/PGA)6 film and the glass substrate.
HCS-2/8 culture
HCS-2/8 human chondrosarcoma cells derived from a chondrocyte-like cell line were grown in Gibco BRL's minimum essential medium with Eagle's salts (MEM, Life Technologies, France), 10% fetal calf serum (FCS, Gibco, France), 50 U/mL penicillin, and 50 U/mL streptomycin (Bio-Whittaker) in a 5% CO2 and 95% air atmosphere at 37 °C. The cells were detached with 0.04% trypsin/EDTA solution (Gibco BRL, UK) and resuspended in a MEM medium supplemented with antibiotics. Cells were distributed into 24-well plates containing the film coated glass slides (105 cells per well) in a total volume of 1mL of MEM supplement. The cells were incubated at 37°C under a 5% CO2 humidified atmosphere. Different times were tested: 4 hours, three days and five days.
Rat chondrocytes culture
Rat chondrocytes were isolated from femoral head caps of Wistar rats. Femoral head caps were digested in 2 mg/mL pronase (Sigma, P5147, France), diluted in physiological serum for 2h at 37°C, 5% CO2 incubator and in 1.5 mg/mL B collagenase (Boehringer, Germany), and then diluted in 1/1 MEM/Ham's F12 medium supplemented with 10% Fetal bovine serum (FBS, Gibco, France) and 100 U/mL penicillin (Life Technologies, France) for 15h at 37°C, 5% CO2 incubator. The supernatant was collected and gentle centrifuged (5 min. at 1000g) and suspended in 1/1 MEM/Ham's F12 medium supplemented with 10% FBS in presence of 100 U/mL penicillin and then seeded in a culture flask.
After reaching confluence, cells were washed with PBS, detached with 0.04% trypsin and resuspended in a MEM:F12 medium supplemented with antibiotics. The cells were distributed into 24-well plates containing the film coated glass slides (2.5×104 cells per well) in a total volume of 1mL of MEM supplemented. The cells were incubated at 37°C under a 5% CO2 humidified atmosphere. Different times were tested: 4 hours, three days and five days. At these times, each well was washed with 1 mL PBS and the medium was changed.
For all the cell culture experiments, the primary chondrocytes were used for each experiment at passage 1. Three slides per type of film were prepared in each experiment and three independent experiments were performed.
Co-culture
For the co-culture experiments, primary chondrocytes were pre-conditioned in HCS culture medium (i.e. MEM medium) for three days. Before plating, HCS-2/8 cells were detached, suspended in MEM and cell membranes were labelled with the PKH 26 red fluorescent cell marker following the manufacturers instructions (Sigma Aldrich, France). HCS-labelled cells were prepared at 5×104 cells per mL. Chondrocytes were washed with PBS, detached with 0.04% trypsin and resuspended in MEM 5×104 cells per mL. Labelled HCS-2/8 cells and chondrocytes were mixed v/v and the final concentration was 2.5×104 cells for each cell type in 1mL of MEM. This 1mL cell mixture was distributed into 24-well plates containing the film coated glass slides. The two cell types were incubated at 37°C under a 5% CO2 humidified atmosphere and observed under the microscope. The estimated fraction of HCS cells in the total surface covered by the cells was performed using fluorescence microscopy (filters: excitation at 560 nm/emission at 590 nm) and subsequent image analysis of the surface covered by the fluorescent cells (NIH Image).
Cell viability (trypan-blue test)
Cells were washed with PBS and detached with 100μL trypsin/EDTA (Gibco BRL, UK) at 37°C during 5 min. 100μL of MEM was added and the suspension was collected. The wells were rinsed one more time with 100 μL of MEM. 60μL of trypan blue were added to the 300μL of cell suspension and the whole mixture was gently agitated for a few minutes. Non-colored cells (i.e. the viable ones) were finally counted with a Neubauer cell. The experiment was performed in duplicate (with three wells per film condition in each experiment).
Immunofluorescence staining
For collagen II labeling, cells were washed twice and fixed in 3.7% paraformaldehyde (PFA, Sigma, France) in PBS for 15 minutes. Samples were incubated for ten min. in 0.1% Triton X100 (Sigma, France) and 3.7 % PFA in PBS to permeabilize the cell membrane and then the membrane was blocked with 10% FBS in PBS. Cells were washed in PBS and incubated with the primary anti-human collagen type II antibody (Novocastra Laboratories, UK) diluted at 1:30 in PBS for 60 min. at room temperature. After PBS washing, the cells were incubated with the secondary anti-rabbit Cy3 antibody (Interbiotech, France) diluted at 1:500 in PBS. After three washes, samples were mounted in Vectashield (Vector Burlingame, CA) and observed with a Nikon E200 microscope. Total cell number was determined by Hoechst stain (2 μL/mL) of nuclei and cell population expressing Collagen II was counted on the same image. The percentage value was obtained after counting ten different fields, each cell being clearly identified due to Hoechst staining of the nucleus.
