Abstract
An increase in the level of active, GTP-bound Ras is not necessary for transformation of chicken embryo fibroblasts (CEF) by v-Src. This suggests that other Ras-independent pathways contribute to transformation by v-Src. To address the possibility that activation of phosphatidylinositol-3-kinase (PI3K) and the mammalian target of rapamycin (mTOR/FRAP), represents one of these pathways, we have examined the effect of simultaneous inhibition of the Ras-MAPK and PI3K-mTOR pathways on transformation of CEF by v-Src. Transformation was assessed by the standard parameters of morphological alteration, increased hexose uptake, loss of density inhibition, and anchorage-independent growth. Inhibition of the Ras-MAPK pathway by expression of the dominant-negative Ras mutant HRasN17 or by addition of the MAPK kinase (MEK) inhibitor PD98059 reduced several of these parameters but failed to block transformation. Similarly, inhibition of the PI3K-mTOR pathway by addition of the PI3K inhibitor 2-[4-morpholinyl]-8-phenyl-4H-1-benzopyran-4-one (LY294002) or the mTOR inhibitor rapamycin, although reducing several parameters of transformation, also failed to block transformation. However, simultaneous inhibition of signaling by the Ras-MAPK pathway and the PI3K-mTOR pathway essentially blocked transformation. These data indicate that transformation of CEF by v-Src is mediated by two parallel pathways, the Ras-MAPK pathway and the PI-3K-mTOR pathway, which both contribute to transformation. The possibility that simultaneous activation of other pathways is also required is not excluded.
INTRODUCTION
Expression of the transforming nonreceptor tyrosine kinase v-Src (pp60v-src) results in tyrosine phosphorylation of numerous substrates and activation of multiple signaling pathways. One of these pathways is the Ras-Raf-MAPK-MAPK kinase (MEK) pathway. Activation of Ras is critical for transformation of NIH-3T3 mouse fibroblasts by v-Src (Smith et al., 1986; DeClue et al., 1991; Nori et al., 1991; Stacey et al., 1991). However chicken embryo fibroblasts (CEF) expressing a dominant-negative mutant of Ras, N17Ras, which suppresses the activation of MAPK, can be transformed by v-Src (Aftab et al., 1997). Similar observations have been made on Rat-2 cells and rat intestinal epithelial cells (Aftab et al., 1997; Oldham et al., 1998). These observations have suggested the existence of Ras-independent pathways that are activated by v-Src and that can lead to transformation.
Phosphatidylinositol-3-kinase (PI3K) is critical for transformation by Ras (Rodriguez-Viciana et al., 1997), and a retrovirus encoding a gag fusion to the catalytic subunit of PI3K is transforming (Chang et al., 1997). The form of PI3K that is activated by Src and Ras is a heterodimeric kinase that is composed of an 85-kDa regulatory subunit (p85) and a 110-kDa catalytic subunit (p110). PI3K activation by v-Src can be mediated by Ras, which interacts directly with the p110 catalytic subunit (Rodriguez-Viciana et al., 1994). PI3K can also be activated by v-Src independently of Ras (Liu et al., 1993; Rodriguez-Viciana et al., 1994). This enzyme is therefore a possible mediator of Ras-independent transformation by v-Src. Activation of PI3K can result from binding of the Src homology 3 domain of Src with a proline-rich region within p85 (Pleiman et al., 1994) or by interaction of p85 with tyrosine-phosphorylated docking proteins such as Cbl (Dombrosky-Ferlan and Corey, 1997; Jain et al., 1997). Thus direct or indirect interactions of p85 with Src could be responsible for Ras-independent activation of PI3K.
