Abstract
Invariant NKT (iNKT) cells are a population of TCRαβ-expressing cells that are unique in several respects. In contrast to conventional T cells, iNKT are selected in the thymus for recognition of CD1, rather than conventional MHC class I or II, and are selected by CD1-expressing double positive thymocytes, rather than by the thymic stromal cells responsible for positive selection of conventional T cells. We have probed further the requirements for thymic iNKT cell development and find that these cells are highly sensitive to B7-CD28 costimulatory interactions as evidenced by the substantially decreased numbers of thymic iNKT cells in CD28 and in B7 knockout mice. In contrast to the requirement for CD1, B7-CD28 signaling does not affect early iNKT lineage commitment, but exerts its influence on the subsequent intra-thymic expansion and differentiation of iNKT cells. CD28 wildtype/CD28 deficient mixed bone marrow chimeras provided evidence of both cell-autonomous and non-cell autonomous roles for CD28 during iNKT cell development. Paradoxically, transgenic mice in which thymic expression of B7 is elevated have essentially no measurable thymic iNKT cells. Taken together, these results demonstrate that the unique pathway involved in iNKT development is marked by a critical role of B7-CD28 interactions and that disruption or augmentation of this costimulatory interaction has substantial effects on iNKT cell development in the thymus.
Keywords: natural killer T cell, thymus, costimulation, cell differentiation
Introduction
NKT cells are a distinct lineage of T lymphocytes that express a subset of surface receptors also found on NK cells and recognize glycolipid antigens presented by the nonpolymorphic MHC class-I related protein CD1 (1). In mice, most NKT cells in the thymus and periphery express an invariant Vα14-Jα18 TCR rearrangement which is preferentially associated with Vβ8, Vβ7, or Vβ2 (2). These invariant NKT (iNKT) cells expressing the Vα14-Jα18 TCR can be distinguished from other populations of NKT cells by their capacity to bind to the glycolipd α-galactosyl ceramide (and other closely related lipids), when presented by CD1 (3, 4). iNKT cells possess a partially activated (effector/memory) phenotype evidenced in part by their capacity to rapidly secrete cytokines following stimulation (5). This rapid cytokine response allows iNKT cells to function in a regulatory capacity to direct the course of a developing immune response and has been suggested as a means by which iNKT cells may function to suppress or exacerbate autoimmunity (6, 7).
iNKT cells originate in the thymus via a developmental pathway dependent on a unique set of factors and conditions distinct from those required for conventional αβTCR T cell development. Deficiencies in the cytokine IL-15, the transcription factors NF-κB p50, RelB and T-bet, the signaling molecule fyn and the signaling adaptor SAP, all impinge more significantly on thymic development of iNKT cells than on conventional αβTCR T cells (8-17). In addition, while conventional αβ T cells are positively selected by MHC-expressing thymic stromal cells, iNKT cells are selected on CD1d-expressing CD4+CD8+ double positive (DP) thymocytes (18-20). Positively selected iNKT cells mature further in the thymus in a series of steps which results in altered cell surface phenotype, including acquisition of memory cell markers, and the capacity to rapidly secrete both IL-4 and IFN-γ when stimulated (21, 22). While both conventional αβTCR T cells and iNKT cells are derived from DP precursors (23-25) the post-DP maturation of thymic iNKT cells is unique in that it is accompanied by a proliferative burst resulting in a massive expansion of this population (21).
These aspects of iNKT cell development suggested that the signaling requirements for differentiation of this lineage might differ from those of conventional T cells, and that differences might include the requirements for costimulatory signaling during thymic development. The proliferative burst that accompanies maturation of lineage-committed TCRαβ-expressing iNKT cells is unique to this population, and the specific signaling requirements for this expansion as well as the acquisition of a memory cell-like phenotype have not been elucidated. We considered the possibility that the B7-CD28 interactions which are critical to the expansion, differentiation, and survival of peripheral T cells might be required for optimal intrathymic development of iNKT cells. Additionally, we were interested in determining whether the restricted TCR repertoire of iNKT cells might render this population highly sensitive to alterations in strength of CD28 costimulatory signals. Although development of conventional αβTCR thymocytes appears largely intact in the absence of CD28 signaling (26, 27) defects in development have been found when examining discrete populations of thymocytes. We recently demonstrated that the absence of CD28 significantly alters selection of HY-TCR+ thymocytes (28), and several groups have described the loss of thymic CD4+CD25+ regulatory T cells in the absence of CD28-B7 interactions (29-31).
To assess the role of CD28-B7 interactions during iNKT cell development we evaluated development of iNKT cells in mice lacking CD28 or both B7.1 and B7.2 (CD80/CD86 double knockouts (B7 DKO)) as well as in mice expressing elevated levels of these costimulatory molecules. Interestingly, we found that thymic iNKT cell numbers were significantly reduced in the absence of B7-CD28 interactions, and that this effect is mediated at stages subsequent to initial selection into the iNKT lineage. Furthermore, analysis of mixed chimeras made by reconstituting irradiated mice with CD28 WT and CD28 knockout (KO) bone marrow, demonstrated that optimal development of thymic iNKT cells was dependent on both cell-autonomous and non-cell autonomous functions of CD28. Paradoxically, elevated expression of either thymic CD28 or B7 in transgenic mice failed to restore, and in the case of B7 actively inhibited, iNKT development in mice of the respective CD28 or B7 knockout background. Overall, our results indicate that physiologic levels of B7-CD28 interactions are critical for the unique events involved in iNKT cell development in the thymus.
Materials and Methods
Reagents
Anti-CD4, CD-8, CD45.1 (Ly5.2), CD45.2 (Ly5.1), CD69, CD3, IFN-γ, IL-4, NK1.1, CD24, CD45, CD11c and Vβ2 (B20.6), Vβ7 (TR310) and Vβ8 (MR5−2) Abs were purchased from BD Pharmingen (San Diego, CA). Anti-B7.2 (GL1) Ab was generated as previously described (32). UEA-1 was purchased from Vector Labs (Burlingame, CA). Mouse PE-conjugated CD1d tetramer unloaded or loaded with PBS-57, an analogue of α-GalCer ligand (33) was supplied by the NIH Tetramer Facility. CD1d tetramer loaded with PBS-57 is referred to as CD1tet.
Mice
C57BL/6 (B6), B6.Ly5.2, and B6.Ly5.1/Ly5.2 mice were obtained from the Frederick Cancer Research Facility (Frederick, MD) and maintained at BioQual (Rockville, MD). CD28 KO mice on a B6 genetic background were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained at Bioqual. B6 mice deficient in both B7.1 and B7.2 (B7 DKO) on a B6 genetic background (34) and B7.2 Line 7 Tg (35) were previously described. The CD28 transgenic lines, CD28.WThigh (expressing high levels of wildtype CD28), CD28.TLhigh (expressing high levels of CD28 with deleted cytoplasmic domain), CD28.WTlow (expressing physiologic low levels of wildtype CD28) and CD28.TLlow (expressing physiologic low levels of CD28 with deleted cytoplasmic domain) were generous gifts of Ryo Abe (CD28high, (36) ) and Alfred Singer (CD28low, (30)) and were maintained on an endogenous CD28 KO B6 background. The Line I2 and E4 B7 tg lines were made using cytoplasmic domain-deleted B7.2 and B7.1, respectively.
Cytoplasmic-domain-deleted B7.1 and B7.2 cDNAs were made as previously described (37) and were cloned into the EμPμΔhGH vector containing the EμPμ Ig -μ promoter-enhancer and a mutated nonexpressible segment of the human growth hormone gene, included to enhance expression of the transgene (38). The Line T59 tg line was made using a previously described construct (37) in which the full length B7.2 cDNA was cloned into the expression vector LK444B7 containing the human β-actin promoter and an SV40 polyadenylation signal. Fragments containing the EμPμ Ig-μ promoter-enhancer, hGH, and the inserted B7 cDNAs (Lines I2 and E4) or the human β-actin promoter, SV40 polyadenylation signal and inserted B7 cDNA (line T59) were excised from the plasmid backbone and were microinjected into fertilized B6 oocytes. Founder mice were identified by Southern blot of DNA obtained from tail tissue. The I2 ,E4, and T59 transgenic lines were maintained by crossing with B7 DKO mice on a B6 background, and progeny were screened for the presence of the B7 Tg by staining peripheral blood lymphocytes with anti-B7.1 or anti-B7−2 mAb. Details of the constructs used for the individual Tg lines are described in Table 1.
Table 1.
| Line | B7 construct | Promoter | Reference |
|---|---|---|---|
| Line 7 | B7.2 full length | H-2Kb promoter plus Ig heavy chain enhancer | (35) |
| Line I2 | B7.2 truncated | EμPμ Ih-μ promoter-enhancer | Unpublished |
| Line E4 | B7.1 truncated | EμPμ Ih-μ promoter-enhancer | Unpublished |
Cell Preparations and FACS Analysis
Single-cell suspensions were prepared from thymus, spleen and liver and resuspended in FACS buffer (0.2% BSA and 0.01% sodium azide in HBSS without phenol red). Hepatic leukocytes were isolated from livers perfused with PBS and gently passed through a 70 micron cell strainer. Total liver cells were washed once in RPMI with 5% FCS then resuspended in 40% isotonic (1 part 10X PBS, 9 parts Percoll) Percoll underlaid with 60% isotonic Percoll. Following centrifugation for 30 minutes at 800g, liver leukocytes were recovered from the 40%/60% Percoll interphase and washed once in RPMI with 5% FCS. Thymic stromal cell preparations from control and B7 tg mice were made by digestion with Liberase 1 at 1U/ml plus DNase 1 (Roche; Mannheim, Germany) in 4 sequential incubations at 37°C. Reactions were stopped by addition of FCS to 20% and remaining tissue was disrupted by passage through a 25g needle. Cells were washed twice and then used for staining. Staining for iNKT cells was performed by incubating cells with CD1tet for 45 minutes at 4°C. Cells were then washed and incubated with anti-FcR mAb 24G2 (to prevent FcR-mediated binding) and FITC-, APC- and biotin-labeled mAbs for 30 min. Following extensive washing, cells were incubated with streptavidin-PerCP conjugate. Data were collected using a FACSCalibur or FACSAria flow cytometer and analyzed using either CellQuest (BD Biosciences) or FloJo (TreeStar, San Carlos, CA) FACS analysis software.