Analysis of BrdU incorporation
Cell incorporation of BrdU (5-bromo-2′-deoxyuridine) was determined using a cell proliferation kit (Amersham Biosciences, England) 36. Briefly, the cells (chondrocytes and HCS) were distributed into 24-well plates containing the film coated glass slides (105 cells per well for the HCS and 2.5×104 cells per well for the chondrocytes) in a total volume of 1mL of MEM supplemented. The cells were incubated at 37°C under a 5% CO2 humidified atmosphere for three days. The cells were washed with PBS and incubated for 18h with 1/1000 BrdU in MEM supplemented. The cells were fixed in acid-ethanol for 30 min. on ice. The cells were probed with anti-5-bromo-2′-deoxyuridine/nuclease for one hour, then the cells were incubated with the peroxidase anti-mouse IgG2a for 30 min. and stained with a solution of 3-3′ Diaminobenzidine Tetrahydrochloride (DAB) for 5 min. Total cell number and cell population expressing BrdU were counted on the same image. The percentage value was obtained after counting ten different fields (each field containing approximately a dozen cells).
Statistical analysis
Data were analyzed using the Kruskal-Wallis one way analysis of variance from SigmaStat 2.0 (Jandel corporation, Germany) with significance at p<0.05. Cells cultured on bare glass slides were always taken as reference for the tests.
Results and discussion
Chemical grafting of PGA-lactose and PGA-mannose
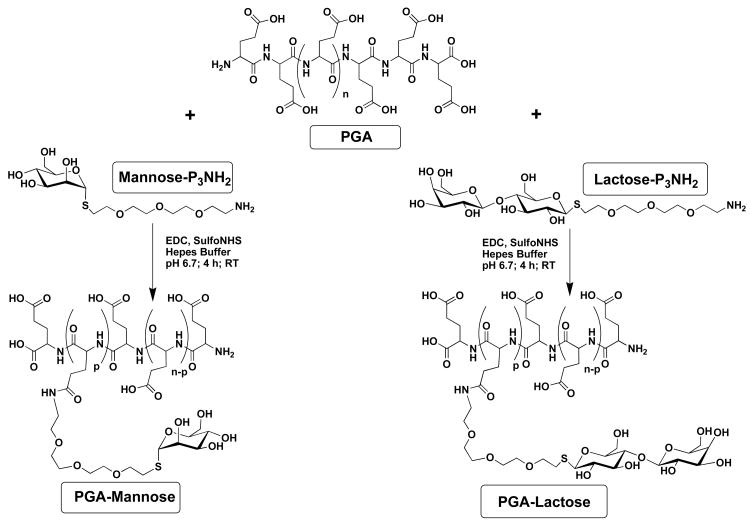
The two kinds of modified poly(L-glutamic) acid, namely the PGA-α-mannose and the PGA-β-lactose, were prepared as depicted in Figure 1 using a PEG spacer arm. The degree of modification was chosen to be of the order of 10% such as to keep some free carboxylic groups (negative charges) uncoupled for promoting the (PLL/PGA) multilayer film buildup. The degree of modification, as measured by 1H-Nuclear Magnetic Resonance analysis, was found to be 10% for the PGA mannose conjugate and 11% for the PGA lactose conjugate. These ligands, when exposed at the surface of polymers, could in principle engage in multiple interactions, and thus increase their apparent affinity for the cells expressing mannose receptors (MR). The lengths of the spacer arms between the α-mannose and β-lactose groups and the scaffold were chosen to provide a good flexibility and accessibility of the ligands to the MR carbohydrate recognition domains.
FIGURE 1.
Synthesis of the PGA-α-mannose (left) and the PGA-β-lactose (right). The final products contain both the saccharide functions and carboxylic sites that have a polyelectrolyte character. The grafting ratio was 10% for mannose modified poly(L-glutamic) acid (n ≈ 540, p ≈ 54) and 11 % for lactose modified poly(L-glutamic) acid (n ≈ 540, p ≈ 59).
Films characteristics
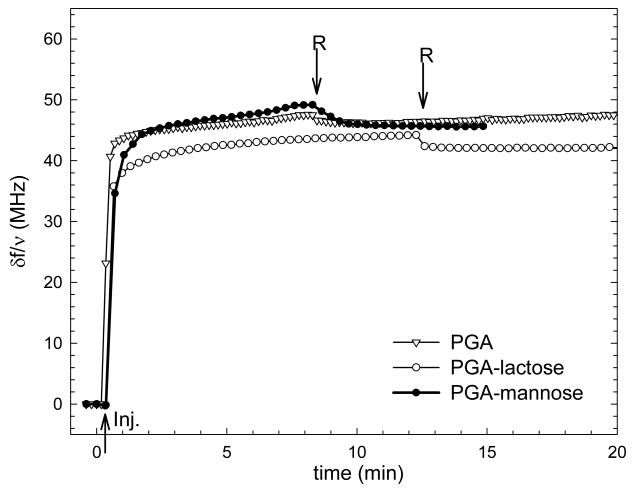
Different types of films were investigated: unmodified PGA (native films), cross-linked after film deposition (CL), or by PGA covalently coupled to either mannose (∼ mannose) and lactose (∼ lactose). Films containing six layer pairs have been used as we have already shown that (PLL/PGA)6 films built in Hepes buffer do entirely cover a glass substrate and have a thickness of 31 nm as measured by in situ optical spectroscopy 17. The buildup of the different films was also followed by QCM-D. The frequency shifts are represented only for the last layer adsorbed on top of (PLL/PGA)5-PLL films in order to compare the adsorption of the glycated polyelectrolytes. The frequency shifts measured for the PGA-mannose and PGA-lactose layers vary between 42Hz and 48 Hz, which can be considered to be similar to that of a PGA layer (Figure 2) due to the experimental errors associated to the technique (reproducibility at about 5Hz). This confirms that the coupling of the sugar residue at a moderate grafting ratio has not modified the polyelectrolyte behaviour of the PGA.