PI3K phosphorylates inositol lipids at the D-3 position of the inositol ring (Liscovitch and Cantley, 1994). The heterodimeric (p85–p110) form of PI3K phosphorylates phosphatidylinositol-4-monophosphate (PI-4-P) and phosphatidylinositol-4,5-bisphosphate (PI-4,5-P2) to form phosphatidylinositol-3,4-bisphosphate (PI-3,4-P2) and phosphatidylinositol-3,4,5-trisphosphate (PI-3,4,5-P3), respectively. PI-3,4-P2 and PI-3,4,5-P3 are present at low concentrations in quiescent cells but are rapidly generated upon stimulation with mitogens (Auger et al., 1989). These PI3K products are elevated in v-Src–transformed cells (Whitman et al., 1988), and the interaction between v-Src and PI3K correlates with transformation by v-Src (Wages et al., 1992). One of the functions of the PI3K-dependent phospholipid products is to recruit to the membrane proteins that contain pleckstrin homology domains (August et al., 1997). In particular, PI-3,4-P2 and PI-3,4,5-P3 recruit the serine-threonine kinase Akt to the membrane (Klippel et al., 1997), where it is activated by PI3K-dependent kinase 1 (PDK1) and PDK2 (Anderson et al., 1998). Akt in turn activates another kinase, the mammalian target of rapamycin (mTOR) (Vonmanteuffel et al., 1996; Gingras et al., 1998). Activation of mTOR results in the phosphorylation of ribosomal protein S6 kinase, which is also regulated by phosphorylation by PDK1 (Pullen et al., 1998). Activation of mTor also results in phosphorylation and inactivation of eukaryotic initiation factor 4E-binding protein 1 (eIF4E-BP1), an inhibitor of the translation initiation factor eIF4E (Beretta et al., 1996; Brunn et al., 1997). Some of the downstream targets of PI3K, such as Akt and eIF4E, are transforming when overexpressed (Lazariskaratzas et al., 1990). Furthermore, overexpression of eIF4E-BP1 can block transformation by Src (Rousseau et al., 1996a). These observations suggest that the PI3K-Akt-mTOR-eIF4E-BP1 pathway may be one of the signaling pathways involved in transformation by v-Src.
We show here that transformation of CEF by v-Src is not blocked by inhibition of the Ras-MAPK pathway at the level of Ras or MEK or by inhibition of the PI3K-mTor pathway at the level of PI3K or mTOR. However, when both pathways are simultaneously inhibited, the phenotype of nontransformed cells is largely restored. We conclude that both the Ras-MAPK pathway and the PI3K-mTor pathway contribute to transformation by v-Src.
MATERIALS AND METHODS
Cells, Viruses, and Expression Vectors
Primary cultures of CEF were prepared from 10-d-old embryos and cultured as described (DeClue and Martin, 1989; Liebl et al., 1992). Cells were grown in a 2:1 mixture of F-10 and Dulbecco’s modified Eagle’s medium supplemented with 2% tryptose phosphate broth, 1% bovine calf serum, and 1% chicken serum (abbreviated 2.1.1). The RCAN-BH and RCAS-BH expression vectors are replication-competent, helper-independent retroviral vectors modified by substitution of the Bryan high-titer virus (BH) polymerase gene (Petropoulos and Hughes, 1991; Federspiel and Hughes, 1994). To express dominant-negative Ras in CEF, a cDNA encoding the N17 mutant of H-Ras (provided by C. Der, University of North Carolina, Chapel Hill, NC) was subcloned into the helper-independent retroviral vector RCAS(B)-BH (Petropoulos and Hughes, 1991; Federspiel and Hughes, 1994), which encodes an envelope subgroup B virus. Wild-type Schmidt–Ruppin A v-src and the temperature-sensitive (ts) mutant tsUP1-src (Maroney et al., 1992) were subcloned into the vector RCAN(A)-BH, which encodes an envelope subgroup A virus. CEF were transfected with these plasmids by polybrene-DMSO shock, and virus stocks were harvested 5–6 d after transfection as described by Liebl et al. (1992). To coexpress v-Src and H-RasN17, cells were infected with the N17Ras virus (in the presence of 2 μg/ml polybrene) 2 d before infection with the Src virus. For infection with retrovirus encoding tsUP1Src, CEF were infected at a multiplicity of infection of >10, maintained at the nonpermissive temperature, 41°C, for an additional 12 h, and then transferred to the permissive temperature, 37°C.
Pharmacological Inhibitors
2-[4-Morpholinyl]-8-phenyl-4H-1-benzopyran-4-one (LY294002; Calbiochem, La Jolla, CA) was added to 10 μM final concentration. PD98059 (New England Biolabs, Beverly, MA; Dudley et al., 1995) was added to 50 μM final concentration. Wortmannin (Calbiochem) was added to 50 nM final concentration. Rapamycin (Calbiochem) was added to 50 nM concentration.
MAPK Assays
The immune complex kinase activity of the MAP kinase Erk-2 was measured as described (Aftab et al., 1997).