Enrichment of CD1tet+ thymocytes
Thymocytes were enriched for CD1tet-binding cells by two different methods. For analysis of HSAhi thymic iNKT cells, CD1tet-binding cells were enriched by incubating thymocyte cell suspensions with PE-conjugated CD1tet followed by enrichment with anti-PE magnetic microbeads (Miltenyi Biotec, Auburn, CA) following the manufacturer's instructions. The Vβ repertoire of thymic iNKT cells was assessed using CD8-depleted thymocytes prepared by incubating thymocyte cell suspensions with anti-CD8 microbeads (Miltenyi Biotec, Auburn, CA) following the manufacturer's instructions.
Cytokine Assay
Thymocytes plated at 2 x 106 cells/ml were stimulated with PMA (200 ng/ml) and ionomycin (300 ng/ml) for 5 hours. Cells were stained with PE-conjugated CD1tet as described above and processed for intracellular cytokine staining according to the manufacturer's protocol (Fixation/Permeabilization kit with GolgiStop; BD Biosciences). Cells were stained with anti-IFN-γ-FITC and anti-IL-4-allophycocyanin for 30 min at 4°C.
Generation and analysis of radiation bone marrow chimeras
Radiation bone marrow chimeras were prepared as described (39). Chimeras were generated by reconstituting lethally irradiated (950 rad) B6.Ly5.1/Ly5.2 mice with 107 T-depleted bone marrow cells from CD28 WT (Ly5.2) and CD28 KO (Ly5.1) mice on a B6 background. Chimeric mice were analyzed 6−10 weeks following reconstitution. For analysis of thymic NKT cells, single cell suspensions of thymocytes were stained with anti-CD45.2 (Ly5.1), anti-CD28, and anti-NK1.1 for analysis of NKT cells. The CD28WT and CD28KO donors were identified as CD28 positive and CD45.2 positive, respectively. For analysis of CD4+CD25+ T regulatory thymocytes, single cell suspensions were stained with anti-CD45.1 or anti-CD45.2, anti-CD25, anti-CD4 and anti-CD8. The CD28WT and CD28KO donors were identified as CD45.2 negative or CD45.1 negative, respectively.
Statistical analysis
Comparisons between paired values were performed using a Wilcoxon signed rank test while comparisons between two groups of animals were performed using an exact Wilcoxon rank sum test. Unless otherwise stated, all p-values are two-tailed and have not been adjusted for multiple comparisons. Statistical evaluations of the relationship of CD28KO/WT NKT cells to CD28KO/WT total thymocytes were performed using standard linear regression methods in order to determine the simplest model which adequately described the data.
Results
Thymic iNKT cell development is impaired in CD28 KO and B7 DKO mice
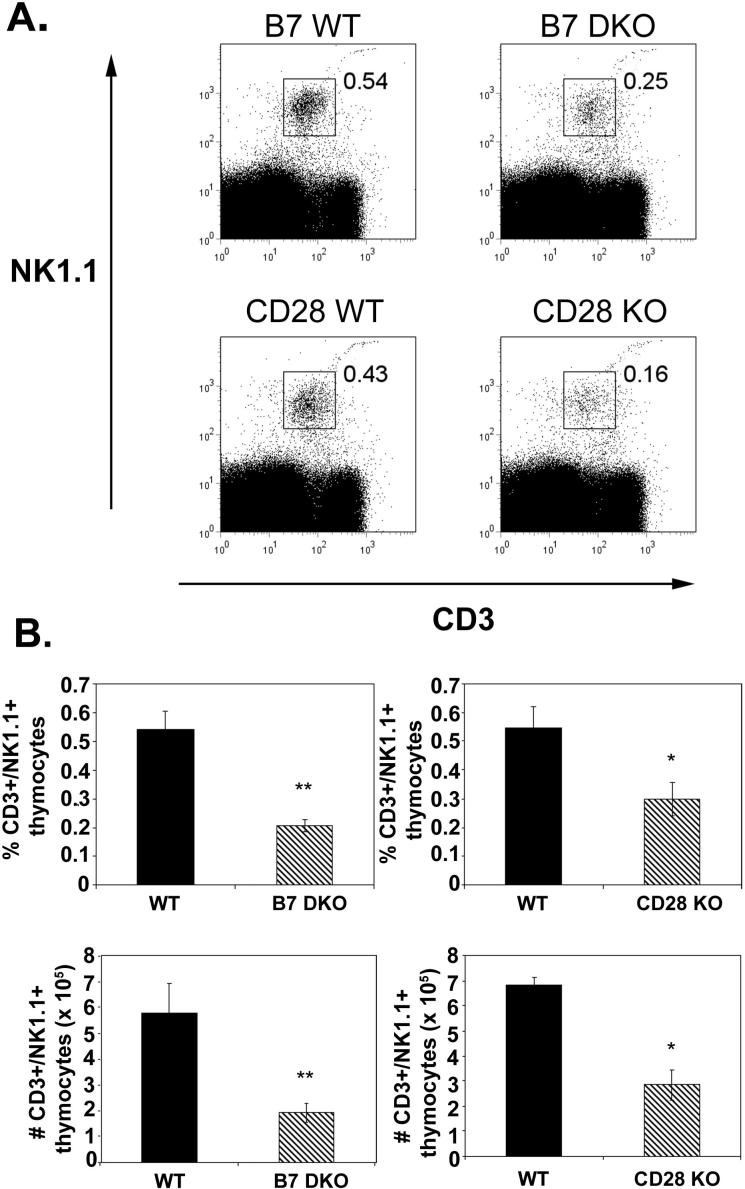
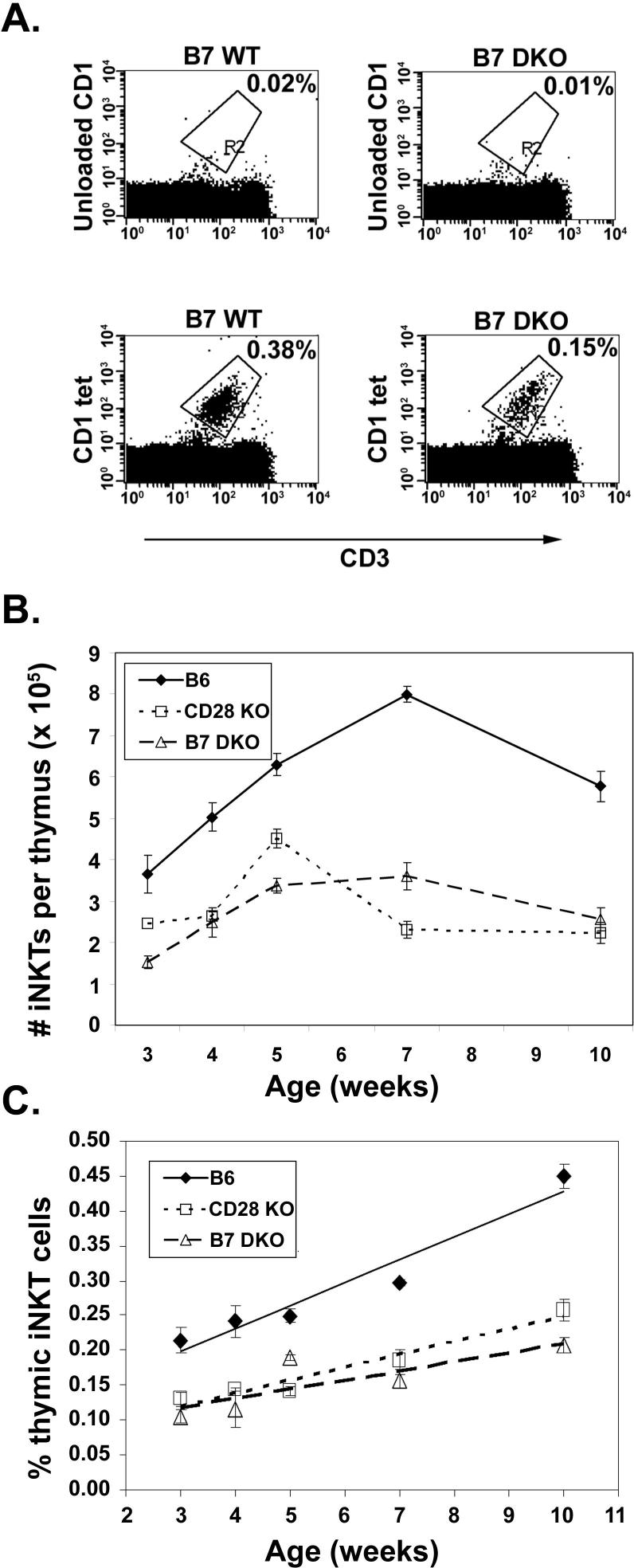
To determine whether the B7-CD28 costimulatory pathway is critical to thymic NKT cell development, frequencies and numbers of thymic NKT cells from 7−12 week old CD28 KO and B7 DKO mice were compared with wildtype litter-mate controls using NK1.1 as a marker for NKT cells. We found a significant decrease in both the number and frequency of CD3+NK1.1+ thymic NKT cells in both CD28KO and B7 DKO mice relative to littermate controls (Fig. 1A and 1B). Thymic NKT cells become detectable approximately 8 days after birth and increase in frequency thereafter (22). To further assess differences in thymic NKT frequencies in B7 DKO, CD28KO and wildtype mice we determined the frequencies of thymic iNKT cells at various timepoints after birth using CD1tet (33) which permits the assessment of iNKT frequencies independent of acquisition of the NK1.1 marker, a relatively late event in NKT cell maturation (40, 41). As can be seen in Fig 2B and C, there is a substantial decrease in the numbers and frequency of thymic iNKT cells in CD28 KO and B7 DKO mice relative to wildtype mice at all ages examined. While the proportion of CD3+ cells in the periphery of WT and CD28 KO mice at all ages examined were comparable (data not shown), reductions in iNKT cell frequencies similar to those observed in the thymus are seen in the spleen and liver of young (3−5 week old) CD28 KO mice relative to age-matched wildtype mice (Fig 3). The differences observed between peripheral populations of iNKT cells in KO and wildtype mice become less significant with age (6−12 weeks; Fig 3), suggesting that there may be differences in factors that act to drive expansion or survival of peripheral versus thymic iNKT cells. Because peripheral iNKT cells originate in the thymus (21, 23, 24) we have focused our studies on requirements for B7-CD28 interactions during differentiation of thymic iNKT cells.
