Abstract
Introduction
Warfarin-associated intracranial hemorrhage (ICH) requires rapid normalization of clotting function. Current therapies are associated with significant complications and/or prolonged time to correction of coagulopathy. Recombinant factor VIIa (FVIIa) might allow faster and safer correction of coagulopathy.
Methods
This article presents a retrospective chart review of all patients with warfarin-associated ICH treated in a neurology/neurosurgery intensive care unit over an 11-month period.
Results
All patients were treated to rapidly reverse the warfarin effect. Fifteen patients received vitamin K and fresh frozen plasma (FFP) alone (FFP group). Twelve patients also received FVIIa (FVIIa group). The median times from presentation to an international normalization ratio (INR) of less than 1.3 were 32.2 and 8.8 hours in the FFP the FVIIa groups, respectively (p = 0.016). INR normalized slowly (at 110 and 130 hours, respectively) in two patients with end-stage renal failure who were given FVIIa, one of whom developed disseminated intravascular coagulation after three doses of FVIIa. No other complications occurred from FVIIa administration. One patient in the FFP group developed severe pulmonary edema.
Conclusion
FVIIa may be an effective adjunct to FFP in warfarin-related ICH, facilitating faster correction of INR and decreasing FFP requirements. A prospective, randomized trial is needed to confirm these preliminary findings and to determine whether there is a clinical benefit.
Keywords: Intracranial hemorrhage, warfarin, coagulopathy, fresh frozen plasma, factor VIIa
Introduction
Intracranial hemorrhage (ICH) is among the most serious complications of warfarin therapy. Estimations of its frequency range from 0.3 to 4.6% per year (1,2). The absolute rate of ICH in patients older than age 60 years who are treated with conventional intensities of anticoagulation ranges from 0.3 to 1% per year, and 60% of cases are fatal (3). Mortality increases with higher INR (4). Initial management of warfarin-associated ICH focuses on rapid reversal of the anticoagulant effects of warfarin to prevent expansion of hemorrhage and to allow rapid surgical intervention, when indicated (5). Vitamin K, fresh frozen plasma (FFP), and prothrombin complex conjugate (PCC) are currently used. These treatment options have several limitations, including a prolonged time to correct coagulopathy (vitamin K and FFP) and significant side effects (FFPand PCC) (6,7).
Recombinant activated factor VII (FVIIa) (NovoSeven®, Novo Nordisk, Copenhagen, Denmark) was developed for the treatment of bleeding in hemophiliacs with inhibitors to FVII and factor IX, but it also appears to be effective in various clinical conditions associated with a hemorrhagic diathesis (8,9). We recently began to use FVIIa as an adjunct to vitamin K and FFP in selected patients with warfarin-associated ICH. To better understand preliminary safety and laboratory efficacy data in these patients, we compared their clinical and laboratory data with those of patients with warfarin-associated ICH admitted over the same time interval but who were not treated with FVIIa.
Materials and Methods
Patients with warfarin-associated hemorrhage were identified by reviewing discharge summaries of all patients with ICH admitted over an 11-month period (March 2002 through January 2003). Patients were included in this study if they had an acute ICH, were taking warfarin, and had an INR greater than 1.3. All patients were treated in the neurology/neurosurgery intensive care unit with vitamin K (10 mg IV/SQ then 10mg SQ daily for an additional 2 days) and FFP. Additionally, selected patients received FVIIa. These were patients who had suffered clinical deterioration from hematoma expansion (or those who were considered to be at especially high risk), those with increased risk of developing FFP-related complications (history of heart failure), or those who required urgent neurosurgical intervention. Patients who received FVIIa (FVIIa group) were identified through our blood bank (they must release FVIIa for each use) and were compared to patients who received vitamin K and FFP alone (FFP group). A chart review was performed on all patients by a single investigator (D. L. Brody).
The clinical variables analyzed included age, gender, associated medical comorbidities, reason for warfarin therapy, INR on presentation, clinical presentation (presenting symptoms, initial Glasgow Coma Scale [GCS] score, initial acute physiology and chronic health evaluation II [APACHE II] score [10]), site of ICH (subdural, supratentorial cerebral, infratentorial cerebral, and mixed), volume of FFP administered, hospital course (length of stay and presence of neurological deterioration), and status on discharge (discharge GCS and hospital disposition).