Immunoblotting
Infected CEF were lysed in 0.5 ml modified radioimmunoprecipitation assay buffer (150 mM NaCl, 10 mM Tris-HCl, 1 mM EDTA, 1% Nonidet P-40, 1% sodium deoxycholate, 0.05% SDS, 1 mM sodium orthovanadate, 0.5 mg/ml chymostatin, 0.5 mg/ml leupeptin, 0.5 mg pepstatin, pH 7.4). Lysates were clarified at 14,000 × g for 10 min. Equal quantities of protein (50 μg) were resolved by SDS-PAGE on 10% polyacrylamide gels. Proteins were transferred to Immobilon membranes (Millipore, Bedford, MA), and the membranes were blocked for 1 h at room temperature in blocking buffer containing 3% BSA, fraction V (ICN Biochemicals, Costa Mesa, CA). Membranes were then incubated for 1 h at room temperature with 1 mg/ml mAb 2–17 (ascites fluid from a hybridoma supplied by Microbiological Associates, Bethesda, MD) to detect Src, with mAb 4G10 (Upstate Biotechnology, Lake Placid, NY) to detect cellular phosphotyrosyl-proteins, with polyclonal antibody ERK 2 (C-14; Santa Cruz Biotechnology, Santa Cruz, CA) to detect phosphorylated (activated) Erk2, or with polyclonal anti-phospho-Akt (pSer473) antibody (New England Biolabs) to detect phosphorylated (activated) Akt. Membranes were washed three times in blocking solution and then incubated for an additional 1 h either in HRP-conjugated rabbit anti-mouse secondary antibody to detect mAbs or in goat anti-rabbit secondary antibody to detect rabbit polyclonal antibodies. Finally membranes were washed three times in the blocking solution, and antibody was detected by enhanced chemiluminescence (ECL kit; Amersham, Arlington Heights, IL).
Deoxyglucose Uptake
Cells were rinsed twice in warm PBS and incubated for 10 min at 41°C in 1 ml PBS containing 40 pM deoxy-d-2-[1,2-3H] glucose (specific activity, 26 Ci/mmol). They were then washed three times with cold PBS and lysed in 250 μl 1% SDS/60-mm dish. Lysates were sonicated for 5 s to shear cellular DNA. One hundred-microliter samples were counted by liquid scintillation spectrometry, whereas the remaining lysates were used to quantitate the protein concentration by the bicinchoninic acid method (Pierce, Rockford, IL).
Colony Assays
CEF were infected with RCAN(A)-BH-v-Src at a multiplicity of infection of 0.1, trypsinized, and resuspended at 1.5 × 106 cells/ml in F-10/Dulbecco’s modified Eagle’s medium (2:1) containing 10% tryptose phosphate broth, 4% bovine calf serum, 1% chicken serum. Cell suspension (0.5 ml) was added to 1 ml of the same medium containing 0.66% agar at 44°C, with or without pharmacological inhibitors. Agar-suspended cells were poured over a base agar layer, with or without pharmacological inhibitors, and the top layer was allowed to set. Suspension cultures were incubated for 10–12 d at 41°C. Assays were fed every 4 d with agar overlay, with or without pharmacological inhibitors, for up to 10–12 d.
RESULTS
Effects of N17Ras and LY294002 on Src Expression and Kinase Activity and on MAPK and Akt Activation
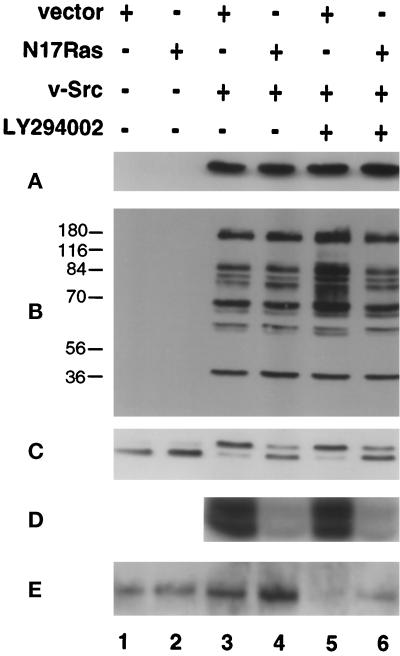
To examine the possibility that PI3K mediates Ras-independent transformation by v-Src, we used coexpression of N17Ras to inhibit Ras signaling and LY294002 or, in some experiments, wortmannin to inhibit PI3K signaling. Initial experiments were carried out to determine whether these inhibitors affected v-Src expression or kinase activity. CEF were infected at 41°C with retroviruses expressing a temperature-sensitive mutant of v-Src, tsUP1 [RCAN(A)-BH-tsUP1Src], and a dominant-negative mutant of Ras, HRasN17 [RCAS(B)-BH-N17Ras]. The coinfected CEF, or CEF infected with tsUP1 alone, were shifted to the permissive temperature, 37°C, and treated with the PI3K-specific inhibitor LY294002. Lysates were prepared after 48 h and resolved by SDS-PAGE. v-Src expression and the total level of phosphotyrosyl-protein were monitored by immunoblotting using antibodies directed against v-Src or phosphotyrosine. As shown in Figure 1, A and B, neither the level of expression of v-Src nor the overall level or pattern of tyrosine phosphorylation was significantly altered when Ras activation was inhibited by coexpression of N17Ras, when PI3K activity was inhibited by addition of LY294002, or when both Ras activation and PI3K activity were simultaneously inhibited. In some experiments LY294002 increased the tyrosine phosphorylation of some but not all bands (Figure 1B, lane 5), but this effect was not always observed (see Figure 4B, lane 4).