FIGURE 2.
Differences in the QCM frequency shifts -δf/ν measured at 15 MHz during the adsorption and rinsing steps of the last layer deposited on a (PLL/PGA)5-PLL film : (∇) PGA, (○) PGA-lactose and (●) PGA- mannose. The arrows indicate the injection of the polyelectrolytes (Inj.) and the rinsing by the buffer solution (R)
Film surfaces were imaged by AFM, which confirmed the presence of a uniform film covering (data not shown). Film thickness, which was estimated by imaging a scratched zone of the film (scratch with a needle) and film roughness are summarized in table 1. The thickness is similar for native, ∼mannose and ∼lactose films (≈ 30 nm) indicating a similar adsorption for the PGA coupled to sugar molecules. Film thickness is slightly increased for the cross-linked films (≈ 35 nm), which may be explained by the swelling of the film during the cross-linking reaction due to the lowering of the pH (at pH5) 18. With respect to the roughness, it is slightly higher for native PGA films than for mannose and lactose ending films, whereas CL films have the highest roughness (increased two-fold as compared to the glycated films). It has to be noted that such an increase in film roughness upon cross-linking has recently been observed for PLL/HA films 37.
Table 1.
Film thickness and roughness estimated by AFM for the different types of films : native, CL, ∼ mannose, ∼ lactose
| Type of film | Thickness
(nm) |
Roughness
(nm) |
|---|---|---|
| Native | 30.3 ± 0.6 | 3.9 ± 0.1 |
| CL | 35.9 ± 0.7 | 4.4 ± 0.1 |
| ∼ mannose | 29.0 ± 0.6 | 2.2 ± 0.1 |
| ∼ lactose | 30.8 ± 0.6 | 1.9 ± 0.1 |
Chondrosarcoma cells and chondrocyte adhesion and proliferation
Chondrosarcoma cells and primary chondrocytes were grown on the different types of films ending either by PGA (native or cross-linked) or functionalized by mannose or lactose (e.g. ending by PGA-mannose and PGA-lactose).
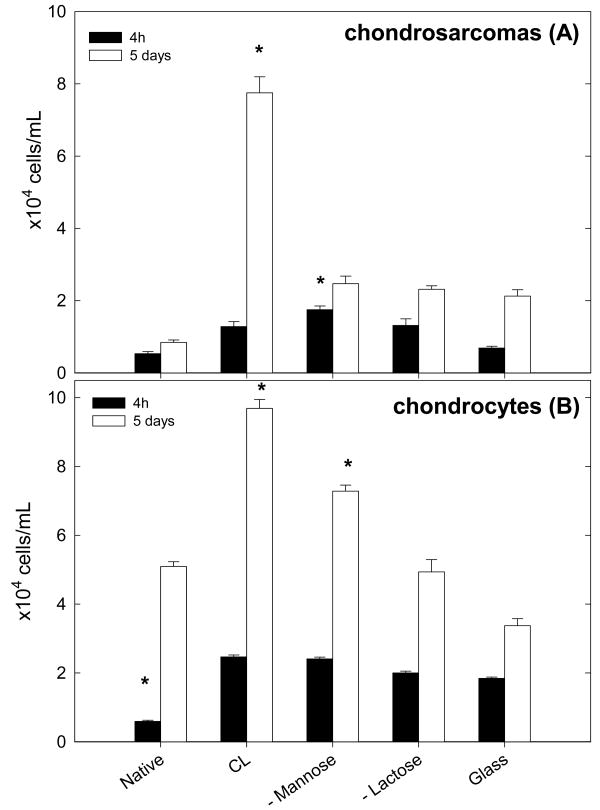
Type II collagen production was also controlled. About 50 to 60% of the chondrocytes were stained by the anti-type II collagen after three days of culture on the different type of films, which shows that the chondrocyte phenotype was conserved for most part of the cells. It is not surprising that all cells were not stained after this time period as chondrocytes are known to dedifferentiate in fibroblasts when grown in monolayers over a week, with dedifferentiation being already visible at day three where cells exhibit a spread and elongated morphology 38. Cell viability was first estimated by the trypan-blue test performed after four hours and five days of culture (Figure 3). After four hours of culture, one could already observe by optical microscopy that native films are the less favorable with respect to chondrosarcoma cells and chondrocytes adhesion whereas the cross-linked ones and the sugar functionalized films are most favorable. After five days in culture, the highest number of cells were found on the cross-linked films for both cell types. For chondrosarcoma cells, the lowest cell number was respectively obtained on native PGA films, ∼mannose, ∼lactose and glass surfaces (Figure 3A). For the primary cells, the highest cell number is found on the CL films, followed by ∼mannose ending films. These surfaces were significantly different from the glass substrate. Cell number was similar on glass and ∼lactose ending films (Figure 3B).
FIGURE 3.
Trypan-blue viability test for the HCS cells (A) and for primary cells (B). Results are shown for four hours in culture (black bars) and after five days in culture (white bars). *Adhesion was statistically different as compared to glass (Kruskal-Wallis oneway analysis of variance, *p < 0.05).
