Abstract
We report a one-flask route for synthesis of dinucleoside tetra and pentaphosphates, in isolated yields of 50 to 85%. This route relies on a mixture of PIII and PV chemistries, using phosphitylation of a protected nucleoside with 2-chloro-4H-l,3,2-benzo-dioxaphosphorin-4-one (salicylchlorophosphite), followed by sequential reaction with inorganic pyrophosphate, and a nucleoside 5′ mono- or diphosphate.
Dinucleoside polyphosphates (5′-5‴-NpnN, n=2-7) have been proposed as signaling and regulatory molecules for many different biological functions in most forms of life.1 Although the most abundant and best characterized of these specialized RNA molecules are Ap3A, Ap4A, and Ap5A, examples with other nucleosides are known, but are typically found at lower concentrations. Gp3G and Gp4G are exceptional in occurring at high concentrations in the dehydrated embryonic cysts of brine shrimp.2 The main source of most cytoplasmic Ap4N is the ‘back-reaction’ of NTPs with various adenylated intermediates, such as aminoacyl-adenylate, catalyzed by aminoacyl-tRNA synthetase,3 and luciferin, catalyzed by firefly luciferase.4 The intracellular levels of NpnN are controlled by a variety of hydrolyzing enzymes, including Ap4A hydrolase (a MuT motif protein) and Ap3A hydrolase (a product of the FHIT tumor suppressor gene).5 Potent extracellular activities for Ap4A and Ap5A are well known,6 and many of their receptors have been established.7 Two examples with important therapeutic potential are inhibition of platelet aggregation8 and regulation of vasoactivity.9 A high yield synthesis for NpnN and their analogs would facilitate studies of their possible medical applications.
Enzymatic approaches10 are limited by scale and to naturally occurring nucleosides. The most widely used chemical approach for the synthesis of Np4N has been the reaction of a nucleoside triphosphate with a nucleotide activated as the morpholidate, diphenylphosphorochloridate, or imidazolate,11 but the yields have been modest. Blackburn pioneered synthesis of Np4N analogs, for the most part by more specialized routes, and also in modest yields.12
Orgel reported many years ago that treatment of adenosine 5′-tetraphosphate with a carbodiimide formed a cyclic trimetaphosphate intermediate that could hydrolyze back to starting material or eliminate inorganic trimetaphosphate to give adenosine monophosphate.13 Nucleoside triphosphates have also been cyclized to the trimetaphosphate using carbodiimides,14 and recently, a series of nucleoside-dye oligophosphates were prepared using intermediates made in this way.15
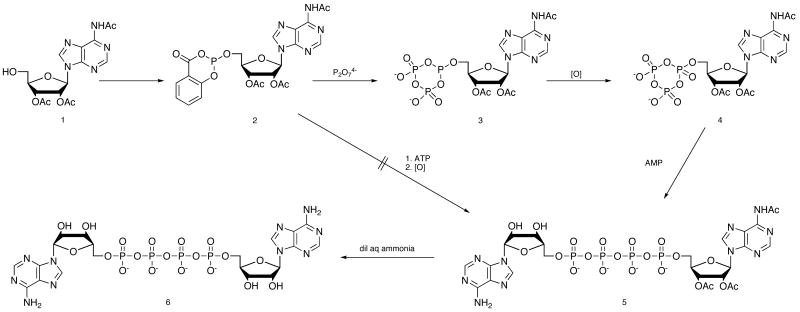
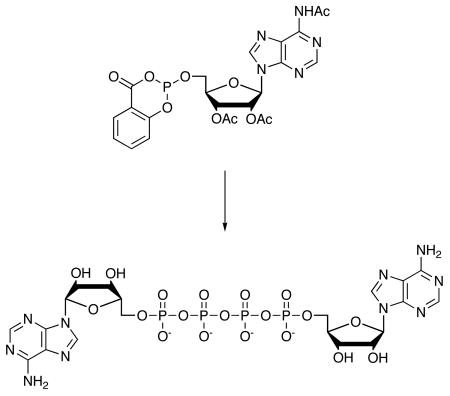
We have developed a new, one-flask route, shown in Scheme 1 for preparation of Ap4A that makes the trimetaphosphate in a more efficient synthesis. Our route begins with the Eckstein procedure for preparation of triphosphates by phosphitylation of triacetyl adenosine (1) with 2-chloro-4H-l,3,2-benzo-dioxaphosphorin-4-one (salicylchlorophosphite) followed by reaction with inorganic pyrophosphate to give the cyclic derivative (3). For triphosphate synthesis, 3 is oxidized to 4 with concomitant hydrolysis of 4,16 and modified di17 and triphosphates18 have also been made using this approach. We first tried reaction of 2 with adenosine 5′-triphosphate (ATP), followed by oxidation, but this route gave complex mixtures in which only traces of 5 could be detected.