Figure 1.
Effects of LY294002 and N17Ras expression on v-Src expression and activity and on the activation of Erk2 and Akt. (A and B) CEF were infected with RCAS(B)-BH (lanes 1, 3, and 5) or RCAS(B)-BH-N17Ras (lanes 2, 4, and 6) 2 d before superinfection with RCAN(A)-BH (lanes 1 and 2) or RCAN(A)-BH-tsUP1Src (lanes 3–6) at the nonpermissive temperature, 41°C. After 12 h coinfected CEF were shifted to the permissive temperature, 37°C, and LY294002 was added (lanes 5 and 6). Cell lysates were prepared 48 h after the temperature shift, resolved by SDS-PAGE, and analyzed by immunoblotting with anti-Src (A) and anti-phosphotyrosine (B) mAbs. (C–E) CEF were coinfected and treated with LY294002 as described above. 24 h after the temperature shift, the CEF were transferred to serum-free medium, incubated for an additional 24 h, and then lysed. Erk2 activity was analyzed by immunoblotting with an antibody to Erk2 to detect the lower-mobility phosphorylated form (C) or by an immune complex myelin basic protein kinase assay (D). Akt activation was analyzed by immunoblotting with an antibody specific for phosphorylated Akt (E). Scanning densitometry indicated that LY294002 reduced the level of phospho-Akt to 47% (lane 5) or 60% (lane 6) of the level in untreated cells expressing empty vector (lane 1) (mean of two determinations).
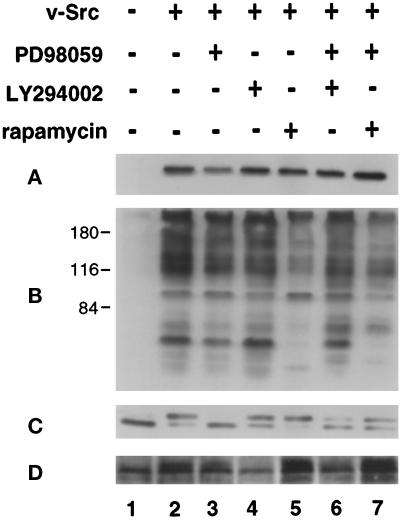
Figure 4.
Effects of PD98059, LY294002, and rapamycin on tsUP1Src expression and kinase activity and on the activation of Erk2 and Akt. (A and B) CEF were infected for 12 h at the nonpermissive temperature, 41°C, with RCAN(A)-BH (lane 1) or RCAN(A)-BH-tsUP1Src (lanes 2–6), shifted to the permissive temperature, 37°C, and incubated with or without inhibitors as indicated. Cell lysates were prepared after 48 h and examined by immunoblotting with anti-Src mAb (A) or anti-phosphotyrosine mAb (B). (C and D) CEF were coinfected and treated with inhibitors as described above. Twenty-four hours after the temperature shift, the CEF were transferred to serum-free medium, incubated for an additional 24 h in the continued presence or absence of inhibitors, and then lysed. Lysates were examined by immunoblotting with anti-Erk2 antibody (C), or anti-phospho-Akt (D).
To determine the effects of N17Ras and LY294002 on Ras and PI3K signaling in CEF expressing v-Src, we examined the effects of these inhibitors on the activation of downstream targets of Ras and PI3K, namely the MAPK Erk2 and Akt. Lysates were prepared as described above from CEF expressing tsUP1, in the presence or absence of coexpressed N17Ras or LY294002. Activated Erk2, which has a slightly lower electrophoretic mobility than the nonphosphorylated form was detected by immunoblotting. Activated Akt was detected by immunoblotting with an antibody specific for phosphorylated Akt. In addition, Erk2 was immunoprecipitated from the lysates, and immune complex kinase assays were performed using myelin basic protein as substrate.
MAPK activity was elevated in CEF expressing v-Src, in the presence or absence of LY294002, as judged by both gel shift and kinase assays (Figure 1, C and D, lanes 3 and 5). Coinfection with a retrovirus expressing N17Ras inhibited MAPK activation (Figure 1, C and D, lanes 4 and 6), confirming that N17Ras inhibits Ras activation in these cells; in some instances MAPK activity was reduced to basal or subbasal levels, whereas in others a low level of residual activation was detected (see Figure 1C). Akt is phosphorylated in CEF expressing v-Src (Figure 1E, lane 3) and in CEF coinfected with retroviruses expressing v-Src and N17Ras (Figure 1E, lane 4), confirming that v-Src activates the PI3K-Akt pathway and that this activation can occur when activation of Ras signaling is blocked by N17Ras. In the presence of LY294002 the level of active Akt is reduced to the level present in CEF infected with the empty retroviral vector (Figure 1E, lanes 5 and 6), whereas Erk2 activation is unaffected, confirming that Akt activation is specifically inhibited by addition of LY294002.