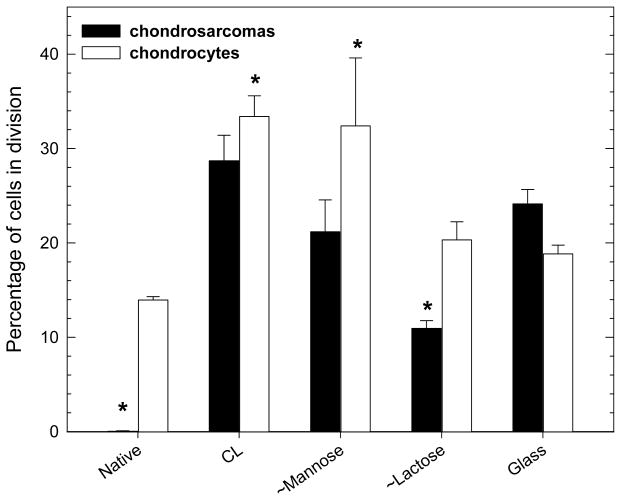
Regarding the proliferation of the two cell types, a BrdU test was performed (Figure 4). For HCS 2/8 cells, proliferation was highest on CL films, followed by glass and ∼mannose surfaces. There was no proliferation at all on the native PGA films. On the lactose ending films, the cells proliferated moderately (10% of the cells were in division). With respect to the primary cells, the proliferation test gave slightly different results. Whereas the native surface still leads to a poor proliferation, the ∼mannose and CL films present the highest number of dividing cells (about one third). Again, proliferation on ∼lactose was moderate (about 20%) but similar to glass. The microscopic observation of the cells on the different film types qualitatively confirms all these findings (Figure 5). Few cells are present on native films whereas the number of cells is much higher on CL ones, for both chondrosarcoma and chondrocytes. PGA-mannose and PGA-lactose films lead to a good chondrocyte proliferation and viability but are less favourable to chondrosarcoma cells. It has to be noted that cells clearly exhibited a round shape on lactose ending films with the eventual formation of large cell aggregates. This may be related to the recent observation of aggregates when chondrocytes are cultured on chitosan chemically modified with lactose 28.
FIGURE 4.
Results of the BrdU proliferation test for chondrosarcoma cells (black bars) and chondrocytes (white bars). *The percentage of cells in division was statistically different as compared to that on top of glass (Kruskal-Wallis oneway analysis of variance, *p < 0.05).
FIGURE 5.
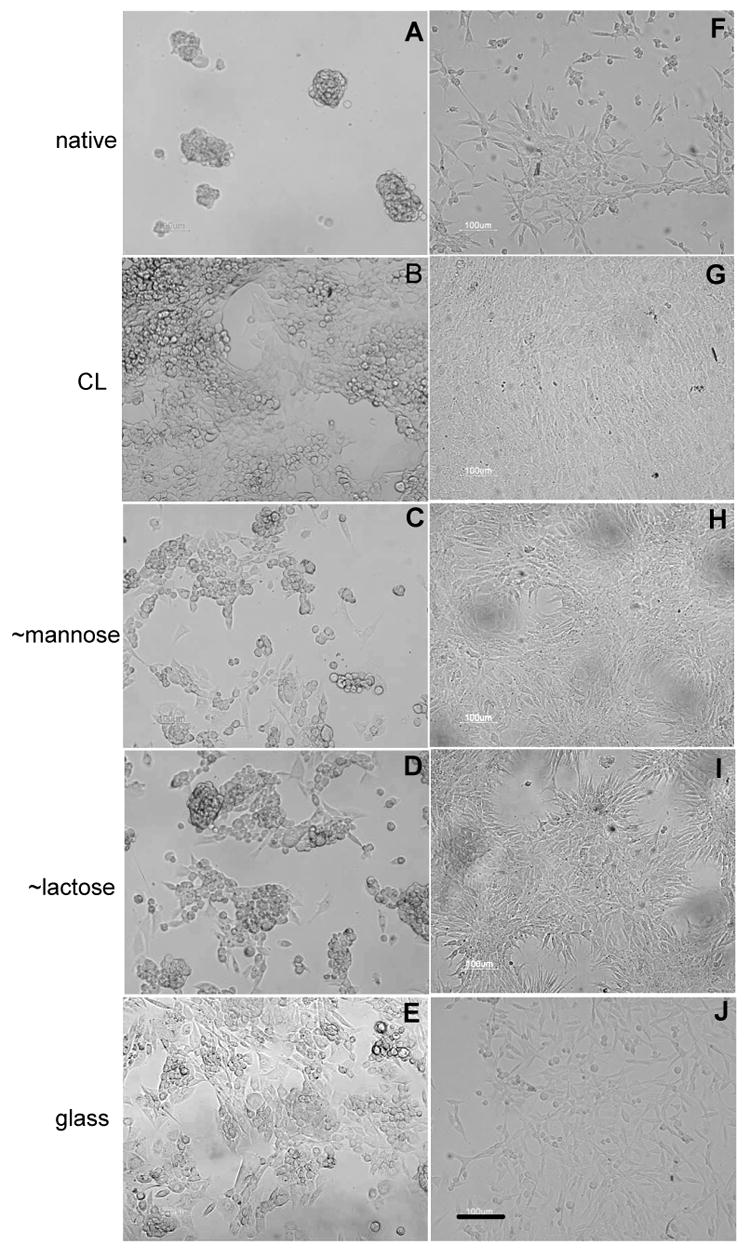
Observation of the human chondrosarcoma cells (A-E) and of primary cells (F-J) by microscopy (×20 objective, scale bar is 100 μm). The cells have been cultured for five days on native films (A, F), cross-linked films (B, G) and films ending by ∼ PGA-mannose (C, H), ∼ PGA-lactose (D, I) as compared to bare glass (E, J).
