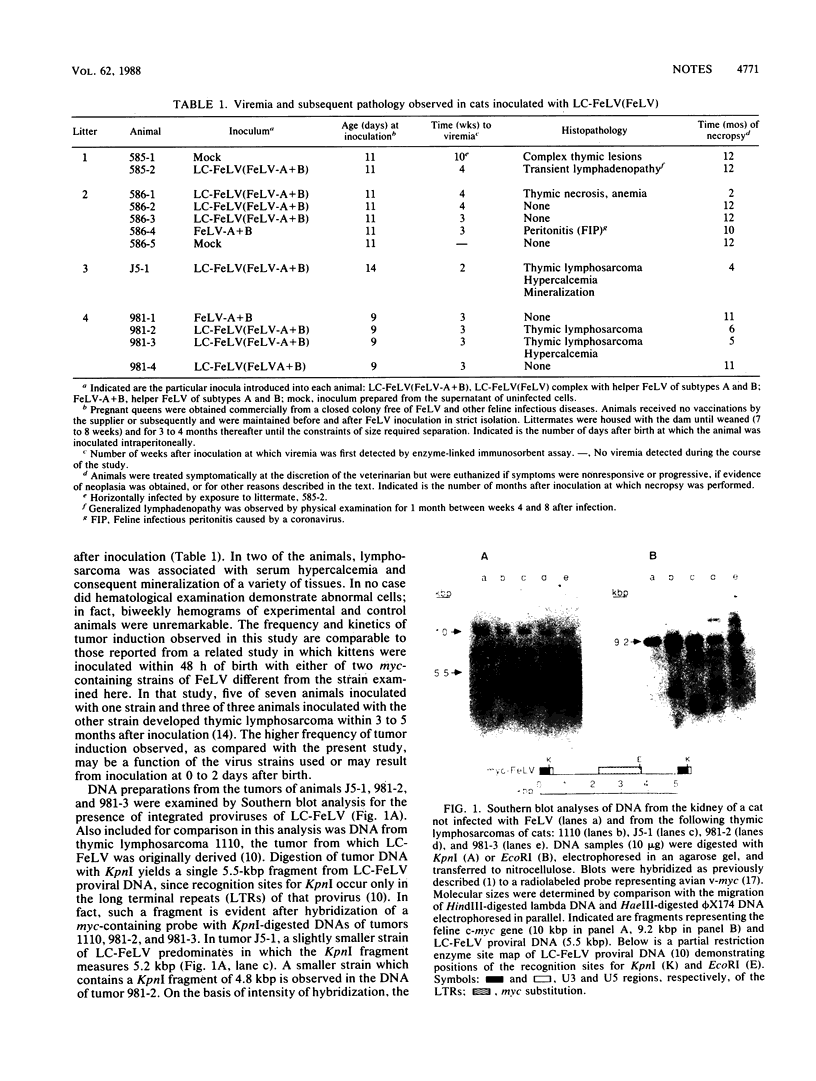
Abstract
The oncogenic capacity of a myc-containing strain of feline leukemia virus (FeLV), termed LC-FeLV, has been examined after inoculation of the virus into neonatal kittens. Like other myc-containing strains of FeLV, LC-FeLV may induce with relatively short latency, but does not necessarily induce, thymic lymphosarcoma in viremic animals. Naturally occurring and experimentally induced tumors are T-cell lymphomas which contain clonally integrated LC-FeLV proviral DNA and which cannot readily be cultivated in vitro in the presence or absence of exogenously supplied interleukin-2. Acquisition of myc by FeLV decreases the period of latency before the appearance of tumors but does not expand the spectrum of tumors induced by FeLV alone.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonham L., Lobelle-Rich P. A., Henderson L. A., Levy L. S. Transforming potential of a myc-containing variant of feline leukemia virus in vitro in early-passage feline cells. J Virol. 1987 Oct;61(10):3072–3081. doi: 10.1128/jvi.61.10.3072-3081.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M. J., Deininger P. L., Casey J. W. Nucleotide sequence of a transduced myc gene from a defective feline leukemia provirus. J Virol. 1985 Jul;55(1):177–183. doi: 10.1128/jvi.55.1.177-183.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesGroseillers L., Rassart E., Jolicoeur P. Thymotropism of murine leukemia virus is conferred by its long terminal repeat. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4203–4207. doi: 10.1073/pnas.80.14.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrietto P. J., Payne L. N., Hayman M. J. A recovered avian myelocytomatosis virus that induces lymphomas in chickens: pathogenic properties and their molecular basis. Cell. 1983 Dec;35(2 Pt 1):369–379. doi: 10.1016/0092-8674(83)90170-8. [DOI] [PubMed] [Google Scholar]
- Essex M. Horizontally and vertically transmitted oncornaviruses of cats. Adv Cancer Res. 1975;21:175–248. doi: 10.1016/s0065-230x(08)60973-2. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J. Avian acute leukemia viruses. Curr Top Microbiol Immunol. 1983;103:109–125. doi: 10.1007/978-3-642-68943-7_5. [DOI] [PubMed] [Google Scholar]