In summary, these assays indicated that in CEF expressing v-Src, N17Ras and LY294002 can be used to specifically inhibit the Ras-MAPK and PI3K pathways, respectively, without any major effects on v-Src expression or activity. These assays also indicate that v-Src can activate the PI3K-Akt pathway in the absence of an increase in Ras activation.
Effect of PI3K Inhibition on Morphological Transformation by v-Src
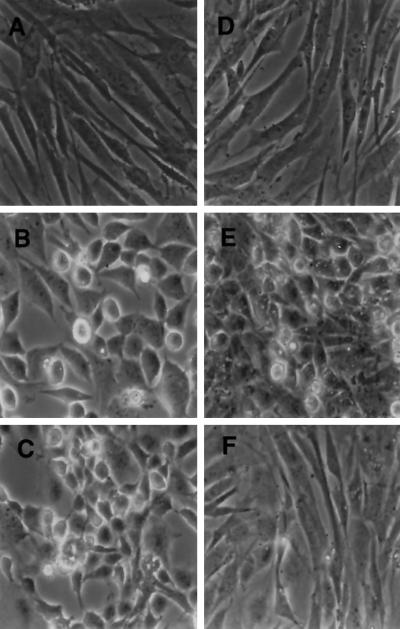
To examine the effects of PI3K inhibition on Ras-independent transformation by v-Src, CEF were again infected at 41°C with vectors expressing tsUP1Src and N17Ras. The coinfected CEF or CEF infected with tsUP1 alone were shifted to the permissive temperature, 37°C, and treated with a PI3K-specific inhibitor, either LY294002 or wortmannin. Morphology was examined after 2 d (Figure 2). CEF infected with a retrovirus encoding tsUP1 and shifted to the permissive temperature were transformed to the rounded, refractile morphology characteristic of Src transformation (Figure 2B). Consistent with our previous results (Aftab et al., 1997), CEF coinfected with retroviruses expressing v-Src and N17Ras also appeared morphologically transformed, although slightly flatter than CEF expressing v-Src alone (Figure 2E). Similarly, CEF expressing v-Src and treated with LY294002 appeared morphologically transformed (Figure 2C). In contrast, CEF coinfected with retroviruses expressing v-Src and N17Ras and treated with LY294002 were morphologically normal (Figure 2F). Comparable results were obtained with CEF transformed by wild-type v-Src when the inhibitors were added after transformation had become established (our unpublished results). Similar results were also obtained when the PI3K inhibitor wortmannin was used in place of LY294002 (our unpublished results). These results indicated that morphological transformation by v-Src is not blocked by inhibition of either PI3K or Ras activity but is blocked by simultaneous inhibition of both PI3K and Ras activation.
Figure 2.
Effects of LY294002 and N17Ras expression on the morphology of CEF expressing v-Src. CEF were infected and grown as described in the legend to Figure 1. (A–C) Morphology of CEF infected with RCAS(B)-BH and coinfected with RCAN(A)-BH (A), coinfected with RCAN(A)-BH-tsUP1Src (B), or coinfected with RCAN(A)-BH-tsUP1Src and incubated with LY294002 (C). (D–F) Morphology of CEF infected with RCAS(B)-BH-N17Ras and coinfected with RCAN(A)-BH (D), coinfected with RCAN(A)-BH-tsUP1Src (E), or coinfected with RCAN(A)-BH-tsUP1Src and incubated with LY294002 (F). Images were acquired using a Zeiss (Thornwood, NY) Axiovert microscope.