Scheme 1. Synthesis of Ap4A.
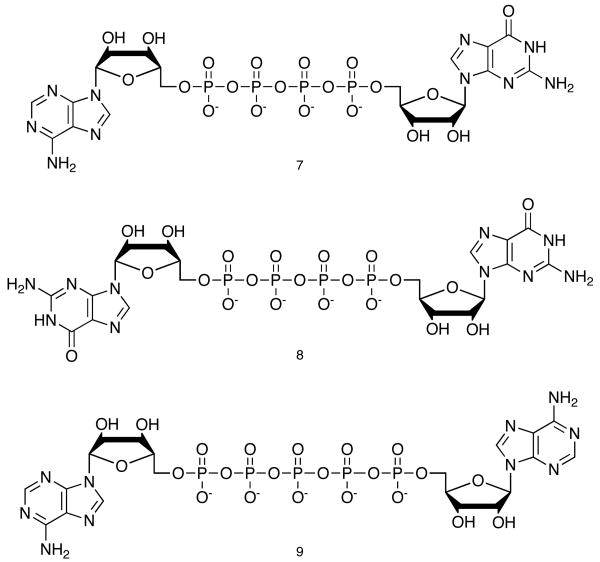
We found instead that careful oxidation of 3 to 4, under conditions that do not bring about hydrolysis, followed by reaction of 4 with AMP in dry DMF, catalyzed by ZnCl2, gives clean conversion to the partially protected tetraphosphate 5. MgCl2 was less effective than ZnCl2, but better than no catalyst. After mild ammonia treatment to remove the acetyl groups, and ion exchange to remove the Zn2+ while it is solubilized as an ammonium complex, the final tetraphosphate 6 is isolated in yields of 85%.19 This yield compares very well to previously reported tetraphosphate syntheses, with which we were seldom able to obtain yields as high as 25%. Ap4G20 (7) and Gp4G21 (8) are prepared in a similar manner, and the Ap5A22 (9) is prepared by addition of ADP to 4. NMR characterization for Ap4A agrees with previously published data.23 In the complex second order 31P NMR spectra for all four compounds, resonances for the end phosphates are well separated from those of the middle phosphates. In the case of the pentaphosphate Ap5A, the 2nd and 3rd phosphates are not resolved, and the envelope appears as a broad singlet. This result is consistent with 31P NMR data for Na7P5O16 in a study of a series of polyphosphates, in which the difference in chemical shifts for the 2nd and 3rd phosphates was less than their coupling constant.24
The approach described here can also be used to prepare a variety of modified dinucleoside polyphosphates, and such work is underway in our laboratory.
Supplementary Material
Spectra (UV, MS, 1H NMR, 13C NMR and 31P NMR). This material is available free of charge via the Internet at http://pubs.acs.org.
Figure 1.
A p4G (7), Gp4G (8), and Ap5A (9), prepared by the route shown in Scheme 1
Acknowledgments
This work was supported by NIH grant EB002809.
