Abstract
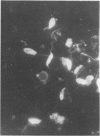
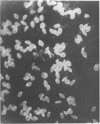
Patients with Indian kala-azar were investigated for the presence of circulating immune complexes by the platelet aggregation test, complement deviation test, and polyethylene glycol precipitation test. Circulating antibodies were tested by the conventional indirect immunofluorescence test using leptomonad forms of Leishmania donovani and Crithidia luciliae. The serum complement level (C3) was measured using the Mancini technique. The results indicate that a large number of patients with Indian kala-azar carry circulating immune complexes with a significant lowering of complement levels in their sera. These complexes may be intimately linked with the depressed cell-mediated immune responses that are commonly observed in these patients. The study warrants a detailed immunohistopathological examination of the kidneys for the presence of tissue-bound complexes in chronic patients. Further, it is revealed that Crithidia luciliae and Leishmania donovani share common antigens and the former can be used as a substitute for determining anti-leishmania antibody by the indirect fluorescence assay.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikat B. K., Sahaya S., Pathania A. G., Bhattacharya P. K., Desai N., Prasad L. S., Mishra S., Jain S. Clinical profile of cases of kala-azar in Bihar. Indian J Med Res. 1979 Oct;70:563–570. [PubMed] [Google Scholar]
- Aikat B. K., Sehgal S., Mahajan R. C., Pathania A. G., Bhattacharya P. K., Sahaya S., Choudhury A. B., Pasricha N., Prasad L. S. The role of counter immunoelectrophoresis as a diagnostic tool in kala-azar. Indian J Med Res. 1979 Oct;70:592–597. [PubMed] [Google Scholar]
- BONE G. J., STEINERT M. Isotopes incorporated in the nucleic acids of Trypanosoma mega. Nature. 1956 Aug 11;178(4528):308–309. doi: 10.1038/178308a0. [DOI] [PubMed] [Google Scholar]
- Berger M., Birch L. M., Conte N. F. The nephrotic syndrome secondary to acute glomerulonephritis during falciparum malaria. Ann Intern Med. 1967 Dec;67(6):1163–1171. doi: 10.7326/0003-4819-67-6-1163. [DOI] [PubMed] [Google Scholar]
- Casali P., Lambert P. H. Purification of soluble immune complexes from serum using polymethylmetacrylate beads coated with conglutinin or C1q. Application to the analysis of the components of in vitro formed immune complexes and of immune complexes occurring in vivo during leishmaniasis. Clin Exp Immunol. 1979 Aug;37(2):295–309. [PMC free article] [PubMed] [Google Scholar]
- Digeon M., Laver M., Riza J., Bach J. F. Detection of circulating immune complexes in human sera by simplified assays with polyethylene glycol. J Immunol Methods. 1977;16(2):165–183. doi: 10.1016/0022-1759(77)90051-5. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Lucas Z. J. Immune response to a mammary adenocarcinoma. V. Sera from tumor-bearing rats contain multiple factors blocking cell-mediated cytotoxicity. J Immunol. 1978 Dec;121(6):2485–2489. [PubMed] [Google Scholar]
- Perry L. L., Benacerraf B., Greene M. I. Regulation of the immune response to tumor antigen. IV. Tumor antigen-specific suppressor factor(s) bear I-J determinants and induce suppressor T cells in vivo. J Immunol. 1978 Dec;121(6):2144–2147. [PubMed] [Google Scholar]
- SANDERSON A. R. APPLICATIONS OF ISO-IMMUNE CYTOLYSIS USING RADIOLABELLED TARGET CELLS. Nature. 1964 Oct 17;204:250–253. doi: 10.1038/204250a0. [DOI] [PubMed] [Google Scholar]
- Sehgal S., Aikat B. K., Pasricha N. Diagnostic significance of antinuclear antibodies. Indian J Med Res. 1978 Jan;67:116–123. [PubMed] [Google Scholar]
- Sehgal S., Kumar B. Circulating and tissue immune complexes in leprosy. Int J Lepr Other Mycobact Dis. 1981 Sep;49(3):294–301. [PubMed] [Google Scholar]
- Small M. Characteristics of the immature cells involved in T cell-mediated enhancement of syngeneic tumor growth. J Immunol. 1977 May;118(5):1517–1523. [PubMed] [Google Scholar]