Effect of PI3K Inhibition on v-Src–induced Hexose Uptake
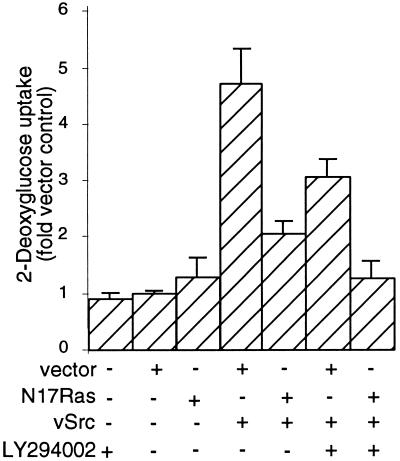
Transformation by v-Src is also characterized by an increase in hexose uptake, which is due to an increase in the number of glucose transporters at the membrane; in CEF this results from both a decrease in the rate of degradation of glucose transporters (Shawver et al., 1987) and an increase in the transcription of the mRNA encoding the GLUT3 transporter (Wagstaff et al., 1995). We therefore determined whether inhibition of PI3K, alone or in conjunction with N17Ras expression, affects hexose uptake by CEF expressing v-Src. CEF infected with retroviral vectors alone exhibited a basal level of hexose uptake that was not significantly altered by the addition of LY294002 (Figure 3). CEF infected with a retroviral vector encoding wild-type v-Src [RCAN(A)-BH-Src] exhibited an ∼4.5-fold increase in the rate of hexose uptake. CEF coexpressing v-Src and N17Ras exhibited a 2-fold increase in hexose uptake. CEF expressing v-Src and incubated with LY294002 exhibited a 3-fold increase in hexose uptake. Thus coexpression of N17Ras or incubation with LY294002 partially inhibits but does not eliminate the increase in hexose uptake induced by v-Src. In contrast, CEF coexpressing both v-Src and N17Ras and then treated with LY294002 exhibited a hexose uptake rate comparable with that of nontransformed CEF infected with the empty retroviral vector, indicating that blockade of both Ras and PI3K signaling completely suppresses this parameter of transformation.
Figure 3.
Effects of LY294002 and N17Ras expression on hexose uptake by CEF expressing v-Src. CEF infected with RCAS(B)-BH or RCAS(B)-BH-N17Ras were superinfected after 48 h with RCAN(A)-BH-v-Src. After 12 h, LY294002 was added to half of the cultures, which were then incubated for a further 24 h. The cells were then washed and incubated for 10 min in the presence of [3H]2-deoxyglucose. The rate of deoxyglucose transport was quantitated as incorporated tritium (counts per minute) per milligram of protein lysate. Results are the mean of three independent experiments each performed in triplicate.
MEK and mTOR as Downstream Mediators of Ras and PI3K
The preceding experiments suggested that Ras and PI3K might activate two distinct signaling pathways that could each mediate transformation by v-Src. MEK can mediate Ras transformation in other systems, whereas mTOR appears to mediate some of the effects of PI3K, suggesting that MEK and mTOR could also mediate transformation by v-Src. To examine this possibility, CEF were infected as before with RCAN(A)-BH-tsUP1 and shifted to 37°C in the presence of the MEK inhibitor PD98059, the mTor inhibitor rapamycin, the PI3K inhibitor LY294002, or PD98059 plus either rapamycin or LY294002.
To determine whether these inhibitors affected v-Src expression or kinase activity, cell lysates were examined as before by immunoblotting with anti-Src or anti-pTyr monoclonal antibodies (Figure 4, A and B). The overall level of v-Src expression was not altered in CEF expressing tsUP1Src and treated with PD98059, LY294002, rapamycin, PD98059 plus LY294002, or PD98059 plus rapamycin (Figure 4A, lanes 2–7). The overall level of tyrosine phosphorylation was not altered by addition of PD98059, LY294002, or both PD98059 and LY294002 (Figure 4B, lanes 2–4 and 6). The level of tyrosine phosphorylation was reduced by the addition of rapamycin (Figure 4B, lanes 5 and 7), suggesting that rapamycin may have nonspecific effects on Src kinase activity; however, despite the change in the level of tyrosine phosphorylation, CEF infected with a retrovirus expressing v-Src and treated with rapamycin appeared morphologically transformed (see below), indicating that the level of Src kinase activity in the presence of rapamycin is sufficient to allow transformation.
To further address the specificity of the pharmacological inhibitors, their effects on Erk2 and Akt activation were examined by immunoblot analysis (Figure 4, C and D). Erk2 was activated in CEF expressing tsUP1Src. Addition of LY294002 or rapamycin did not inhibit Erk2 activation, but, as expected, addition of PD98059 significantly reduced activation (Figure 4C, lanes 2–5). (The inhibition of Erk2 by PD98059 was incomplete when rapamycin was also added [Figure 4C, lane 7]; the reason for this is unclear, although it may reflect an effect of rapamycin on some pathway that down-regulates Erk2 activity.) Akt was activated in CEF infected with a retrovirus expressing tsUP1Src, and Akt activation was not blocked by addition of either PD98059 or rapamycin (Figure 4D, lanes 2, 3, and 5); addition of PD98059 slightly reduced Akt phosphorylation, but the level of activation remained elevated relative to that observed in CEF infected with the empty retroviral vector. Addition of LY294002 to CEF expressing v-Src blocked Akt activation (Figure 4D, lane 4). These results confirm that PD98059 specifically inhibits MEK signaling to Erk2 and LY294002 inhibits PI3K signaling to Akt.