The charts of patients in both groups were reviewed to determine the time required to normalize the INR. This threshold was based on previous literature on the topic (6,7). Two time intervals were evaluated: (a) time from diagnosis (for already hospitalized patients) or presentation to our institution to normalization of the INR (<1.3) and (b) time from initial order to administer FVIIa or FFP to normalization of the INR. Both time intervals were determined because, in many patients, the decision to administer FVIIa was made later in the patient’s course, after initial attempts to correct the coagulopathy with vitamin K and FFP were complicated by deteriorating neurological status or signs of FFP-related complications. This allowed us to obtain an estimate of logistical delays for administering an effective dose of both agents.
Asystematic search for thrombotic and other complications attributable to FVIIa was performed and was defined as follows: (a) myocardial infarction: elevated troponin-I (>1.5 ng/mL) occurring after FVIIa was administered; (b) cerebral infarction: a new discrete brain lesion in a vascular territory with signal characteristics of infarction on computed tomography (CT) or magnetic resonance imaging (MRI); (c)deep venous thrombosis diagnosed by lower extremity Doppler scan or pulmonary embolus diagnosed by a ventilation/perfusion scan or a spiral chest CT scan; and (d) other complications (i.e., other adverse events attributed to the administration of FVIIa by the treating physicians).
Complications related to FFP were defined as follows: (a) pulmonary edema (caused by either heart failure or transfusion-related acute lung injury): respiratory distress necessitating oxygen therapy, intubation, or worsening of arterial blood gas levels (change in PaCO2 by >10 mmHg or PaO2 by >20 mm Hg) with no other obvious explanation; (b) transfusion reaction identified by the treating physicians; (c) other complications (e.g., infections attributed to transmission by FFP).
Statistical analysis was performed using SPSS 10.0.5 (SPSS, Chicago, IL). Variables were compared using the Fisher exact test or Mann Whitney U-test (for categorical and non-normally distributed continuous variables) and a Student t-test or ANOVA (for continuous variables); a p-value of less than 0.05 was considered significant.
Results
We treated 28 patients with warfarin-associated ICH over an 11-month period. Thirteen patients received FVIIa (FVIIa group), and 15 did not (FFP group). One patient given FVIIa was excluded because the timing of INR correction could not be accurately determined. The reasons for anticoagulation and medical comorbidities were similar in both groups (see Table 1). Six patients in the FVIIa group had a GCS of 8 or less compared to only one in the FFP group (p = 0.01, Mann Whitney U-test). Mortality was higher and discharge GCS scores were worse in the FVIIa group.
Table 1.
Clinical Characteristics
| FVIIa group | FFP group | p-value | |
|---|---|---|---|
| Age (years): mean ± SD (range) | 71.0 ± 13.1 (50-89) | 76.5 ± 6.9 (60-88) | NS |
| Females | 6 | 7 | NS |
| Hemorrhage type: n (%) | |||
| Intraparenchymal | 4 (33%) | 7 (47%) | |
| Subdural | 4 (33%) | 5 (33%) | NS |
| Posterior fossa | 2 (17%) | 1 (7%) | |
| Mixed or other | 2 (17%) | 2 (13%) | |
| Presentation | |||
| acute | 8 | 5 | NS |
| subacute | 4 | 10 | |
| Admission GCS ≤ 8 (n) | 6 | 1 | 0.01 |
| Admission APACHE II score (mean ± SD) | 20.2 ± 11.6 | 11.2 ± 4.8 | NS |
| Clinical course | |||
| stable | 6 | 10 | NS |
| deterioration | 6 | 5 | |
| Surgical intervention: n (%) | 5 (42%) | 6 (40%) | NS |
| ICU length of stay, days (mean ± SD) | 5.3 ± 3.3 | 3.9 ± 1.7 | NS |
| Hospital length of stay (mean ± SD) | 9.2 ± 5.5 | 9.4 ± 5.1 | NS |
| Discharge GCS of survivors: median (range) | 13.5 (13-15) | 15 (13-15) | NS |
| Disposition: n (%) | |||
| dead | 5 (42%) | 2 (13%) | NS |
| nursing home | 2 (17%) | 3 (20%) | |
| rehabilitation | 3 (25%) | 9 (60%) | |
| home | 2 (17%) | 1 (7%) | |
Abbreviations: SD, standard deviation; M, male; F, female; GCS, Glasgow coma scale; APACHE, acute physiology and chronic health evaluation; ICU, intensive care unit; NS, not significant; FFP, fresh frozen plasma.