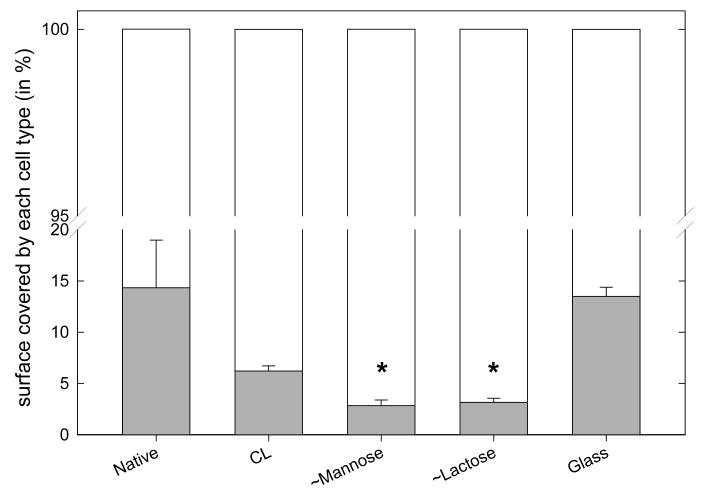
We also investigated whether a given surface is able to induce adhesion of a preferred cell type by preparing a co-culture of chondrosarcomas and chondrocytes, the chondrosarcomas appearing in black in the images (Figure 6). The percentage of cells covered by the chondrosarcoma cells over all the cells present was counted. Noticeably, the ∼mannose and ∼lactose grafted films induced the proliferation of chondrocytes as compared to chondrosarcoma cells (Figure 6).
FIGURE 6.
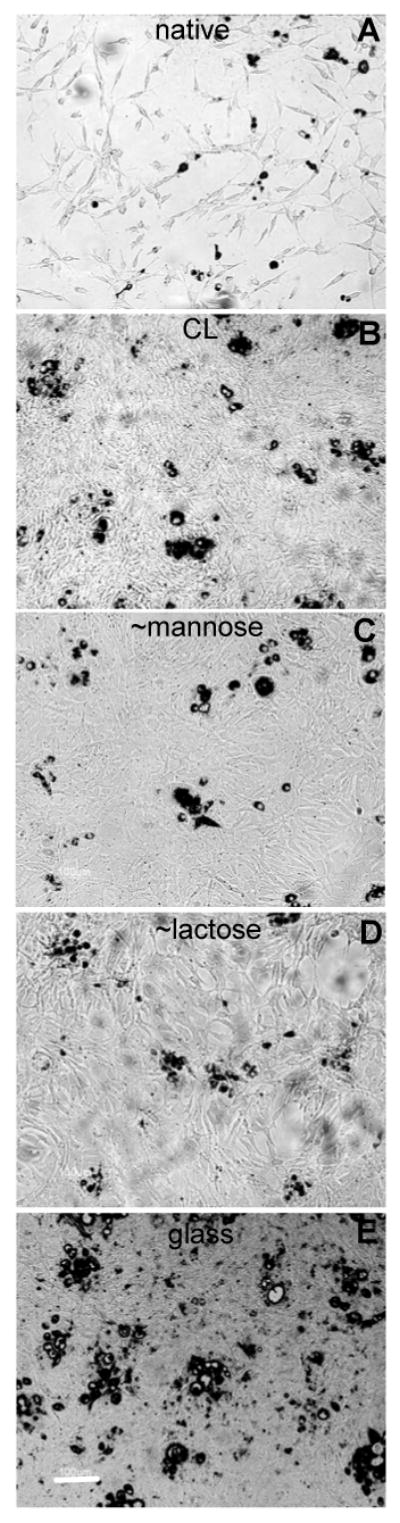
Images of primary chondrocytes and human chondrosacoma co-cultures (×20 objective, scale bar is 100 μm) after five days. Human chondrosarcoma cells have been coloured in red (represented in black in the figure). The films are either native (A), CL (B) or ending by ∼ PGA-mannose (C) or ∼ PGA-lactose (D). The control is glass (E).
All together, these quantitative results on cell adhesion and proliferation confirmed the previous finding that cross-linked films have a drastic effect on cell adhesion, as was already demonstrated for other types of films such as (PLL/HA) multilayer films 18 and for other cell types like primary osteoblasts 17. In the case of chondrosarcomas and chondrocytes, this effect seemed to be cell type specific. The most striking and interesting results came from mannose films, for which high chondrocyte adhesion and proliferation was observed, whereas chondrosarcoma cells adhesion and proliferation was clearly lower. This suggests that mannose may be a ligand for chondrocytes, as was recently suggested by Howard et al. 25. In order to investigate the possibility of specific binding via mannose receptors, soluble mannose (10 μg/mL) was incubated with the cells in suspension for one hour prior to deposition on the film surfaces. However, this did not lead to a change in cell adhesion, as is usually observed when soluble RGD is added on cells that should adhere to RGD coated surfaces 39. This suggests that adhesion on the mannose ending surface is not specific to this receptor but may be multifunctional. Clearly, the nature of the grafted sugar is important as lactose grafted films showed the same trend but with much less differences between chondrocytes and chondrosarcomas behaviours. The surface chemistry may also influence the way proteins from the serum adsorb onto the film as was recently evidenced for fibronectin adsorption on synthetic PEM films 40. However, as serum is a complex mixture of different sorts of protein, it is difficult to extrapolate data obtained on single protein analysis.