Figure 1.
Decrease in NKT thymocytes in B7 DKO and CD28 KO mice. A. Thymocytes from B7 DKO (age 8−12 wk), CD28 KO (age 7−9 wk old) and littermate control mice were stained with anti-CD3 and anti-NK1.1 and analyzed by flow cytometry. B. Summary of analyses performed as in Panel A for B7 DKO and littermate controls (n=7 pairs) and CD28 KO and littermates controls (n=4 pairs). Data shown represent mean ± SE of each group. Thymic NKT cell frequencies in CD28 KO and B7 DKO mice are statistically different from those in wildtype mice using an exact Wilcoxon rank sum test (*, p < 0.05; **, p < 0.005).
Figure 2.
Decreased number and frequency of iNKT thymocytes with aging in CD28 KO and B7 DKO mice. Thymocytes from wildtype, CD28 KO and B7 DKO mice were harvested at the indicated number of weeks post birth and stained with anti-CD3 and CD1tet. A. Representative flow cytometry dot plots for wildtype and B7 DKO mice at 8 weeks. B. and C. Thymocytes from a minimum of 3 mice per strain at each age were analyzed as shown in Panel A. With the exception of CD28 KO iNKT frequency at 3 weeks, where only 3 mice per group were analyzed, thymic NKT cell numbers and frequencies in CD28 KO and B7 DKO mice are statistically different from those in wildtype mice (p ranging from <0.05 to < 0.005) at all time points using an exact Wilcoxon rank sum test.
Figure 3.
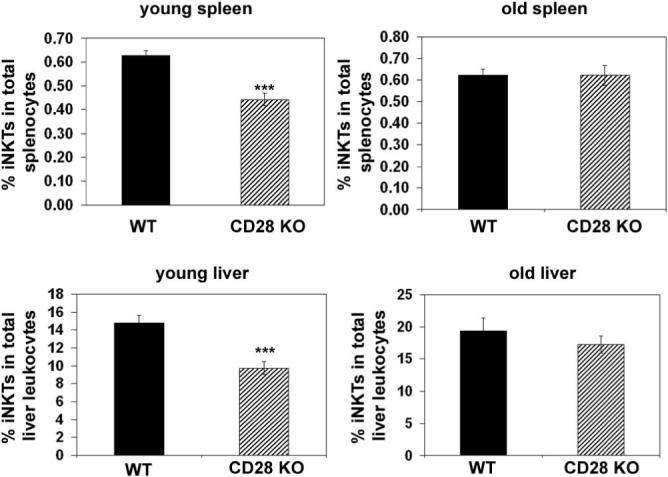
Decreased iNKT frequency in periphery of young (3−5 week) mice. Spleen and liver cells from wildtype and CD28 KO mice were harvested at the indicated ages (young mice, 3−5 weeks; old mice, 6−12 weeks) and stained with anti-CD3 and CD1tet (spleen) or anti-CD45, anti-CD3 and CD1tet (liver). Data shown represent a mean ± SE for each group (minimum of 9 mice per group). Spleen and liver NKT cell frequencies in young CD28 KO mice are statistically different from those in young wildtype mice using an exact Wilcoxon rank sum test (p < 0.005).
The defect in thymic iNKT cell development in CD28 KO and B7 DKO mice is post-selection
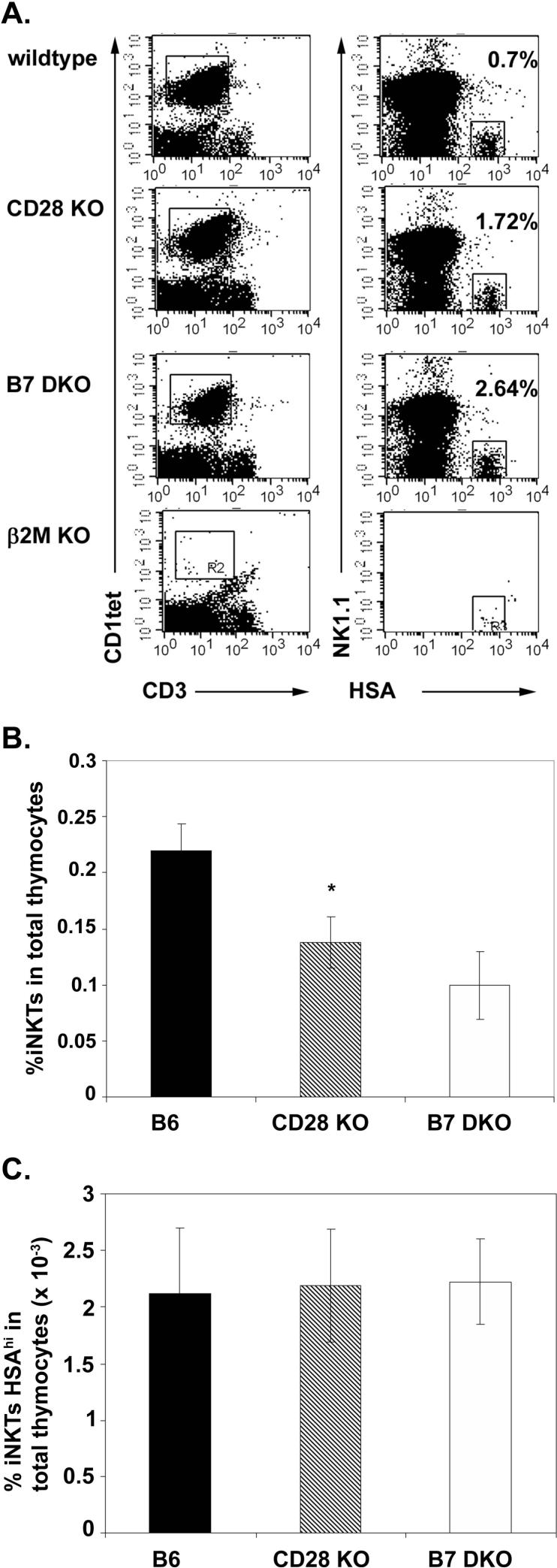
The deficiency in thymic iNKT cell numbers could reflect a deficiency in selection of cells into the iNKT developmental pathway and/or a defect at subsequent stages of maturation and expansion. The earliest discernable population of iNKT cells present in the thymus are CD1tet+ HSAhi thymocytes that, like HSAhi CD69+ CD4 and CD8 SP thymocytes, are thought to represent cells that have been recently positively selected (40). While the majority of CD1tet+ thymocytes are HSAhi in 3−4 day old mice, these levels drop to less than 1% of CD1tet+ cells by 2 weeks of age (40). To assess whether B7-CD28 costimulation is involved in positive selection into the iNKT lineage, we determined the frequency of CD1tet+ HSAhi thymocytes in 3.5−4.5 week old wildtype, CD28 KO and B7 DKO mice. To assess the frequency of this rare population we first enriched for iNKT thymocytes using a CD1tet-based magnetic bead cell sorting strategy. As a control, we performed the CD1tet enrichment procedure on thymocytes from mice lacking CD1 (β2m KO). As observed previously by Benlagha et al. (40) we find that CD1tet+HSAhi cells are absent from mice that do not express CD1 (Fig 4A). When we compared the frequency of HSAhi iNKT cells in the CD1tet-enriched total iNKT populations from wildtype, CD28 KO, and B7 DKO mice we observed an increased frequency in the knockout relative to wildtype mice (Fig 4A). When the frequency of CD1tet+HSAhi iNKT thymocytes (Fig. 4B) is combined with the thymic iNKT frequency in knockout and wildtype (Fig. 4A) to calculate the frequency of CD1tet+HSAhi iNKT cells per thymus, this overall frequency is comparable among the B7 DKO, CD28 KO and wildtype mice (Fig 4C). Taken together, these findings indicate that CD28 costimulation is not involved in the positive selection of this earliest HSAhi iNKT population. Thus, at the level of iNKT cell positive selection, the requirements for signal 1 through TCR and signal 2 through CD28 can be dissociated; positive selection fails to occur in the absence of CD1 (23, 40, 42) but occurs with equal efficacy in the presence and absence of B7-CD28 interactions.
Figure 4.
HSAhi CD1tet+ thymocytes are present at comparable levels in wildype and CD28 KO mice. A. Representative flow cytometry dot blots showing frequencies of HSAhi CD1tet+ thymocytes in wildtype, CD28KO, B7 DKO and β2M KO mice. CD1tet-enriched thymocytes were stained with anti-HSA, CD1tet, anti-CD3 and anti-NK1.1. CD1tet+ -gated thymocytes (left panel) were examined for expression of HSA and NK1.1 (right panel). B. iNKT frequencies in wildtype, CD28 KO and B7 DKO thymocytes prior to CD1tet enrichment. C. Frequency of HSAhiCD1tet+ thymocytes in wildtype, CD28KO and B7 DKO mice. Data shown in B and C represent mean ± SE of 5 separate experiments each using mice at 3.5−4.5 weeks with n=3 to 5 mice per strain for each experiment. B7 DKO mice were evaluated in only 2 of the 5 experiments, precluding statistical analysis. Thymic iNKT cell frequencies in CD28 KO are statistically different from those in wildtype mice using an exact Wilcoxon rank sum test (*, p < 0.05).