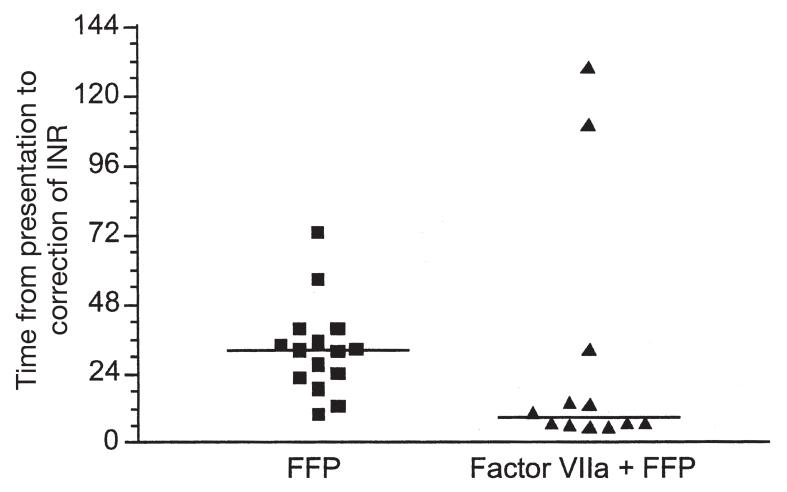
There were clear differences between the two groups in the times to correction of the INR to less than 1.3 (Table 2). The median times from presentation to correction were 8.8 and 32.2 hours for the FVIIa and FFP groups, respectively (p = 0.016, Mann Whitney U-test) (Figure 1). Therefore, addition of FVIIa was associated with approximately fourfold faster correction of warfarin-related coagulopathy, as reflected by the INR. Similarly, once the decision was made to use FVIIa, the median time to INR normalization was 5.1 hours compared to 32.2 hours from the first order for FFP (p = 0.0081, Mann Whitney U-test). However, this comparison is confounded by the fact that the FVIIa patients had already received FFP.
Table 2.
Coagulation Parameters, Time to Correction, and Factor Replacement
| FVIIa group | FFP group | p-value | |
|---|---|---|---|
| Initial INR mean ± SD | 3.7 ± 4.3 | 3.2 ± 1.3 | NS |
| Final INR mean ± SD | 0.9 ± 0.3 | 1.2 ± 0.06 | 0.019 |
| Time from presentation to INR less than 1.3 median (range) | 8.8 (1.8-130) | 32.2 (10.0-72.8) | 0.016 |
| Factor VIIa dose (mg) mean ± SD (range) | 4.8 ± 2.1 (2.4-9.6) | N/A | N/A |
| FFP dose to correct INR (mL) mean ± SD (range) | 1272 ± 782 (0-2475) | 2044 ± 773 (780-3484) | 0.022 |
INR, international normalized ratio; SD, standard deviation; NS, not significant; FFP, fresh frozen plasma; N/A, not applicable.
Fig. 1.
Time to correction of INR. Scatter plot of time interval from presentation to correction of INR (<1.3). Each symbol represents one patient. Median values indicated by horizontal bars (p = 0.016, Mann Whitney U-test).
Of the 11 patients requiring surgical intervention, hemostasis was adequate in 10, according to the operative notes. In the 11th patient, the INR was not completely corrected prior to surgery, and hemostasis was restored after treatment with FFP and FVIIa.