Beside the role of surface chemistry, the role of film mechanical properties in cell adhesion and spreading has recently been evidenced for different types of films such as poly(acrylic acid)/poly(allylamine hydrochloride) films built at different pHs 19 and for (PLL/HA) multilayer films cross-linked at various cross-linker concentrations 41. In order to investigate the possible role of substrate stiffness in the adhesion on the carbohydrates ending surfaces, the surface Young's modulus of the films was estimated by means of nano-indentation experiments using an AFM and a colloidal probe as indenter 35(Figure 8). As the (PLL/PGA)6 films are very thin (≈ 30 nm), they could not be directly used for nano-indentation experiments due to the requirements in terms of indentation depths and sample thickness for applying the modified Hertz model42. There were thus deposited on a PEM precursor film composed of (PLL/HA)24 film acting as a spacer between the (PLL/PGA) film and the glass substrate. This (PLL/HA)24 films has known mechanical properties35 and serves as a basis for comparing the native (PLL/PGA) and glycated films.
FIGURE 8.
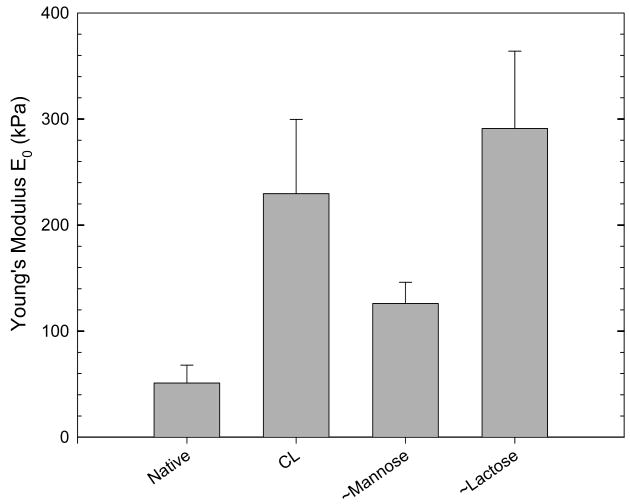
Surface Young's modulus E0 determined by the AFM nano-indentation technique for various films: native, CL, ∼ mannose and ∼lactose.
As expected, E0 was much higher for CL films than for native ones (229.5 ± 70 kPa instead of 51 ± 17 kPa). E0 of ∼mannose films (126 ± 20 kPa) was about half that of CL films whereas E0 of ∼lactose films (290 ± 73 kPa) is even higher than that of CL films. Of note, the simple adsorption of a sugar grafted layer as the end layer was able to induce an important change in the film mechanical properties.
Such moduli are higher than that of common substrates used to study cell attachment such as polyacrylamide gels (1 to 40 kPa depending on the cross-linker concentration) and gelatin gels (1 to 10 kPa) but are smaller than that of other polyelectrolyte multilayer films made of synthetic polymers 19. In addition, Rubner and coworkers recently authors showed that surface conjugation of adhesion peptide sequences such as Arg-Gly-Asp (RGD) does alter the local mechanical properties of weak polyelectrolyte multilayers. In particular, it was found that immersed adsorption of intermediate functionalization reagents significantly decreased compliance of the PEMs (i.e. increased the film stiffness), whereas polymer-on-polymer stamping of these same reagents did not alter compliance of weak PEMs 43. Interestingly, another recent work focused on the preparation of PEM films with PAH as polycation and two ionic polysaccharides (iota and lambda-carrageenans) as polyanions, which have similar chemical composition but different confirmations 44. The (PAH/t-carr)30 films were found to be about three times stiffer (≈ 30 MPa) than the (PAH/λ-carr)30 films (≈ 10 MPa). To the best of our knowledge, these are the only publications, beside the present one, which evidence the interplay between film mechanical properties and film biochemical properties. Our data indicates that film stiffness can not be, in the present case, the principal factor explaining the differences observed on the carbohydrate ending films. In addition, film roughness could neither explain the differences observed in cell adhesion.
We thus suggest that film chemistry and nature of sugar grafting is the most important parameter here, with primary chondrocytes being particularly reactive to ∼mannose ending films. The fact the cells have many different glycoproteins on their surface may explain why specific sugars can have such an important effect on cell adhesion properties. Recently, the role of glycoproteins was underlined in cancer and metastatic processes 21. Glyco-polyelectrolytes can also considerably enhance cell transfection but the exact mechanism for this recognition remains unclear 22. In the present work, we have demonstrated that mannose functionalized films are a good surface for primary cells adhesion and proliferation while the exact molecular mechanism of this recognition remains unclear. Different cell types have different responses depending on the sugar molecules considered. This represents a new and simple way to create cell attractive surfaces, which are favouring the adhesion of primary cells at the expense of the adhesion of tumor cells. These finding may be useful for designing new surfaces with high cell activities for certain types and not for others at a much lower cost than using synthetic peptides. Larger amounts of materials can thus be quickly and inexpensively made available and tested.