B7-CD28 interactions are required for efficient maturation of thymic iNKT cells
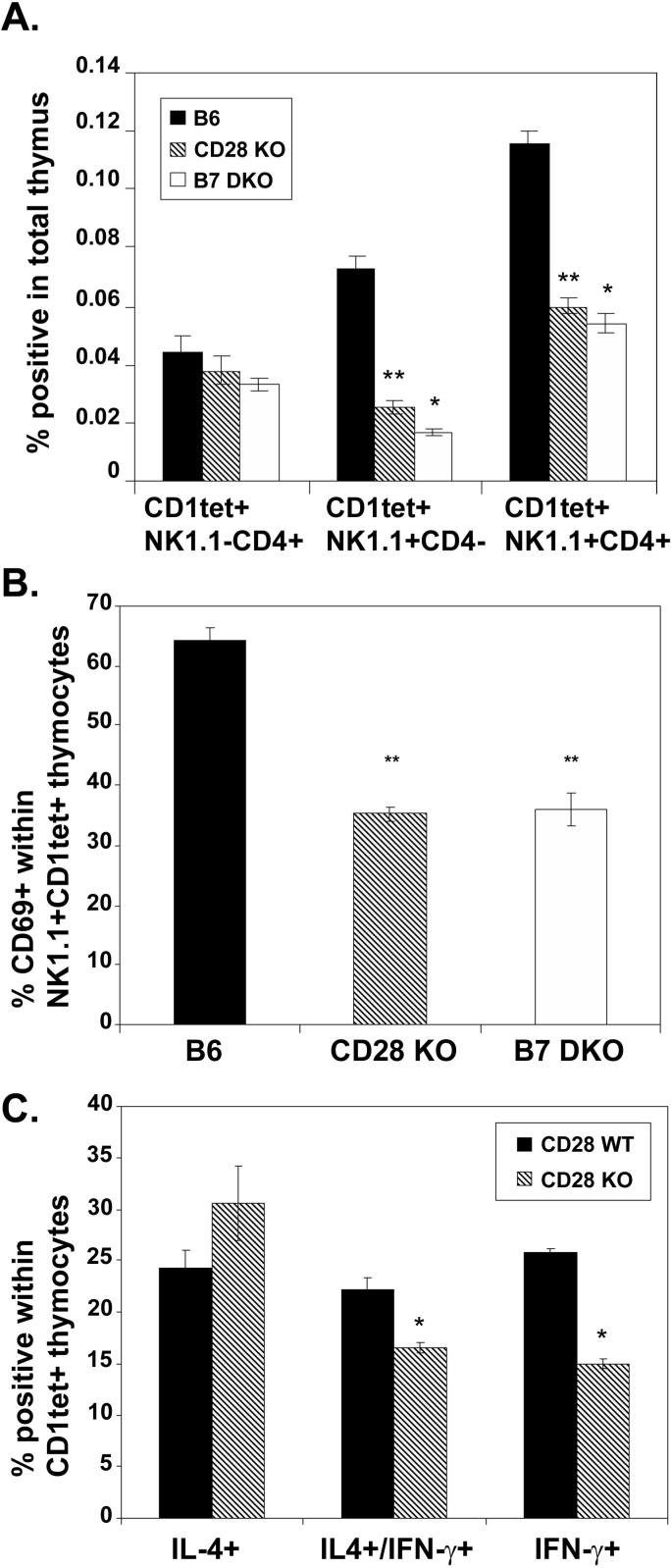
Once committed to the iNKT lineage, iNKT cells progress through a series of distinct maturation steps that have been defined by cytokine expression potential as well as expression of cell surface markers including NK1.1, CD69, and CD4. NK1.1 has been demonstrated to be a marker of relatively more mature iNKT cells (43). Immature iNKT cells are NK1.1neg and CD4+; as these cells mature, they acquire NK1.1 and a fraction of the NK1.1+ cells lose expression of CD4 to become DN (22, 40, 41). When tetramer positive cells from 4−5 wk old wildtype, CD28 KO and B7 DKO mice were compared for expression of NK1.1 and CD4, iNKT cells from the knockout mice had a less mature phenotype: comparable frequencies of the most immature NK1.1neg, CD4+ iNKT cells were observed in wildtype and knockout mice, while the frequencies of more mature CD4negNK1.1+ and CD4+NK1.1+ iNKT cells were lower in the knockout relative to wildtype mice (Fig 5A). CD69 expression has also been correlated with stage of iNKT cell maturation in the thymus. The majority of immature CD1tet+HSAhi iNKTs express CD69 while expression of CD69 in CD1tet+HSAlo iNKTs is correlated with NK1.1 expression; less than 10% of CD1tet+HSAloNK1.1− express CD69 while roughly 70% of more mature CD1tet+HSAloNK1.1+ iNKTs express CD69 (41). When CD69 expression was examined on thymic iNKT cells in wildtype and knockout mice, a significant decrease in the frequency of NK1.1+ iNKT thymocytes expressing CD69 was observed in CD28 KO and B7 DKO mice relative to wildtype mice, again consistent with a less mature phenotype of these knockout iNKT thymocytes (Fig 5B). Expression of NK1.1 on peripheral iNKT populations in the spleen and liver was not significantly different between wildtype and CD28 KO mice at all ages examined (data not shown). Thus, even in young animals (4−6 wks) where peripheral iNKTs cells are significantly reduced in CD28 KO relative to wildtype mice, differences in maturation as reflected by NK1.1 expression were not consistently observed, perhaps reflecting the recent finding that a significant proportion of NK1.1 negative iNKTs in the periphery are a stable, mature population distinct from thymic NK1.1 negative iNKTs (44).
Figure 5.
iNKT thymocytes from CD28 KO and B7 DKO mice are phenotypically less mature than wildtype iNKT thymocytes. Thymocytes from 4−5 week old wildtype, CD28 KO and B7 DKO mice were stained with CD1tet, anti-CD3, anti-CD4 and anti-NK1.1 (A) or CD1tetCD1tet, anti-CD3, anti-CD69 and anti-NK1.1 (B) and analyzed by flow cytometry. C. Total thymocytes from 4−5 week old wildtype and CD28 KO were treated with PMA (200 ng/ml) and ionomycin (300 ng/ml) for 5 hrs then stained with CD1tet and intracellular IL4 and IFN-γ. Data shown is representative of 3 separate experiments performed under similar conditions. Data shown in A-C represent mean ± SE of 3 to 6 mice in each group. The frequency of NK1.1+CD4−, NK1.1+CD4+, and NK1.1+CD69+ iNKT cells in CD28 KO and B7 DKO mice is statistically different from that in wildtype mice using an exact Wilcoxon rank sum test (two-tailed; *, p < 0.05; **, p < 0.005). The frequency of IFNγ+/IL-4+, and IFN-γ+ cells in CD28 KO and B7 DKO mice is statistically different from that in wildtype mice using an exact Wilcoxon rank sum test (one-tailed, consistent with our prior hypothesis (see text); *, p < 0.05).
Unlike most thymic T cells, NKT cells are capable of responding to initial in vitro TCR stimulation by rapidly synthesizing and releasing cytokines, including IFN-γ and IL-4. The relative levels of IFN-γ and IL-4 expressed in iNKT cells has been found to correlate with developmental stage such that cells are skewed towards IL-4 production early in development and towards IFN-γ later in development (21), (41). To test the hypothesis that knockout iNKT thymocyes would be less mature by functional phenotype, similar to the relative immaturity demonstrated by cell surface markers, the cytokine producing potential of thymic iNKT cells was determined. This was accomplished by stimulating thymocytes from 4−5 week old CD28KO and wildtype mice for 5 hours in vitro with PMA and ionomycin and detecting intracellular cytokine production by flow cytometry. When the relative proportions of thymic iNKT cells producing cytokine were assessed, a reduction in frequency of IFN-γ- producing cells as well as IFN-γ and IL-4-producing cells was observed in the CD28KO mice relative to wildtype mice (Fig 5C), consistent with a less mature functional phenotype of iNKT cells in the absence of B7-CD28 interactions. Taken together, the data characterizing selection and maturation of thymic iNKT cells in wildtype, CD28 KO and B7 DKO mice support the conclusion that, in the absence of B7-CD28 interactions, initial selection into the iNKT lineage is unimpaired while the subsequent maturation of this population is compromised.
Cell autonomous and non cell-autonomous roles for CD28 in thymic iNKT development
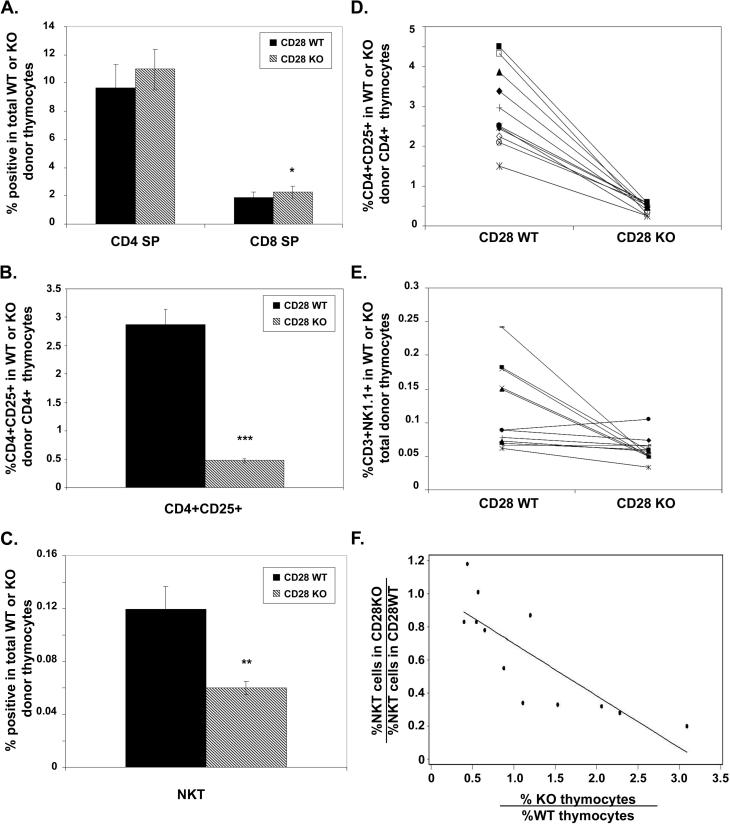
The observed effects of altered CD28/B7 costimulation on iNKT development could reflect cell-intrinsic requirements for signaling through CD28 receptors on developing NKT cells, and/or could reflect an indirect effect of CD28 signaling in the thymus. To determine whether the observed defect in iNKT cell number is cell autonomous, mixed bone marrow chimeric mice were constructed by transferring an equal mix of T cell-depleted bone marrow cells from WT and CD28 KO mice into irradiated WT recipients. Mixed chimeras analyzed 6−10 weeks post reconstitution demonstrated an overall balanced chimerism, though chimerism in individual mice ranged from 1:3 to 3:1, WT:KO. In the thymus, the percent contribution of each donor to CD4+ SP, CD8+ SP, NKT (NK1.1+), and CD4+CD25+ (T regulatory) cells was assessed by gating on each donor population and then determining the frequency of the population of interest within that donor population. On the basis of previous studies, frequencies of CD4+SP and CD8+SP from WT and CD28 KO donors were expected to be equivalent, while in contrast, it was expected that T regulatory thymocytes would be selectively derived from CD28 WT, not CD28 KO, donors (30). Figure 6A shows that the frequencies of CD4+SP thymocytes in the CD28 WT and CD28 KO donor-derived populations were similar, consistent with CD28 being dispensable for conventional T cell development, as previously reported (26, 27). CD8+SP thymocytes from the two donors have very similar means but, in a paired statistical analysis, the frequency of CD8+SPs in the CD28KO is significantly higher than in the CD28 WT. Additional insight into this difference will require further analyses to determine whether the difference resides in the CD8+SP immature (ISP) or mature population. In contrast to CD4+SP and CD8+SP cells, the frequency of CD4+CD25+ thymocytes in the CD28 WT donor-derived population was several-fold higher than in the CD28 KO donor population, in keeping with the previously described role of CD28 costimulation in differentiation of this population of cells (Fig 6B) (29, 30). Interestingly, when NKT cell populations were examined, the frequency of NKT cells was significantly higher in the CD28 WT donor-derived population relative to the CD28 KO donor population (Fig 6C). However, when the frequencies of CD4+CD25+ and NKT thymocytes within the CD28 WT and CD28 KO donor populations were examined in individual chimeras, it was apparent that there was greater variability in the relative frequencies of NKT thymocytes (Fig 6D and E). Overall, roughly one third of the chimeras showed similar frequencies of thymic NKTs in both donors while the remaining two-thirds had higher frequencies in the CD28 WT relative to the CD28 KO donor (Fig 6E). To probe the basis for this heterogeneity among individual chimeric mice, we plotted the ratio of NKT cell frequencies from each donor, as a function of the ratio of the CD28 KO to CD28 WT donor contribution to the total thymocyte population. When analyzed in this way, we noted a significant correlation between overall thymocyte chimerism and the NKT frequency within each donor population (adjusted r-squared = 0.64, p=0.001 for the slope parameter) (Fig 6F). When the ratio of CD28 KO to WT thymocytes was high in a given chimera, i.e. when there were relatively few CD28 WT thymocytes present, the relative frequency of CD28 KO NKT cells was low, evidence of a cell autonomous defect in generation of CD28-deficient NKT cells. In contrast, when the ratio of CD28 KO to WT thymocytes was low, the efficiency of NKT generation was similar in CD28 KO and WT cells, indicating that a non-cell autonomous mechanism was capable of supporting generation of CD28-deficient NKT cells under these conditions. Additional experiments assessing CD1tet+ iNKT cells generated similar results (data not shown), indicating that CD28 mediates both cell autonomous and non-autonomous functions in iNKT development.