References
- 1.Kisselev LL, Justesen J, Wolfson AD, Frolova LY. FEBS Lett. 1998;427:157–163. doi: 10.1016/s0014-5793(98)00420-7. [DOI] [PubMed] [Google Scholar]; McLennan AG. Pharmacol Therapeut. 2000;87:73–89. doi: 10.1016/s0163-7258(00)00041-3. [DOI] [PubMed] [Google Scholar]; McLennan AG, Barnes LD, Blackburn GM, Brenner C, Guranowski A, Miller AD, Rovira JM, Rotllan P, Soria B, Tanner JA, Sillero A. Drug Devel Research. 2001;52:249–259. [Google Scholar]
- 2.Cartwright JL, McLennan AG. Arch Biochem Biophys. 1999;361:101–105. doi: 10.1006/abbi.1998.0970. [DOI] [PubMed] [Google Scholar]
- 3.Goerlich O, Foeckler R, Holler E. Eur J Biochem. 1982;126:135–142. doi: 10.1111/j.1432-1033.1982.tb06757.x. [DOI] [PubMed] [Google Scholar]
- 4.Sillero A, Sillero MAG. Pharmacol Therapeut. 2000;87:91–102. doi: 10.1016/s0163-7258(00)00047-4. [DOI] [PubMed] [Google Scholar]
- 5.Guranowski A. Pharmacol Therapeut. 2000;87:117–139. doi: 10.1016/s0163-7258(00)00046-2. [DOI] [PubMed] [Google Scholar]
- 6.Hoyle CHV, Hilderman RH, Pintor JJ, Schluter H, King BF. Drug Devel Research. 2001;52:260–273. [Google Scholar]
- 7.Pintor J, Diaz-Hernandez M, Gualix J, Gomez-Villafuertes R, Hernando F, Miras-Portugal MT. Pharmacol Therapeut. 2000;87:103–115. doi: 10.1016/s0163-7258(00)00049-8. [DOI] [PubMed] [Google Scholar]
- 8.Chan SW, Gallo SJ, Kim BK, Guo MJ, Blackburn GM, Zamecnik PC. Proc Natl Acad Sci U S A. 1997;94:4034–4039. doi: 10.1073/pnas.94.8.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]; Flores NA, Stavrou BM, Sheridan DJ. Cardiovas Res. 1999;42:15–26. doi: 10.1016/s0008-6363(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 9.Steinmetz M, Janssen AK, Pelster F, Rahn KH, Schlatter E. J Pharmacol Exp Ther. 2002;302:787–7794. doi: 10.1124/jpet.302.2.787. [DOI] [PubMed] [Google Scholar]; Luo J, Jankowski V, Gungar N, Neumann J, Schmitz W, Zidek W, Schluter H, Jankowski J. Hypertension. 2004;43:1055–1059. doi: 10.1161/01.hyp.0000126110.46402.dd. [DOI] [PubMed] [Google Scholar]
- 10.Guranowski A, Sillero MAG, Sillero A. FEBS. 1990;271:215–218. doi: 10.1016/0014-5793(90)80409-c. [DOI] [PubMed] [Google Scholar]; Ortiz B, Sillero A, Gunther Sillero MA. Eur J Biochem. 1993;212:263–270. doi: 10.1111/j.1432-1033.1993.tb17658.x. [DOI] [PubMed] [Google Scholar]; Theoclitou ME, El-Thaher TSH, Miller ADJ. J C S Chem Comm. 1994;5:659–661. [Google Scholar]; Theoclitou ME, Wittung EPL, Hindley AD, El-Thaher TSH, Miller ADJ. J Chem Soc Perkin Trans 1. 1996;16:2009–2019. [Google Scholar]
- 11.Reiss JR, Moffatt JG. J Org Chem. 1965;30:3381–3387. doi: 10.1021/jo01021a029. [DOI] [PubMed] [Google Scholar]; Feldhaus P, Frohlich T, Goody RS, Isakov M, Schirmer RH. Eur J Biochem. 1975;57:197–204. doi: 10.1111/j.1432-1033.1975.tb02291.x. [DOI] [PubMed] [Google Scholar]; Shimazu M, Shinozuka K, Sawai H. Tetrahedron Lett. 1990;31:235–238. [Google Scholar]