Conclusion
In this work, we showed that glycated polymers such as mannose and lactose can be successfully adsorbed onto polyelectrolyte multilayer films made of PLL and PGA and that these glycated PEM films present interesting properties in terms of cell adhesion. These films were compared to ungrafted films (native) and cross-linked films, which are already known to considerably change film adhesive properties 31. Noticeably, ∼mannose grafted films were found to be particularly interesting as they favour chondrocytes adhesion and proliferation whereas chondrosarcoma cells adhesion and proliferation was very limited onto such films. A similar trend was observed for ∼lactose grafted films but in a much lesser extent. The adhesion mediated by mannose molecules is likely to be multifunctional and is not directly related to the modification of film stiffness. These results show that glycated polyelectrolytes may not only be used as non viral vectors but also as cell adhesive or anti-adhesive surfaces depending on the cell type.
FIGURE 7.
Results of the co-culture of chondrosarcoma cells and of primary cells after five days presented as the fraction of cell surface covered by each cell type (the total cell coverage being set at 100%): HCS-2/8 are represented in gray and primary cells in white. The percentage of cells was statistically different as compared to that on top of glass (Kruskal-Wallis oneway analysis of variance, *p < 0.05).
Acknowledgments
This work has been partly supported by the Action Concertée Incitative “Nanosciences” (ACI NR204) from the Ministère de la Jeunesse, de l'Education Nationale et de la Recherche, by NIH (R21 grant) via a subcontract to CP (n°544168A) and by Association pour la Recherche sur la Cancer (ARC, equipment grant n°7916, CP). AS is indebted to the « Region Alsace » and GF is indebted to the “Faculté de Chirurgie Dentaire” for financial support. We thank Bernard Senger for his help in the statistical analysis of the data.
References
- 1.Falconnet D, Csucs G, Michelle Grandin H, Textor M. Biomaterials. 2006;27:3044–3063. doi: 10.1016/j.biomaterials.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Mohammed JS, DeCoster MA, McShane MJ. Biomacromolecules. 2004;5:1745–1755. doi: 10.1021/bm0498631. [DOI] [PubMed] [Google Scholar]
- 3.Fukuda J, Khademhosseini A, Yeh J, Eng G, Cheng J, Farokhzad OC, R L. Biomaterials. 2006;27:1479–1486. doi: 10.1016/j.biomaterials.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Decher G, Hong JD, Schmitt J. Thin Solid Films. 1992;210-211:831–835. [Google Scholar]
- 5.Decher GA, Lvov Y, Schmitt J. Thin Solid Films. 1994;244:772–777. [Google Scholar]
- 6.Lvov L, Haas H, Decher G, Moehwald H, Mikhailov A, Mtchedlishvily B, Morgunova E, Vainshtein B. Langmuir. 1994;10:4232–4236. [Google Scholar]
- 7.Sukhorukov GB, Moehwald H, Decher G, Lvov YM. Thin Solid Films. 1996;284-285:220–223. [Google Scholar]
- 8.Lvov YM. In: Handbook of Surfaces and Interfaces of Materials; Vol 3: Nanostructures Materials, Micelles, and Colloids. Nalwa HS, editor. Academic Press; 2001. [Google Scholar]
- 9.Mendelsohn JD, Yang SY, Hiller J, Hochbaum AI, Rubner MF. Biomacromolecules. 2003;4:96–106. doi: 10.1021/bm0256101. [DOI] [PubMed] [Google Scholar]
- 10.Salloum DS, Olenych SG, Keller TC, Schlenoff JB. Biomacromolecules. 2005;6:161–167. doi: 10.1021/bm0497015. [DOI] [PubMed] [Google Scholar]
- 11.Richert L, Lavalle P, Payan E, Stoltz JF, Shu XZ, Prestwich GD, Schaaf P, Voegel JC, Picart C. Langmuir. 2004;1:284–294. doi: 10.1021/la035415n. [DOI] [PubMed] [Google Scholar]
- 12.Thierry B, Kujawa P, Tkaczyk C, Winnik FM, Bilodeau L, Tabrizian M. Journal of the American Chemical Society. 2005;127:1626–1627. doi: 10.1021/ja045077s. [DOI] [PubMed] [Google Scholar]
- 13.Ratner BD, Hoffman AS, Schoen FJ, Lemons JE. Biomaterials sicence : an introduction to materials in medecine. Academic Press; 1996. [Google Scholar]
- 14.Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 15.Schaffner P, Dard MM. Cellular and Molecular Life Sciences. 2003;60:119–132. doi: 10.1007/s000180300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg MC, Yang SY, Hammond PT, Rubner MF. Langmuir. 2004;20:1362–1368. doi: 10.1021/la0355489. [DOI] [PubMed] [Google Scholar]
- 17.Picart C, Elkaim R, Richert L, Audoin F, Da Silva Cardoso M, Schaaf P, Voegel JC, Frisch B. Advanced Functional Materials. 2005;15:83–94. [Google Scholar]