A standardized time table for INR measurement in the treatment of warfarin-induced bleeding was not used. Therefore, we analyzed the interval between the first and second INR tests during the period of acute illness for each patient. These intervals were 7.0 ± 3.4 hours (mean ± standard deviation) for the FFP group and 8.3 ± 7.5 hours for the FVIIa group. The difference between groups was not statistically significant. (p = 0.92, Mann Whitney U-Test). Thus, we concluded that the difference in time to correction of INR between the two groups could not be accounted for by discrepancies in the frequency of INR measurements.
The total volume of FFP infused differed considerably between the groups. Patients who did not receive FVIIa required almost twice as much FFP to normalize their INR (Table 2). This certainly contributed to the delays in correcting INR in the FFP group, because hospital procedures slow obtaining large volumes of FFP. However, each dose of FVIIa required approval from the laboratory medicine resident-on-call in our hospital, which also added a delay.
One of the patients in the FFP group developed acute respiratory distress during FFP administration, despite the absence of a history of congestive heart failure. She had evidence of pulmonary edema on a chest X-ray and was treated with 100% oxygen and intravenous furosemide. She did not require intubation and recovered to baseline. There was only one complication that was considered as probably related to FVIIa. This patient had severe peripheral vascular disease and renal failure and was the only patient to receive multiple doses of FVIIa (4.8, 2.4, and 4.8 mg over 6 days). After the first dose, his arteriovenous dialysis graft clotted (he had had a history of graft clotting in the past). Approximately 12 hours after the third dose, the patient developed signs of ischemia in his extremities. Laboratory data suggested disseminated intravascular coagulation (DIC), with rising prothrombin time and partial thromboplastin times and falling platelet count. Angiography revealed diffuse severe peripheral vascular disease. Low-dose heparin was started, and the ischemic lesions did not progress. An above-the-knee amputation of the right leg was planned, but the patient’s family declined and elected to discontinue dialysis. He died approximately 2 weeks later. Another patient developed a deep venous thrombosis and symptomatic pulmonary embolism 9 days after receiving FVIIa. The treating physicians did not attribute this complication to use of FVIIa. She did well after inferior vena cava filter placement. The INR corrected very slowly in two patients, even after administration of FVIIa and large volumes of FFP. Both patients had end-stage renal failure and were on dialysis. Neither received heparin during dialysis treatments once the ICH was diagnosed.
Discussion
Activated FVII has recently been used to correct bleeding diatheses in several scenarios, including warfarin-associated intracranial and intraspinal hematomas (11-13). It acts by directly binding to tissue factor to activate thrombin generation, bypassing much of the cascade of coagulation factors (14). There may also be an independent effect on activated platelet function (15). FVIIa can be administered without typing or crossmatching, in minimal volumes of fluid, and with few reported immune-mediated reactions to date (16). It also appears to have a low incidence of major prothrombotic complications, with only 17 thrombotic events reported for more than 480,000 standard doses administered between 1996 and October 2001(17).
In this study, the use of FVIIa in conjunction with FFP was associated with shortened times to correction of INR and reduced the total dose of FFP required for correction of coagulopathy. However, the mortality rate was higher and discharge GCS scores were lower in patients treated with FVIIa. There are several possible explanations for this. There was bias in selecting patients to be treated with FVIIa: they tended to be in worse clinical condition on admission, with a lower GCS and higher APACHE II score. Half of the patients given FVIIa, but only 1 of 15 patients given FFP, had an admission GCS of 8 or less. Additionally, patients who were neurologically stable were generally not administered FVIIa, whereas patients given FVIIa often experienced progressing neurological deficits required urgent surgery, or heart failure.
Although there was a fourfold shorter time to correction with FVIIa, the median time from presentation was still 8.8 hours. This was largely because the decision to administer FVIIa was often not made at presentation. It is likely that with an established protocol, this time can be shortened significantly, to the order of minutes. On the other hand, the median time to correction with FFP alone was 32.2 hours from the time of admission. The long time resulted from numerous factors, including delays in getting FFP from the blood bank, time taken to administer the prescribed dose, and the almost invariable underestimation of the dose of FFP required. Even in a prospective trial where FFP was administered at the maximal tolerated rate according to a protocol, the mean time to correction was 9 hours. Additionally, in that trial, rapid infusion of FFP led to a significant complication rate (five of eight patients) (7).