- Lenz J., Celander D., Crowther R. L., Patarca R., Perkins D. W., Haseltine W. A. Determination of the leukaemogenicity of a murine retrovirus by sequences within the long terminal repeat. 1984 Mar 29-Apr 4Nature. 308(5958):467–470. doi: 10.1038/308467a0. [DOI] [PubMed] [Google Scholar]
- Levy L. S., Gardner M. B., Casey J. W. Isolation of a feline leukaemia provirus containing the oncogene myc from a feline lymphosarcoma. 1984 Apr 26-May 2Nature. 308(5962):853–856. doi: 10.1038/308853a0. [DOI] [PubMed] [Google Scholar]
- Morse H. C., 3rd, Hartley J. W., Fredrickson T. N., Yetter R. A., Majumdar C., Cleveland J. L., Rapp U. R. Recombinant murine retroviruses containing avian v-myc induce a wide spectrum of neoplasms in newborn mice. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6868–6872. doi: 10.1073/pnas.83.18.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Brody D. S., Binari R. C., Jr, Cotter S. M. Viral transduction of c-myc gene in naturally occurring feline leukaemias. 1984 Apr 26-May 2Nature. 308(5962):856–858. doi: 10.1038/308856a0. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Hughes D., McFarlane R., Wilkie N. M., Onions D. E., Lees G., Jarrett O. Transduction and rearrangement of the myc gene by feline leukaemia virus in naturally occurring T-cell leukaemias. 1984 Apr 26-May 2Nature. 308(5962):814–820. doi: 10.1038/308814a0. [DOI] [PubMed] [Google Scholar]
- Onions D., Lees G., Forrest D., Neil J. Recombinant feline viruses containing the myc gene rapidly produce clonal tumours expressing T-cell antigen receptor gene transcripts. Int J Cancer. 1987 Jul 15;40(1):40–45. doi: 10.1002/ijc.2910400108. [DOI] [PubMed] [Google Scholar]
- Papas T. S., Lautenberger J. A. Sequence curiosity in v-myc oncogene. Nature. 1985 Nov 21;318(6043):237–237. doi: 10.1038/318237a0. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Forrest D., McFarlane R., Onions D., Wilkie N., Neil J. C. Conservation of the c-myc coding sequence in transduced feline v-myc genes. Virology. 1986 Oct 15;154(1):121–134. doi: 10.1016/0042-6822(86)90435-6. [DOI] [PubMed] [Google Scholar]
- Vennström B., Moscovici C., Goodman H. M., Bishop J. M. Molecular cloning of the avian myelocytomatosis virus genome and recovery of infectious virus by transfection of chicken cells. J Virol. 1981 Aug;39(2):625–631. doi: 10.1128/jvi.39.2.625-631.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]