- 12.Blackburn GM, Taylor GE, Thatcher GRJ, Prescott M, McLennan AG. Nucleic Acids Res. 1987;15:6991–7004. doi: 10.1093/nar/15.17.6991. [DOI] [PMC free article] [PubMed] [Google Scholar]; McLennan AG, Taylor GE, Prescott M, Blackburn GM. Biochemistry. 1989;28:3868–3875. doi: 10.1021/bi00435a036. [DOI] [PubMed] [Google Scholar]
- 13.Ng KE, Orgel LE. Nucleic Acids Res. 1987;15:3573–3580. doi: 10.1093/nar/15.8.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glonek T, Kleps RA, Myers TC. Science. 1974;185:352–355. doi: 10.1126/science.185.4148.352. [DOI] [PubMed] [Google Scholar]
- 15.Sood A, Kumar S, Nampalli S, Nelson JR, Macklin J, Fuller CW. J Am Chem Soc. 2005;127:2394–2395. doi: 10.1021/ja043595x. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig J, Eckstein F. J Org Chem. 1989;54:631–635. [Google Scholar]
- 17.Li P, Xu Z, Liu H, Wennefors CK, Dobrikov MI, Ludwig J, Shaw BR. J Am Chem Soc. 2005;127:16782–16783. doi: 10.1021/ja055179y. [DOI] [PubMed] [Google Scholar]
- 18.Wang G, Boyle N, Chen F, Rajappan V, Fagan P, Brooks JL, Hurd T, Leeds JM, Rajwanshi VK, Jin Y, Prhavc M, Bruice TW, Cook PD. J Med Chem. 2004;47:6902–6913. doi: 10.1021/jm040116w. [DOI] [PubMed] [Google Scholar]
- 19.Preparation of Ap4A. To a solution of 2′,3′-O-6-N-triacetyladenosine (0.13 g, 0.33 mmol) in 2 mL of anhydrous N,N-dimethylformamide (DMF) was added 2-chloro-4H-l,3,2-benzo-dioxaphosphorin-4-one (0.13 g, 0.64 mmol, 1.9 eq). The solution was stirred for 15 min at RT under N2. A 0.5 M solution of bis(tri-n-butylammonium) pyrophosphate in anhydrous DMF (1.3 mL, 0.65 mmol, 2.0 eq) was vortexed with tri-n-butylamine (0.60 mL, 2.5 mmol, 7.6 eq) and immediately added to the reaction mixture. After 20 min a solution of iodine (0.12 g, 0.47 mmol, 1.4 eq) in 1.5 mL pyridine and 0.01 mL water was added. After 15 minutes, a mixture of adenosine monophosphate monohydrate, proton form, (0.45 g, 1.23 mmol, 3.7 eq) and zinc chloride (0.42 g, 3.1 mmol, 9.4 eq) that had been dried together by evaporation of pyridine and DMF was added with stirring. After 16 hr, 10% aqueous ammonia (20 mL, 118 mmol, 358 eq) was added, and the deprotection was complete after 1 hr. The dilute basic solution was applied to a sodium cation exchange resin (50WX2, 10 mL, 18 eq) to remove Zn2+. The product was concentrated and purified by preparative reverse phase HPLC using 0.1 M ammonium bicarbonate in acetonitrile to give 0.25 g of Ap4A in the ammonium form (0.28 mmol, 85%): UV λmax 260 nm; 1H NMR (D2O, 400 MHz): 8.40 (s, 2H), 8.15 (s, 2H), 6.01 (d, J = 5.73 Hz, 2H), 4.69 (t, J = 5.40 Hz, 2H), 4.54 (t, J = 4.35 Hz, 2H), 4.39-4.34 (m, 2H), 4.33-4.21 (m, 4H); 31P NMR (D2O, 400 MHz): d -10.16, -21.90. The mass was confirmed by ESI-MS in negative mode as m/z (M-1) 835.33 amu (calculated for C20H27N10O19P4-: 835.04).