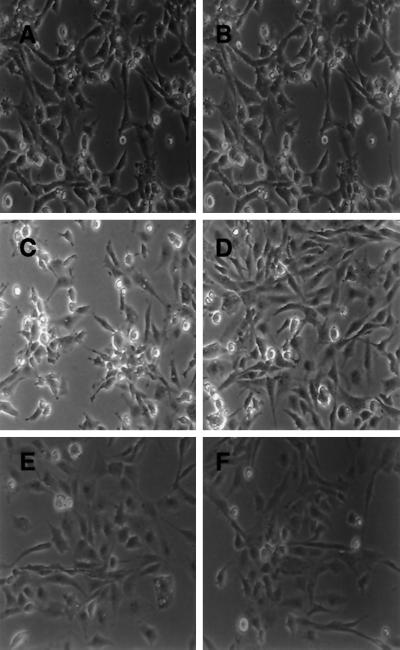
CEF expressing tsUP1Src that were treated with PD98059, rapamycin, or LY294002 individually displayed transformation morphologies indistinguishable from those of CEF expressing v-Src in the absence of any inhibitors (Figure 5, A–D). However, when either rapamycin or LY294002 was added in conjunction with PD98059, morphological transformation by v-Src was suppressed (Figure 5, E and F). These results indicate that inhibition of MEK, mTor, or PI3K is not sufficient to block transformation by v-Src, but that inhibition of both MEK and mTor or both MEK and PI3K blocks morphological transformation.
Figure 5.
Effects of PD98059, LY294002, and rapamycin on morphological transformation by tsUP1Src. CEF were infected with RCAN(A)-BH-tsUP1Src as described in the legend to Figure 4 and incubated with no inhibitor (A), PD98059 (B), LY294002 (C), rapamycin (D), LY294002 and PD98059 (E), or rapamycin and PD98059 (F). Images were acquired using a Zeiss Axiovert microscope.
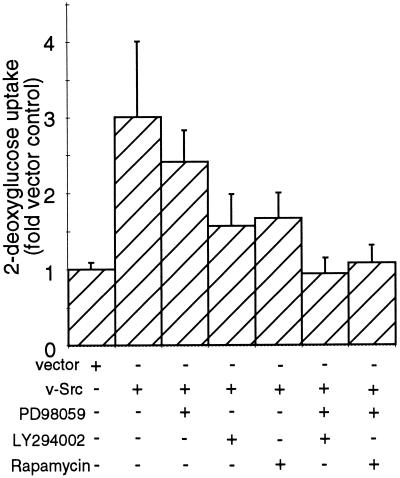
Effect of MEK and mTor Inhibition on Hexose Uptake
The effects of inhibition of MEK and mTor on v-Src–induced hexose uptake were also examined. As shown in Figure 6, addition of PD98059, LY294002, or rapamycin to CEF expressing tsUP1Src reduced the level of induction of hexose uptake, but in each case the cells displayed hexose uptake rates that were still elevated relative to the rate displayed by CEF infected with the empty retroviral vector. However, addition of PD98059 plus either LY294002 or rapamycin to CEF expressing tsUP1Src reduced the hexose uptake rate to a level comparable with that of nontransformed cells. These results indicate that inhibition of MEK and either PI3K or mTor suppresses the v-Src–induced increase in hexose uptake.
Figure 6.
Effects of PD98059, LY294002, and rapamycin on hexose uptake by CEF expressing tsUP1Src. CEF were infected as described in the legend to Figure 4. Twenty-four hours after the shift to the permissive temperature, deoxyglucose uptake was monitored as described in the legend to Figure 3.