Figure 6.
Cell-autonomous and non-cell-autonomous roles for CD28 signaling during iNKT cell development. CD28 WT-CD28 KO mixed chimeras were made by reconstituting lethally irradiated B6.Ly5.2/Ly5.1 host mice with equal numbers of bone marrow cells from B6.Ly5.2 (CD28 WT) and B6.Ly5.1 (CD28 KO) mice. Thymocytes were analyzed 6−10 wk after reconstitution. Analyses of cell populations deriving from CD28 WT and CD28 KO donors were made by gating on each donor-derived population and determining the frequency of either CD4 SP, CD8 SP (A), CD4+CD25+ (B) or CD3+NK1.1+ (C) within the gated population. Data shown in A-C represent the mean ± SE; n=12 mice D. and E. show the frequencies of CD4+CD25+ thymocytes (D) or CD3+NK1.1+ (E) in individual chimeric mice. The frequency of CD4+CD25+ and NKT thymocytes in the CD28KO donor population is statistically different from that in the CD28WT donor by a Wilcoxon signed rank test (*, p < 0.05;**, p < 0.005; ***, p < 0.0005). F. A simple linear regression model for a straight line shows a significant correlation (r-squared = 0.64) between overall chimerism and the relative frequencies of NKT thymocytes within CD28WT and CD28KO donor populations.
Overexpression of CD28 or B7 on thymocytes leads to diminished iNKT cells
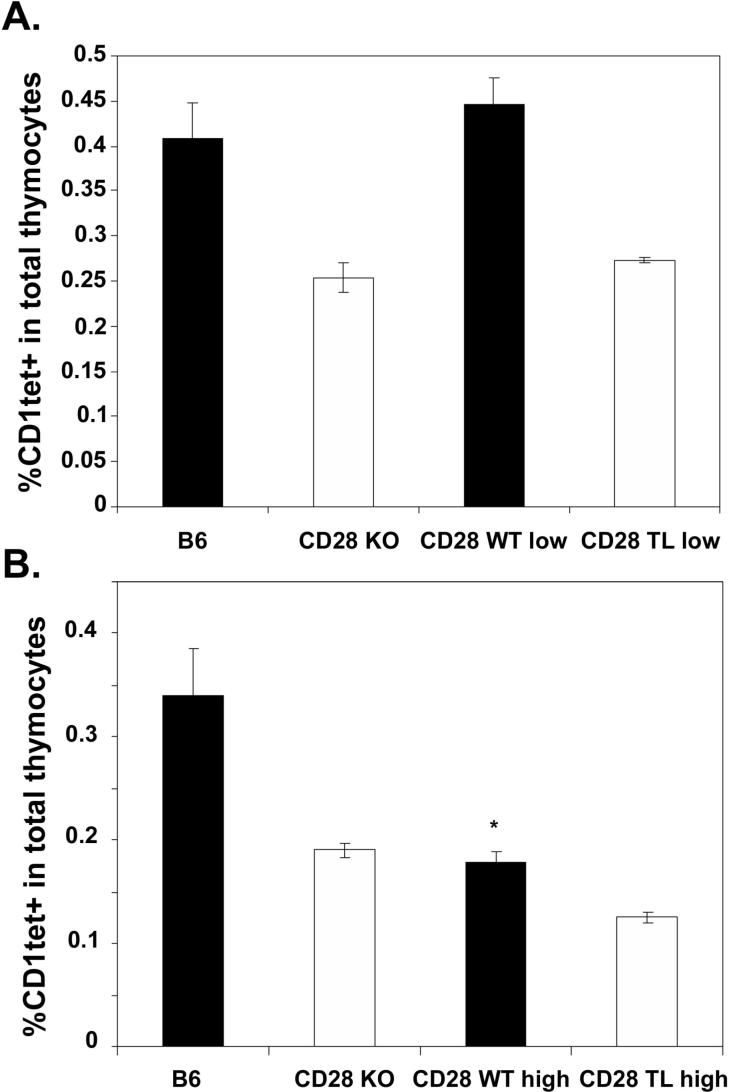
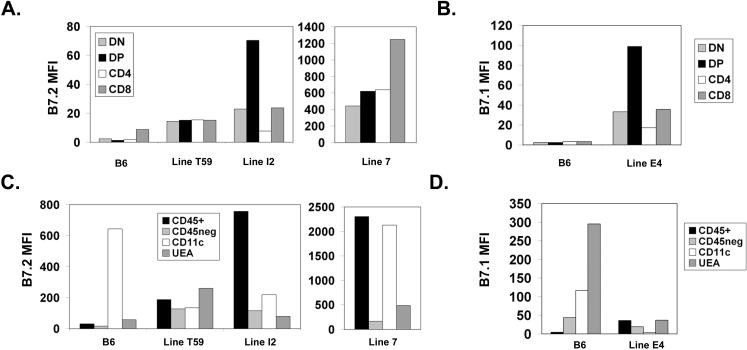
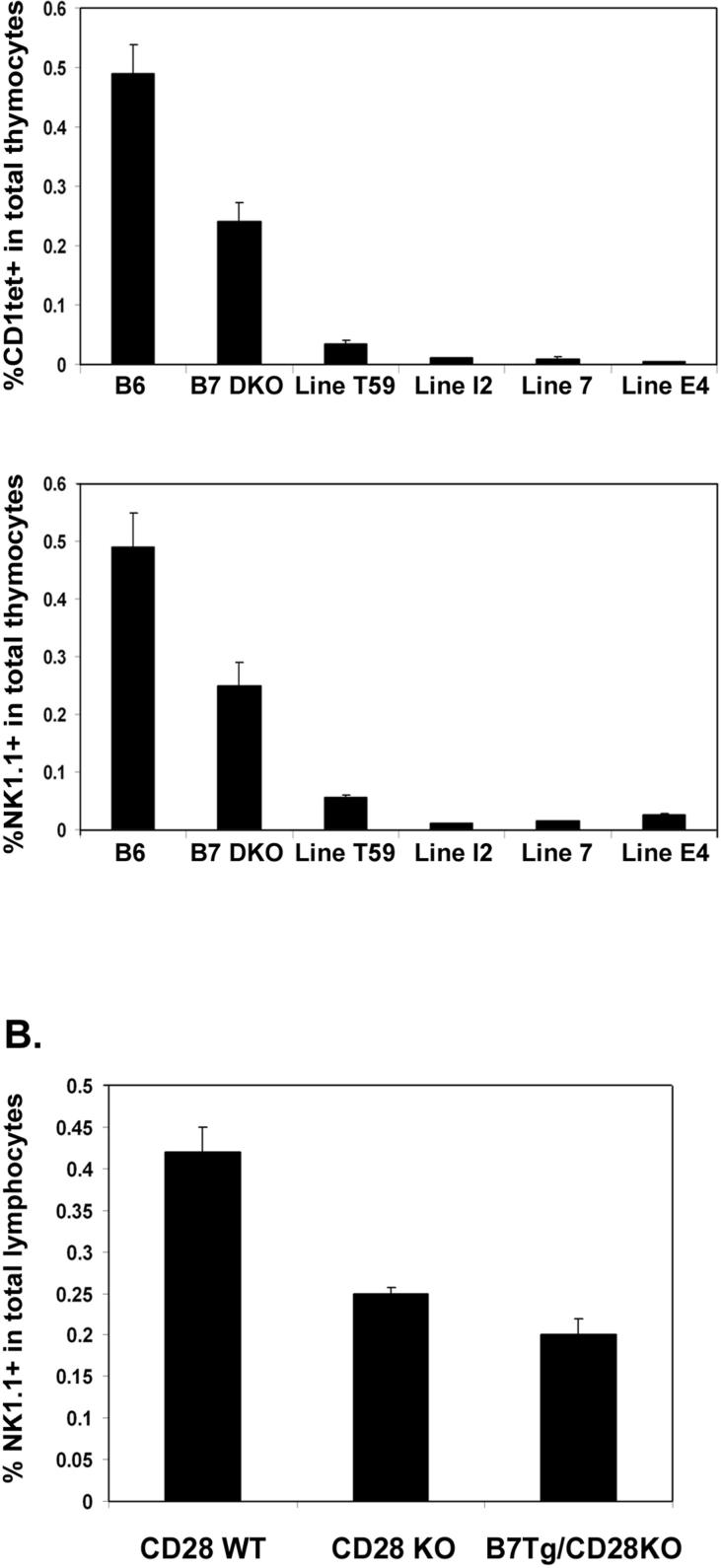
Though the selection process for iNKT cells in the thymus is not fully understood, several reports have suggested that these cells can be subject to negative selection in the presence of high levels of CD1d agonist ligand (45, 46). Thus, while CD1d is clearly critical for development of iNKT cells, as the iNKT cell population is absent from CD1d deficient animals, excess expression of CD1d can apparently result in negative selection of this same population. This strict dependence on ligand density may be related to the very restricted TCR repertoire of the iNKT population. We were therefore interested in determining whether development of iNKT cells would be similarly sensitive to variation in levels of CD28 and its ligand B7. To accomplish this, we analyzed CD28 KO and B7 DKO mice expressing varying levels of CD28 and B7.2 transgenes, respectively. We hypothesized that a strong costimulatory signal, in combination with TCR signaling by antigenic ligand, may also be capable of modulating the balance of positive and negative selection in the iNKT cell compartment, and that increased CD28 signaling might therefore result in decreased NKT cell development. To test this, we analyzed transgenic lines expressing CD28 at low and high levels as well as four transgenic lines expressing variable amounts of B7.1 or B7.2 on thymocytes. In all transgenic lines studied, the major thymocyte subpopulations, DN, DP, CD4 SP and CD8 SP, were present at similar frequencies to those of wildtype control animals (data not shown). We first examined iNKT cells in two CD28 WT tg lines, CD28WTlow, which expresses wildtype CD28 at levels comparable to those of endogenous CD28 (30), and CD28WThigh, which expresses WT CD28 at levels roughly 20-fold the endogenous level (36). We found that the frequency of thymic iNKT cells in the CD28WTlow tg line was comparable to that of wildtype mice, demonstrating that restoring a physiologic level of CD28 expression to CD28 KO mice rescued efficient development of iNKT cells (Fig 7A). By contrast, the frequency of thymic iNKT cells was decreased relative to wildtype mice in the CD28WThigh tg line (Fig 7B). Thymic iNKT cells were not restored in CD28 tgs expressing either low or high levels of a truncated CD28 tg (CD28TL), indicating that the cytoplasmic domain of CD28 plays an essential role in mediating CD28 function in iNKT development. To determine whether expression of transgenic B7 molecules would alter thymic iNKT development, we assessed thymic iNKT cell numbers in mice expressing varying levels of transgenic B7.1 and B7.2. We found that mice expressing high levels of transgenic B7.1 or B7.2 (Fig 8A and B) had dramatically reduced numbers of thymic iNKT cells, even relative to the levels in completely B7-deficient mice (Fig 9A). Interestingly, this reduction in thymic iNKTs correlated with expression of transgenic B7 on thymocytes, and not with expression on CD45− cells (including UEA+ medullary epithelial cells (mTECs)) or dendritic cells in the thymus. This is most clearly illustrated in the Line E4 transgenic where B7.1 transgene expression is elevated relative to endogenous B7.1 on the majority of CD45+ cells but is expressed at levels lower than endogenous B7.1 on DCs (Fig 8A and B). The inhibitory effect of B7 tg expression on thymic iNKT cell development does, however, require CD28 expression, as expressing each of these B7 tgs on a CD28 KO background resulted in numbers of NKT cells comparable to those of CD28 KO mice and greater than numbers observed in B7 tgs on a wildtype CD28 background (Figure 9B). The absence of NKT cells in the B7 tg lines thus results from interactions of the B7 tg with CD28 and not from other, unanticipated effects of the high level of B7 expressed. In addition, the reduction in thymic iNKT cell frequency does not require the cytoplasmic portion of the B7 molecule as both the full length and truncated B7 tgs lines showed reduced thymic iNKT cell frequencies.
Figure 7.