- 18.Richert L, Engler AJ, Discher DE, Picart C. Biomacromolecules. 2004;5:1908–1916. doi: 10.1021/bm0498023. [DOI] [PubMed] [Google Scholar]
- 19.Thompson MT, Berg MC, Tobias IS, Rubner MF, Van Vliet KJ. Biomaterials. 2005;26:6836–6845. doi: 10.1016/j.biomaterials.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular biology of the cell. Garland Publishing Inc; New York: 1994. [Google Scholar]
- 21.Dube DH, Bertozzi CR. Nature Reviews Drug Discovery. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 22.Roche AC, Fajac I, Grosse S, Frison N, Rondanino C, Mayer R, Monsigny M. Cell and Molecular Life Science. 2003;60:288–297. doi: 10.1007/s000180300024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.East L, Isacke CM. Biochimica Biophysica Acta. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 24.McGreal EP, Martinez-Pomares L, Gordon S. Molecular Immunology. 2004;41:1109–1121. doi: 10.1016/j.molimm.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 25.Howard MJ, Chambers MG, Mason RM, Isacke CM. Osteoarthritis and Cartilage. 2004;12:74–82. doi: 10.1016/j.joca.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Schultz P, Vautier D, Richert L, Jessel N, Haikel Y, Schaaf P, Voegel JC, Ogier J, Debry C. Biomaterials. 2005;26:2621–2630. doi: 10.1016/j.biomaterials.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 27.Khademhosseini A, Suh KY, Yang JM, Eng G, Yeh J, Levenberg S, Langer R. Biomaterials. 2004;25:3583–3592. doi: 10.1016/j.biomaterials.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 28.Donati I, Stredanska S, Silvestrini G, Vetere A, Marcon P, Marsich E, Mozetic P, Gamini A, Paoletti S, Vittur F. Biomaterials. 2005;26:987–998. doi: 10.1016/j.biomaterials.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Tryoen-Toth P, Vautier D, Haikel Y, Voegel JC, Schaaf P, Chluba J, Ogier J. Journal of Biomedical Materials Research. 2002;60:657–667. doi: 10.1002/jbm.10110. [DOI] [PubMed] [Google Scholar]
- 30.Vautier D, Karsten V, Egles C, Chluba J, Schaaf P, Voegel JC, Ogier J. Journal of Biomaterials Science Polymer Edition. 2002;13:713–732. doi: 10.1163/156856202320269175. [DOI] [PubMed] [Google Scholar]
- 31.Richert L, Boulmedais F, Lavalle P, Mutterer J, Ferreux E, Decher G, Schaaf P, Voegel JC, Picart C. Biomacromolecules. 2004;5:284–294. doi: 10.1021/bm0342281. [DOI] [PubMed] [Google Scholar]
- 32.Elbert DL, Hubbell JA. Biomacromolecules. 2001;2:430–441. doi: 10.1021/bm0056299. [DOI] [PubMed] [Google Scholar]
- 33.Hook F, Rodahl M, Brzezinski P, Kasemo B. Journal of Colloid and Interface Science. 1998;208:63–67. doi: 10.1006/jcis.1998.5774. [DOI] [PubMed] [Google Scholar]
- 34.Rodahl M, Kasemo B. Review of Scientific Instruments. 1996;67:3238–3241. [Google Scholar]
- 35.Francius G, Hemmerle J, Ohayon J, Schaaf P, Voegel JC, Picart C, Senger B. Microscopy Research and Techniques. 2006;69:84–92. doi: 10.1002/jemt.20275. [DOI] [PubMed] [Google Scholar]
- 36.Gratzner HG, Leif RC, Ingram DJ, Castro A. Experimental Cell Research. 1975;95:88–94. doi: 10.1016/0014-4827(75)90612-6. [DOI] [PubMed] [Google Scholar]
- 37.Schneider A, Francius G, Obeid R, Schwinté P, Frisch B, Schaaf P, Voegel JC, Senger B, Picart C. Langmuir. 2005;2006:1193–1200. doi: 10.1021/la0521802. [DOI] [PubMed] [Google Scholar]
- 38.Brodkin KR, Garcia AJ, Levenston ME. Biomaterials. 2004;25:5929–5938. doi: 10.1016/j.biomaterials.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 39.Grzesik WJ, Robey PG. Journal of Bone and Mineral Research. 1994;9:487–496. doi: 10.1002/jbmr.5650090408. [DOI] [PubMed] [Google Scholar]
- 40.Olenych SG, Moussallem MD, Salloum DS, Schlenoff JB, Keller TC. Biomacromolecules. 2005;6:3252–3258. doi: 10.1021/bm050298r. [DOI] [PubMed] [Google Scholar]
- 41.Schneider A, Francius G, Obeid R, Schwinté P, Frisch B, Schaaf P, Voegel JC, Senger B, Picart C. Langmuir. 2006;22:1193–1200. doi: 10.1021/la0521802. [DOI] [PubMed] [Google Scholar]
- 42.Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS. Biophys J. 2002;82:2798–2810. doi: 10.1016/S0006-3495(02)75620-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson MT, Berg MC, Tobias IS, Lichter JA, Rubner MF, Van Vliet KJ. Biomacromolecules. 2006;7:1990–1995. doi: 10.1021/bm060146b. [DOI] [PubMed] [Google Scholar]
- 44.Schoeler B, Delorme N, Doench I, Sukhorukov GB, Fery A, Glinel K. Biomacromolecules. 2006;7:2065–2071. doi: 10.1021/bm060378a. [DOI] [PubMed] [Google Scholar]