Despite the high incidence of coronary artery and cerebrovascular disease in the FVIIa group, there was only one thrombotic complication. A patient with severe peripheral vascular disease and end-stage renal disease who received multiple doses of FVIIa developed DIC and peripheral thrombotic complications. Both peripheral vascular disease and renal failure might have contributed to this complication. Pickard et al. (18) reported a case of middle cerebral artery stroke after administration of FVIIa in a patient with subarachnoid hemorrhage and polycystic kidney disease with renal failure.
Our study had several drawbacks. It was retrospective in nature. There were no predetermined criteria for the administration of FVIIa, and the timing of administration of FVIIa and FFP and the timing of determination of the INR were not standardized. The patient population is heterogeneous regarding the location of the bleed, presentation, degree of anticoagulation, and comorbidities. Radiological data, such as serial CT scans to determine the degree of hemorrhage growth, would have been helpful but were not systematically available because of the retrospective nature of this report. Finally, because most of the patients who received FVIIa received it after a certain amount of FFP had already been infused, it is also possible that corrected INR reflects, to a certain extent, the FFP already infused.
It can also be argued that using the INR to define correction of coagulopathy is an unreliable indicator of the risk of ongoing bleeding. In fact, the INR is extremely sensitive to FVII levels and does not accurately reflect the prothrombin level (19). Therefore, faster correction of INR may not necessarily translate to faster correction of coagulopathy. However, FVIIa also increases the plasma activities of factors IX and X in a dose-dependent manner (20). Observations of our patients and several previously published case series have demonstrated the efficacy of FVIIa in normalizing the INR as well as in stopping bleeding in a variety of clinical situations, including bleeding associated with excessive anticoagulation (9,11-13). Therefore, we believe that correction of the abnormal INR is likely to be associated with a correction of the in vivo coagulopathy as well.
Conclusion
FVIIa in addition to FFP can be used to rapidly correct the INR in patients with warfarin-associated ICH. Time to correct the INR is significantly shorter and volume of FFP infused is lower in patients who receive FVIIa compared to those who receive FFP alone. Caution should be exercised in using repeated doses of FVIIa in patients with peripheral vascular disease.
Acknowledgments
We would like to acknowledge assistance from Dr. J. Lewis, the nurses of the Barnes-Jewish Hospital NNICU, the neurosurgical chief residents at Washington University, and Dr. William J. Powers. Supported in part by NIH grant 2 P01 NS035966-06.
Footnotes
Dr. Diringer receives financial compensation for consulting and speaking for Novo Nordisk.
References
- 1.Dahl T, Abildgaard U, Sandset PM. Long-term anticoagulant therapy in cerebrovascular disease: does bleeding outweigh the benefit? J Intern Med. 1995;237(3):323–329. doi: 10.1111/j.1365-2796.1995.tb01182.x. [DOI] [PubMed] [Google Scholar]
- 2.Evans A, Kalra L. Are the results of randomized controlled trials on anticoagulation in patients with atrial fibrillation generalizable to clinical practice? Arch Intern Med. 2001;161(11):1443–1447. doi: 10.1001/archinte.161.11.1443. [DOI] [PubMed] [Google Scholar]
- 3.Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke. 1995;26(8):1471–1477. doi: 10.1161/01.str.26.8.1471. [DOI] [PubMed] [Google Scholar]