Differential reconstitution of iNKT thymocytes in mice expressing CD28 transgenes at low and high levels. A. Thymocytes from 9−10 week old wildtype, CD28 KO, CD28WTlow, and CD28TLlow mice were stained with CD1tet, anti-CD3 and anti-NK1.1 and analyzed by flow cytometry. B. Thymocytes from 6−7 week old wildtype, CD28 KO, CD28WThigh, and CD28TLhigh mice were stained with CD1tet, anti-CD3 and anti-NK1.1 and analyzed by flow cytometry. Data shown represent mean ± SE of at least 3 mice in each group. Thymic iNKT cell frequencies in CD28WThigh mice are statistically different from those in wildtype mice using an exact Wilcoxon rank sum test (*, p < 0.05).
Figure 8.
Patterns of B7.1 and B7.2 expression in B7 transgenic mice. A.and B. Thymocytes from 9−11 week old wildtype, B7 DKO and B7 transgenic mice were stained with anti-B7−2 (A) or anti-B7−1 (B) and CD4 and CD8. The mean fluorescence intensity (MFI) for each thymocyte subpopulation for each strain is presented as the MFI for that strain minus the MFI for the B7 DKO. C. and D. Collagenase-digested thymus preparations were stained with CD45, CD11c, UEA-1 and B7.2 (C) or anti-B7−1 (D). The MFI for each thymus cell subpopulation (CD45+, CD45neg, CD11c+ (CD45+), and UEA+ (CD45 neg )) for each strain is presented as the MFI for that strain minus the MFI for the B7 DKO. Data shown represent the mean MFI of 2−4 mice for each strain.
Figure 9.
Effect of B7 transgene expression on iNKT development. A. Thymocytes from 9−11 week old wildtype, B7 DKO and B7 transgenic mice were stained with CD1tet, anti-CD3, anti-NK1.1 and analyzed by flow cytometry. All B7tg Lines are on a B7 DKO background. B. Thymocytes from 9−10 week old littermates from backcrosses of each of three B7 transgenic lines (Lines I2, E4 and 7) to CD28 KO mice were stained with anti-CD3 and anti-NK1.1. The CD28WT (CD28+/−) and CD28KO (CD28−/−) groups represent transgene negative littermates from the three crosses. The B7Tg/CD28KO group represents pooled data from B7 transgenic lines I2, E4 and 7 lines on a CD28−/− background. Data shown are the mean ± SE of 3 mice in each group (except Figure 9B, CD28WT where n=2).
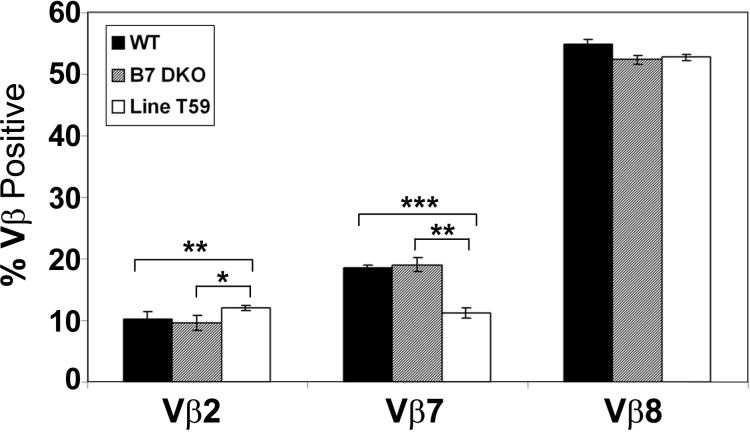
It has been previously demonstrated that iNKT cells in mice overexpressing a CD1d tg are subject to negative selection and that those iNKT cells that do persist have an altered pattern of TCR Vβ expression compared to that observed in wildtype mice. To determine whether the surviving iNKTs in B7 tgs expressed an altered pattern of TCR Vβ expression, we examined Vβ expression on iNKTs in the B7.2 tg line T59 in which deletion of iNKTs is not complete. As shown in Fig. 10, we find that while iNKT thymocytes in Line T59 do preferentially utilize Vβ2, 7 and 8, the relative expression of these Vβs is altered relative to wildtype thymic iNKT cells; in particular, we observe a significant decrease in the proportion of thymic iNKT cells expressing Vβ7 and an increase in thymic iNKT cells expressing Vβ2 in Line T59 relative to both wildtype B6 and B7 DKO mice. Interestingly, despite the clear defects in development of thymic iNKT cells in the absence of B7-CD28 interactions, we do not find significant differences in Vβ usage when comparing thymic iNKT cells in wildtype and B7 DKO mice. Overall, these results indicate that maintenance of physiologic levels of CD28 and B7 are critical for normal development of thymic iNKT cells.
Figure 10.
Vβ chain usage in wildtype, B7 DKO and Line T59 thymic iNKT cells. CD8-depleted thymocytes from 6−12 week old wildtype, B7 DKO and Line T59 mice were incubated with CD1tet, anti-CD3 and anti-Vβ antibody. The percent Vβ+ was calculated from within CD1tet+ gated cells. Frequencies of Vβ2+ and Vβ7+ thymic iNKT cells in the Line T59 mice are statistically different from those in wildtype and B7 DKO mice using an exact Wilcoxon rank sum test (*, p < 0.05;**, p < 0.005; ***, p < 0.0005).
Discussion
We have analyzed iNKT cell populations in wildtype mice and in mice deficient in the B7-CD28 costimulatory pathway and have found a unique role for CD28 signaling in thymic development of these cells. CD28 KO and B7 DKO mice display a 50−75% reduction in the frequency and absolute numbers of thymic iNKT cells. This reduction in iNKT development is observed early in post-natal life, and these differences persist through adult life. By assessing cell surface markers associated with maturation of iNKT cells, we have demonstrated that, at all ages examined, thymic iNKT populations present in CD28 and B7 DKO mice are skewed toward a less mature phenotype than those present in wildtype mice. These results demonstrate that physiologic levels of CD28 signaling are required for optimal expansion and maturation of thymic iNKT cells. Peripheral iNKT cell populations are decreased in CD28 and B7 KOs relative to wildtype mice in young (4−6 wk) mice, but these differences become undetectable as the mice age, perhaps reflecting differences in factors driving expansion/proliferation of iNKTs in thymic and peripheral compartments. In young mice, peripheral populations of iNKT cells may more directly reflect maturation of recently exported thymic iNKT precursors, a process we have demonstrated to be dependent on CD28 signaling, while in older animals homeostatic mechanisms, perhaps less dependent on CD28, may be critical determinants of iNKT cell numbers.