- 20.Preparation of Ap4G. Starting with 2′,3′-O-2-N-triacetylguanosine (0.14 g, 0.34 mmol) to make the trimetaphosphate intermediate, Ap4G was prepared by the same procedure described above. Following HPLC purification, 0.20 g of Ap4G in the ammonium form was obtained (0.22 mmol, 65%): UV λmax 256 nm; 1H NMR (D2O, 400 MHz): 8.40 (s, 1H), 8.12 (s, 1H), 8.00 (s, 1H), 6.04 (d, J = 5.51 Hz, 1H), 5.80 (d, J = 5.94 Hz, 1H), 4.71 (t, J = 5.45 Hz, 2H), 4.58-4.50 (m, 2H), 4.39-4.18 (m, 6H); 31P NMR (D2O, 400 MHz): d -10.17, -21.82. The mass was confirmed by ESI-MS in negative mode as m/z (M-1) 851.22 amu (calculated for C20H27N10O20P4-: 851.04).
- 21.Preparation of Gp4G. Starting with 2′,3′-O-2-N-triacetylguanosine (0.13 g, 0.32 mmol), Gp4G was prepared by the same procedure described above, except that the cation exchange resin was in the Li+ form rather than Na+ to minimize aggregation of the product. Following HPLC purification, 0.14 g of Gp4G in the ammonium form was obtained (0.15 mmol, 47%): UV λmax 253 nm with shoulder at 275 nm; 1H NMR (D2O, 400 MHz): 8.04 (s, 2H), 5.84 (d, J = 5.93 Hz, 2H), 4.74 (t, J = 5.80 Hz, 2H), 4.54 (t, J = 4.11 Hz, 2H), 4.35-4.30 (m, 2H), 4.30-4.20 (m, 4H); 31P NMR (D2O, 400 MHz): d -9.15, -20.84. The mass was confirmed by ESI-MS in negative mode as m/z (M-1) 867.37 amu (calculated for C20H27N10O21P4-: 867.03).
- 22.Preparation of Ap5A. Starting with 2′,3′-O-6-N-triacetyladenosine (0.12 g, 0.31 mmol), the intermediate trimetaphosphate was prepared as described above. Adenosine diphosphate (0.29 g, 0.68 mmol, 2.2 eq) was used in the coupling instead of AMP. Following HPLC purification, 0.15 g of Ap5A in the ammonium form was obtained (0.15 mmol, 48%): UV λmax 259 nm; 1H NMR (D2O, 400 MHz): 8.45 (s, 2H), 8.16 (s, 2H), 6.02 (d, J = 5.76 Hz, 2H), 4.70 (t, J = 5.42 Hz, 2H), 4.56 (t, J = 4.24 Hz, 2H), 4.43-4.35 (m, 2H), 4.34-4.20 (m, 4H); 31P NMR (D2O, 400 MHz): d -10.18, -21.61. The mass was confirmed by ESI-MS in negative mode as m/z (M-1) 915.17 amu (calculated for C20H28N10O22P5-: 915.01).
- 23.Kolodny NH, Collins LJ. J Biol Chem. 1986;261:14571–14575. [PubMed] [Google Scholar]
- 24.Glonek T, Costello AJR, Myers TC, Van Wazer JR. J Phys Chem. 1975;79:1214–1218. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spectra (UV, MS, 1H NMR, 13C NMR and 31P NMR). This material is available free of charge via the Internet at http://pubs.acs.org.