- 4.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120(11):897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Broderick JP, Adams HP, Jr., Barsan W, et al. American Heart Association Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council. Stroke. 1999;30(4):905–915. doi: 10.1161/01.str.30.4.905. [DOI] [PubMed] [Google Scholar]
- 6.Baglin T. Management of warfarin (coumarin) overdose. Blood Rev. 1998;12(2):91–98. doi: 10.1016/s0268-960x(98)90020-0. [DOI] [PubMed] [Google Scholar]
- 7.Boulis NM, Bobek MP, Schmaier A, Hoff JT. Use of factor IX complex in warfarin-related intracranial hemorrhage. Neurosurgery. 1999;45(5):1113–1118. doi: 10.1097/00006123-199911000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Hedner U. NovoSeven as a universal haemostatic agent. Blood Coagul Fibrinolysis. 2000;11(Suppl 1):S107–S111. doi: 10.1097/00001721-200004001-00020. [DOI] [PubMed] [Google Scholar]
- 9.Deveras RA, Kessler CM. Reversal of warfarin-induced excessive anticoagulation with recombinant human factor VIIa concentrate. Ann Intern Med. 2002;137(11):884–888. doi: 10.7326/0003-4819-137-11-200212030-00009. [DOI] [PubMed] [Google Scholar]
- 10.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 11.Lin J, Hanigan WC, Tarantino M, Wang J. The use of recombinant activated factor VII to reverse warfarin-induced anticoagulation in patients with hemorrhages in the central nervous system: preliminary findings. J Neurosurg. 2003;98(4):737–740. doi: 10.3171/jns.2003.98.4.0737. [DOI] [PubMed] [Google Scholar]
- 12.Veshchev I, Elran H, Salame K. Recombinant coagulation Factor VIIa for rapid preoperative correction of warfarin-related coagulopathy in patients with acute subdural hematoma. Med Sci Monit. 2002;8(12):CS98–CS100. [PubMed] [Google Scholar]
- 13.Park P, Fewel ME, Garton HJ, Thompson BG, Hoff JT. Recombinant activated factor VII for the rapid correction of coagulopathy in nonhemophilic neurosurgical patients. Neurosurgery. 2003;53:34–38. doi: 10.1227/01.neu.0000068830.54968.a8. [DOI] [PubMed] [Google Scholar]
- 14.van’t Veer C, Mann KG. The regulation of the factor VII-dependent coagulation pathway: rationale for the effectiveness of recombinant factor VIIa in refractory bleeding disorders. Semin Thromb Hemost. 2000;26(4):367–372. doi: 10.1055/s-2000-8454. [DOI] [PubMed] [Google Scholar]
- 15.Monroe DM, Hoffman M, Oliver JA, Roberts HR. Apossible mechanism of action of activated factor VII independent of tissue factor. Blood Coagul Fibrinolysis. 1998;9(Suppl 1):S15–S20. [PubMed] [Google Scholar]
- 16.Key NS, Aledort LM, Beardsley D, et al. Home treatment of mild to moderate bleeding episodes using recombinant factor VIIa (Novoseven) in haemophiliacs with inhibitors. Thromb Haemost. 1998;80(6):912–918. [PubMed] [Google Scholar]
- 17.Erhardtsen E. Ongoing NovoSeven® trials. Intensive Care Med. 2002;28(Suppl 2):S248–S256. doi: 10.1007/s00134-002-1472-6. [DOI] [PubMed] [Google Scholar]
- 18.Pickard JD, Kirkpatrick PJ, Melsen T, et al. Potential role of NovoSeven in the prevention of rebleeding following aneurysmal subarachnoid haemorrhage. Blood Coagul Fibrinolysis. 2000;11(Suppl 1):S117–S120. doi: 10.1097/00001721-200004001-00022. [DOI] [PubMed] [Google Scholar]
- 19.Lind SE, Callas PW, Golden EA, Joyner KA, Jr., Ortel TL. Plasma levels of factors II, VII and X and their relationship to the international normalized ratio during chronic warfarin therapy. Blood Coagul Fibrinolysis. 1997;8(1):48–53. doi: 10.1097/00001721-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Erhardtsen E, Nony P, Dechavanne M, Ffrench P, Boissel JP, Hedner U. The effect of recombinant factor VIIa (NovoSeven) in healthy volunteers receiving acenocoumarol to an International Normalized Ratio above 2.0. Blood Coagul Fibrinolysis. 1998;9(8):741–748. doi: 10.1097/00001721-199811000-00003. [DOI] [PubMed] [Google Scholar]