Results of mixed bone marrow chimeras revealed cell-autonomous as well as non-cell-autonomous roles for CD28 in the development of thymic iNKT cells. The requirement for cell-autonomous expression of CD28 was evident in chimeras in which the CD28 KO donor predominated over the wildtype donor. In these animals, the frequency of CD28 KO iNKT cells was not rescued by the presence of wildtype thymocytes. In contrast, chimeras in which the wildtype donor predominated had similar frequencies of iNKT cells within each donor population. We interpret these findings to suggest that when wildtype thymocytes dominate in the thymus, sufficient levels of B7-CD28 signaling are achieved to allow an indirect effect on development of iNKT cells. CD28 signaling could function indirectly via production of cytokines and other growth factors and/or upregulation of cell-surface molecules that can rescue efficient development of CD28 deficient iNKT cells. In chimeric thymi in which CD28-expressing thymocytes are less frequent, indirect effects of CD28 signaling on iNKT development are minimized, and the cell-autonomous function of CD28 in promoting iNKT cell development is evident. These results are in contrast to the role described for CD28 signaling in the generation of CD4+CD25+ T regulatory cells in the thymus where, under the conditions tested, no role for non-cell autonomous CD28 signaling was found ((30) and our own data).
To determine whether the compromised development of iNKT cells in the absence of B7-CD28 interactions is due to a failure to efficiently positively select these cells into the iNKT lineage and/or results from a failure of iNKT cells to expand and mature subsequent to initial selection, we compared the frequencies of HSAhi CD1tet+ thymocytes in wildtype, CD28 KO and B7 DKO animals. HSAhi CD1tet+ thymocytes are thought to correspond to the earliest defined stage of iNKT development and to represent cells which have recently been positively selected (40). Like their recently selected conventional thymocyte counterparts, HSAhi CD1tet+ NKT cells are CD69+, and they are not present in the absence of their selecting ligand, CD1 (40). We observe a similar frequency of HSAhi iNKT thymocytes in wildtype and knockout mice indicating that the earliest steps of development including positive selection of thymic iNKT cells proceeds normally in the absence of B7-CD28 interactions. Once positively selected, nascent iNKT cells decrease HSA expression to become HSAlo, NK1.1−, CD4+ and we find that the frequency of these NK1.1−CD4+ iNKT thymocytes is also not significantly different between wildtype, CD28 KO and B7 DKO mice. It is only as iNKT cells mature further to become NK1.1+ and skewed to IFN-γ production that we find a significant reduction in these more mature thymic iNKT cells when comparing knockout and wildtype mice. Taken together with previously published studies regarding the role of CD1 in selection of iNKT cells, our results indicate that the roles for TCR-CD1 interactions (signal 1) and B7-CD28 costimulatory interactions (signal 2) during thymic development of iNKT cells can be dissociated; CD1 is required both for positive selection (of HSAhi iNKT cells) and for continued maturation of thymic iNKT cells (40, 47), whereas B7-CD28 interactions are not required during positive selection but are necessary for efficient subsequent maturation and/or survival of these cells once selected. Interestingly, Horai et al. (48) have recently described a similar dependency on CD28 signaling for the innate-type CD8 cells that develop in Itk-deficient mice. Similar to NKT cells, these innate-type CD8 cells are selected intrathymically on hematopoietic cells and are characterized by expression of memory markers, dependency on IL-15 and rapid production of cytokines (48).
Though both conventional αβTCR T cells and iNKT cells develop in the thymus from immature double positive thymocytes, these two populations differ in several respects. Unlike conventional αβTCR T cells, iNKT cells express a highly restricted TCR repertoire, undergo a massive proliferative burst subsequent to TCRαβ expression in the DP stage and acquire a memory cell-like phenotype characterized by expression of memory cell surface markers as well as the capacity to rapidly secrete cytokines rapidly following TCR stimulation (reviewed in (1)). Similar to conventional αβTCR T cells that require CD28 signaling for optimal survival, proliferation and memory cell formation, thymic iNKT cells may require CD28 to expand and survive. Among the mechanisms by which CD28 signals to promote survival is the upregulation of the anti-apoptoic molecule, Bcl-xl (49). While the requirements for survival of thymic iNKT cells are not completely understood, evidence that Bcl-xl may have a critical role was provided by studies of B6-ΔNtg mice in which NF-κB signaling is inhibited. Interestingly, the compromised thymic iNKT cell development that characterizes these mice can be partially restored by forced expression of Bcl-xl but not Bcl-2 (10). Based on data linking the proliferative responses of cytokine-stimulated iNKT cells to cell-cycle regulated Bcl-xl, and not Bcl-2 expression, Stanic et al. (10) hypothesize that cycling (NK1.1−) iNKT cells may depend on Bcl-xl rather than Bcl-2 for survival. The signaling motifs within the cytoplasmic tail of the CD28 molecule responsible for the proliferative and anti-apoptotic signals mediated by CD28 have been mapped (50, 51). Whether the CD28 signaling motif known to be important for upregulation of Bcl-xl is involved in CD28's role in promoting thymic iNKT cell development is currently under investigation.
In contrast to conventional T cells that are believed to be positively selected by weak TCR interactions, iNKTs cells are thought to be positively selected by thymic encounter with TCR agonists. However, when developing thymic iNKT cells are exposed to supra-physiologic levels of TCR ligand in model systems where agonist antigen is presented at high concentrations or where CD1d expression is increased above physiologic levels, dramatically reduced numbers of thymic iNKT cells are found (45). Thus it appears that intense TCR signals may promote negative selection of thymic iNKT cells. Our findings in mice transgenic for CD28 or B7 suggest that enhanced costimulatory interactions may also result in negative selection or impaired development of the thymic iNKT population. In mice expressing T-cell restricted CD28 transgenes in the absence of endogenous CD28, we found that the capacity of the transgene to restore the thymic iNKT population was sensitive to the level of CD28 transgene expression. In mice expressing a CD28 transgene at levels comparable to that of endogenous CD28, thymic iNKT cell numbers were restored to wildtype levels, while in mice expressing a CD28 transgene at levels at least 20-fold higher than endogenous CD28, iNKT cell numbers remained similar to those of the CD28 KO. Thus physiologic levels of CD28 support iNKT cell development while higher levels appear to impair development. We also examined mice expressing B7 transgenes; in these mice, the cell-type specific expression of the B7 transgenes differs from endogenous B7 by virtue of the promoter systems used (see Table 1) and results, in all lines examined, in appreciable levels of B7 expression on thymocytes, in contrast to the absent or minimal expression of endogenous B7 on thymocytes. When iNKT cell numbers were assessed in these B7 transgenics, all lines exhibited decreases in iNKT cell numbers relative to B7 DKO mice. This effect of B7 transgene expression was CD28-dependent, reinforcing the conclusion that excessive CD28 signaling severely impairs iNKT development. Interestingly, we observed dramatically impaired development of thymic iNKT cells even in the Line E4 B7 transgenic mice in which elevated expression of B7 levels relative to wildtype are observed only on thymocytes, not CD11c+ DC or CD45neg thymic stromal cells. This contrasts with the findings in CD1d transgenic mice where expression on DCs was important for mediating negative selection of thymic iNKT cells (54) suggesting that distinct cell populations are involved in negative selection mediated by excess signal 1 (CD1d) versus excess signal 2.
We hypothesize that during normal iNKT cell development, nascent iNKT cells are positively selected by CD1-expressing DP thymocytes. The role of B7-CD28 interactions at this stage is likely minimal as similar numbers of HSAhi+CD1tet+ are present in wildtype and knockout animals, consistent with our inability to detect expression of endogenous B7 molecules on DP thymocytes (data not shown). Thus, we propose a model of B7-CD28 engagement in promoting iNKT development in which encounter of the TCR-ligand, CD1d, occurs on DP thymocytes in the absence of CD28 costimulation, resulting in positive selection into the iNKT lineage, while physiologically important B7-CD28 interactions would happen later, perhaps as maturing iNKT cells move from thymic cortex to medulla and encounter B7.1 and B7.2 on thymic DCs and mTECs.
While the importance of the CD28/B7 costimulatory pathway for activation of mature T cells is well established, the role(s) for CD28/B7 interactions during normal thymic development have been more difficult to define. A clear exception is the development of thymic CD4+CD25+ T regulatory cells; B7 or CD28-deficient mice lack significant numbers of T regulatory cells (29, 30). Elegant work by Tai et al. (30) has suggested that it is the capacity of CD28 signaling to induce FoxP3, the T regulatory cell “master gene”, that makes it indispensable to the T regulatory cell developmental program. While clearly important for thymic T regulatory cell development, initial findings in CD28-deficient mice provided no clear evidence of a requirement for B7-CD28 interactions in either positive or negative selection of conventional αβ TCR thymocytes. Negative selection of superantigen-reactive thymocytes and both positive and negative selection of class I- and class II-restricted TCR Tg mice have been reported to remain intact in the absence of CD28 (27). At odds with these findings, however, are those that describe a reduction in deletion or death of double-positive (DP) thymocytes in response to TCR-mediated stimulus in the absence of CD28 (52, 53) and our own findings of a novel negative regulatory role for CD28 in inhibiting differentiation of CD8 SP thymocytes, probably through inhibition of thymic positive selection (28). More definitive conclusions regarding the role of B7-CD28 interactions in selecting the conventional αβ TCR compartment may await methodologies permitting more precise assessment of the αβTCR repertoires that develop in the presence and absence of this costimulatory pathway.
iNKT cells from wildtype mice preferentially express Vβ 2, 7, or 8 together with invariant TCRα chain. When we examined the distribution of Vβ usage on thymic iNKT cells from B7 DKO and wildtype mice, we found no significant differences in the percentages of cells expressing Vβ2, 7 or 8 suggesting that the absence of B7-CD28 interactions does not profoundly affect the iNKT Vβ repertoire. We did find, however, that Vβ usage in a B7.2 transgenic line (Line T59) in which thymic iNKT cells are substantially reduced was significantly altered. In this case, Vβ7 was under-represented while Vβ2 was over-represented when comparing thymic iNKT cells from Line T59 with either B7 DKO or wildtype mice. A similar change in iNKT cell Vβ repertoire was observed in CD1d transgenic mice in which overexpression of CD1d resulted in reduced numbers of iNKT cells (45).
Our findings with respect to the role of B7-CD28 interactions in development of thymic iNKT cells indicate that, similar to requirements for CD28 signaling in T regulatory cell development, the absence of CD28 signals result in a significantly reduced level of this specialized T cell subpopulation. The work of Chung et al., published during revision of our report, similarly identifies a decrease in thymic iNKT development in absence of costimulation (54). Additionally, our data from transgenic mice expressing elevated levels of thymic CD28 or B7 suggest that thymic iNKT cells can be deleted in the presence of high level B7-CD28 interactions similar to what has been reported for DP thymocytes exposed to robust TCR signals in the presence of CD28 signals (52, 53). The role of B7-CD28 interactions in promoting thymic iNKT cell development may relate to the unusual features that characterize intrathymic development of this population including the massive expansion post-positive selection and acquisition of memory cell-like phenotype, features not shared by conventional αβ TCR thymocytes.
Acknowledgments
We thank Karen Hathcock, Pam Schwartzburg and Alfred Singer for critical reading of the manuscript. We thank Genevieve Sanchez-Howard and staff at Bioqual for excellent animal care and husbandry, and National Institutes of Health tetramer core facility for PBS57-loaded CD1d-tetramer.
Abbreviations used in this paper
- α-GalCer
a-galactosylceramide
- DP
double positive
- HSA
heat stable antigen
- iNKT cell
invariant natural killer T cell
- KO
knockout
- Tg
transgenic
Footnotes
JML and JAW contributed equally to this work.
References
- 1.Bendelac A, Savage PB, Teyton L. The Biology of NKT Cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what's in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 3.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 4.Burdin N, Brossay L, Koezuka Y, Smiley ST, Grusby MJ, Gui M, Taniguchi M, Hayakawa K, Kronenberg M. Selective ability of mouse CD1 to present glycolipids: alpha-galactosylceramide specifically stimulates V alpha 14+ NK T lymphocytes. J Immunol. 1998;161:3271–3281. [PubMed] [Google Scholar]
- 5.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mieza MA, Itoh T, Cui JQ, Makino Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, Koseki H, Taniguchi M. Selective reduction of V alpha 14+ NK T cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- 7.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 8.Ohteki T, Ho S, Suzuki H, Mak TW, Ohashi PS. Role for IL-15/IL-15 receptor beta-chain in natural killer 1.1+ T cell receptor-alpha beta+ cell development. J Immunol. 1997;159:5931–5935. [PubMed] [Google Scholar]
- 9.Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. J Exp Med. 2003;197:1613–1621. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanic AK, Bezbradica JS, Park JJ, Matsuki N, Mora AL, Van Kaer L, Boothby MR, Joyce S. NF-kappa B controls cell fate specification, survival, and molecular differentiation of immunoregulatory natural T lymphocytes. J Immunol. 2004;172:2265–2273. doi: 10.4049/jimmunol.172.4.2265. [DOI] [PubMed] [Google Scholar]
- 11.Elewaut D, Shaikh RB, Hammond KJ, De Winter H, Leishman AJ, Sidobre S, Turovskaya O, Prigozy TI, Ma L, Banks TA, Lo D, Ware CF, Cheroutre H, Kronenberg M. NIK-dependent RelB activation defines a unique signaling pathway for the development of V alpha 14i NKT cells. J Exp Med. 2003;197:1623–1633. doi: 10.1084/jem.20030141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 13.Gadue P, Morton N, Stein PL. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J Exp Med. 1999;190:1189–1196. doi: 10.1084/jem.190.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberl G, Lowin-Kropf B, MacDonald HR. Cutting edge: NKT cell development is selectively impaired in Fyn- deficient mice. J Immunol. 1999;163:4091–4094. [PubMed] [Google Scholar]
- 15.Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, de Saint-Basile G, Latour S. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 18.Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei DG, Lee H, Park SH, Beaudoin L, Teyton L, Lehuen A, Bendelac A. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202:239–248. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J Immunol. 2000;164:2412–2418. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- 21.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 22.Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- 24.Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, Bendelac A, Littman DR. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Bezbradica JS, Hill T, Stanic AK, Van Kaer L, Joyce S. Commitment toward the natural T (iNKT) cell lineage occurs at the CD4+8+ stage of thymic ontogeny. Proc Natl Acad Sci U S A. 2005;102:5114–5119. doi: 10.1073/pnas.0408449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 27.Walunas TL, Sperling AI, Khattri R, Thompson CB, Bluestone JA. CD28 expression is not essential for positive and negative selection of thymocytes or peripheral T cell tolerance. J Immunol. 1996;156:1006–1013. [PubMed] [Google Scholar]
- 28.Vacchio MS, Williams JA, Hodes RJ. A novel role for CD28 in thymic selection: elimination of CD28/B7 interactions increases positive selection. Eur J Immunol. 2005;35:418–427. doi: 10.1002/eji.200424918. [DOI] [PubMed] [Google Scholar]
- 29.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 30.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 31.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 32.Hathcock KS, Laszlo G, Dickler HB, Bradshaw J, Linsley P, Hodes RJ. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993;262:905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Goff RD, Zhou D, Mattner J, Sullivan BA, Khurana A, Cantu C, 3rd, Ravkov EV, Ibegbu CC, Altman JD, Teyton L, Bendelac A, Savage PB. A modified alpha-galactosyl ceramide for staining and stimulating natural killer T cells. J Immunol Methods. 2006;312:34–39. doi: 10.1016/j.jim.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7−1 and B7−2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 35.Fournier S, Rathmell JC, Goodnow CC, Allison JP. T cell-mediated elimination of B7.2 transgenic B cells. Immunity. 1997;6:327–339. doi: 10.1016/s1074-7613(00)80335-0. [DOI] [PubMed] [Google Scholar]
- 36.Harada Y, Tokushima M, Matsumoto Y, Ogawa S, Otsuka M, Hayashi K, Weiss BD, June CH, Abe R. Critical requirement for the membrane-proximal cytosolic tyrosine residue for CD28-mediated costimulation in vivo. J Immunol. 2001;166:3797–3803. doi: 10.4049/jimmunol.166.6.3797. [DOI] [PubMed] [Google Scholar]
- 37.Yu X, Abe R, Hodes RJ. The role of B7-CD28 co-stimulation in tumor rejection. Int Immunol. 1998;10:791–797. doi: 10.1093/intimm/10.6.791. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt EV, Pattengale PK, Weir L, Leder P. Transgenic mice bearing the human c-myc gene activated by an immunoglobulin enhancer: a pre-B-cell lymphoma model. Proc Natl Acad Sci U S A. 1988;85:6047–6051. doi: 10.1073/pnas.85.16.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singer A, Hathcock KS, Hodes RJ. Cellular and genetic control of antibody responses. V. Helper T-cell recognition of H-2 determinants on accessory cells but not B cells. J Exp Med. 1979;149:1208–1226. doi: 10.1084/jem.149.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gadue P, Stein PL. NK T cell precursors exhibit differential cytokine regulation and require Itk for efficient maturation. J Immunol. 2002;169:2397–2406. doi: 10.4049/jimmunol.169.5.2397. [DOI] [PubMed] [Google Scholar]
- 42.Eberl G, Lees R, Smiley ST, Taniguchi M, Grusby MJ, MacDonald HR. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]
- 43.Matsuda JL, Gapin L. Developmental program of mouse Valpha14i NKT cells. Curr Opin Immunol. 2005;17:122–130. doi: 10.1016/j.coi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 44.McNab FW, Pellicci DG, Field K, Besra G, Smyth MJ, Godfrey DI, Berzins SP. Peripheral NK1.1 NKT cells are mature and functionally distinct from their thymic counterparts. J Immunol. 2007;179:6630–6637. doi: 10.4049/jimmunol.179.10.6630. [DOI] [PubMed] [Google Scholar]
- 45.Chun T, Page MJ, Gapin L, Matsuda JL, Xu H, Nguyen H, Kang HS, Stanic AK, Joyce S, Koltun WA, Chorney MJ, Kronenberg M, Wang CR. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med. 2003;197:907–918. doi: 10.1084/jem.20021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schumann J, Pittoni P, Tonti E, Macdonald HR, Dellabona P, Casorati G. Targeted expression of human CD1d in transgenic mice reveals independent roles for thymocytes and thymic APCs in positive and negative selection of Valpha14i NKT cells. J Immunol. 2005;175:7303–7310. doi: 10.4049/jimmunol.175.11.7303. [DOI] [PubMed] [Google Scholar]
- 47.McNab FW, Berzins SP, Pellicci DG, Kyparissoudis K, Field K, Smyth MJ, Godfrey DI. The influence of CD1d in postselection NKT cell maturation and homeostasis. J Immunol. 2005;175:3762–3768. doi: 10.4049/jimmunol.175.6.3762. [DOI] [PubMed] [Google Scholar]
- 48.Horai R, Mueller KL, Handon RA, Cannons JL, Anderson SM, Kirby MR, Schwartzberg PL. Requirements for selection of conventional and innate T lymphocyte lineages. Immunity. 2007;27:775–785. doi: 10.1016/j.immuni.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 50.Okkenhaug K, Wu L, Garza KM, La Rose J, Khoo W, Odermatt B, Mak TW, Ohashi PS, Rottapel R. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol. 2001;2:325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- 51.Burr JS, Savage ND, Messah GE, Kimzey SL, Shaw AS, Arch RH, Green JM. Cutting edge: distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- 52.Noel PJ, Alegre ML, Reiner SL, Thompson CB. Impaired negative selection in CD28-deficient mice. Cell Immunol. 1998;187:131–138. doi: 10.1006/cimm.1998.1332. [DOI] [PubMed] [Google Scholar]
- 53.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chung Y, Nurieva R, Esashi E, Wang YH, Zhou D, Gapin L, Dong C. A critical role of costimulation during intrathymic development of invariant NK T cells. J Immunol. 2008;180:2276–2283. doi: 10.4049/jimmunol.180.4.2276. [DOI] [PubMed] [Google Scholar]