Effects of Pharmacological Inhibitors on Density-independent Growth
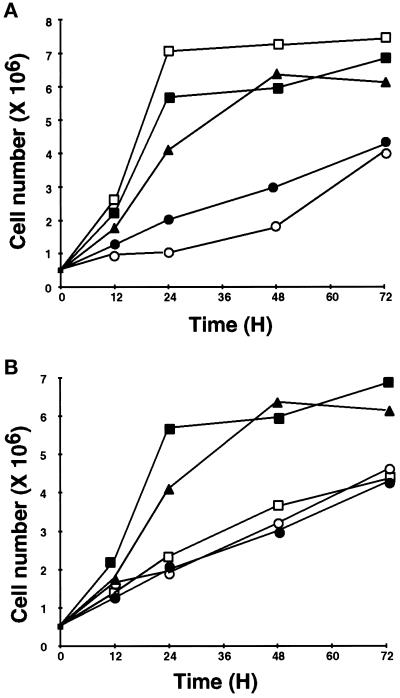
Another characteristic of CEF transformed by v-Src is the ability to grow in a density-independent manner. Under the culture conditions used here (2% serum), the growth of normal CEF is slowed at confluency (∼1–2 × 106 cells/60-mm dish) but does not cease; in contrast, CEF transformed by v-Src continue to proliferate at high cell densities. To examine the role of MEK, PI3K, and mTOR in v-Src–induced density-independent growth, we followed the growth of CEF infected with RCAN(A)-BH-tsUP1 and treated with PD98059, LY294002, rapamycin, PD98059 plus LY294002, or PD98059 plus rapamycin. In dense cultures CEF expressing tsUP1Src and treated with either PD98059 or LY294002 exhibited growth rates comparable to that of transformed CEF growing in the absence of any inhibitor (Figure 7A). However, CEF expressing tsUP1Src and treated with rapamycin, PD98059 plus LY294002, or PD98059 plus rapamycin exhibited growth rates comparable with that of CEF infected with the empty retroviral vector (Figure 7, A and B). Under certain conditions, inhibition of PI3K and Akt can lead to apoptosis, because Akt has an antiapoptotic function (Kulik et al., 1997). However, the decrease in growth rate in cultures exposed to the LY294002 did not appear to be due to apoptosis, because we were unable to detect the DNA fragmentation characteristic of apoptosis in cultures exposed to LY294002, either alone or in combination with other inhibitors (our unpublished results).
Figure 7.
Effects of PD98059, LY294002, and rapamycin on growth of CEF expressing tsUP1Src. (A) CEF were infected as described in the legend to Figure 4 with either RCAN(A)-BH (●) or RCAN(B)-BH-tsUP1Src. CEF infected with RCAN(A)-BH-tsUP1Src were incubated with no inhibitor (▪), PD98059 (▴), LY294002 (□), or PD98059 and LY294002 (○). (B) CEF were infected with either RCAN(A)-BH (●) or RCAN(A)-BH-tsUP1Src. CEF infected with RCAN(A)-BH-tsUP1Src were treated with no inhibitor (▪), PD98059 (▴), rapamycin (□), or PD98059 and rapamycin (○). Data points are means of triplicate determinations; error bars are omitted for clarity.
These data indicate that inhibition of either MEK or PI3K is not sufficient to block v-Src–induced density-independent growth, but inhibition of both MEK and PI3K restores the growth properties of normal, nontransformed cells. Addition of rapamycin alone reduces the growth of CEF expressing Src to a rate characteristic of normal CEF, indicating that, although inhibition of mTor fails to block morphological transformation, it has a more dramatic effect on cell growth, presumably because cell growth is particularly sensitive to inhibition of protein synthesis.
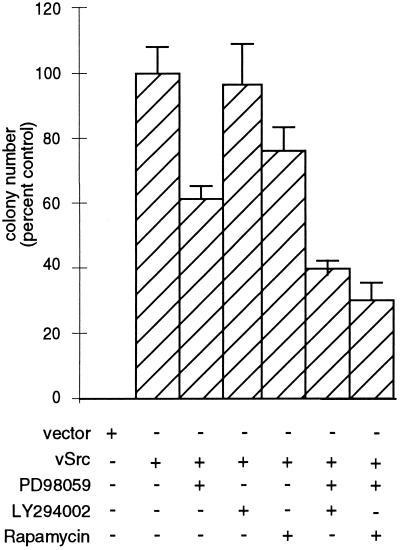
Anchorage-independent Growth Is Blocked by Inhibition of Both the PI3K-mTor and Ras-MAPK Pathways
CEF expressing v-Src can grow in an anchorage-independent manner. Addition of PD98059, LY294002, or rapamycin to CEF infected with a retrovirus encoding v-Src did not prevent the formation of colonies in soft agar. Addition of PD98059 reduced colony formation by 40%; LY294002 reduced it by 5–10%; and rapamycin reduced it by 20–25% (Figure 8). However, addition of both PD98059 and either LY294002 or rapamycin reduced the number of colonies in soft agar by 60–70%; furthermore, the colonies growing in the presence of two inhibitors were significantly reduced in size. We cannot exclude the possibility that long-term exposure to the inhibitors results in nonspecific toxicity. However, these results are consistent with the hypothesis that both the Ras-MAPK pathway and the PI3K-mTOR pathway contribute to anchorage-independent growth by v-Src.
Figure 8.
Effects of PD98059, LY294002, and rapamycin on agar colony formation by CEF expressing v-Src. CEF infected with RCAN(A)-BH-v-Src at 0.1 multiplicity of infection were trypsinized and resuspended in agar suspension containing no inhibitor, PD98059, LY294002, rapamycin, PD98059 and LY294002, or PD98059 and rapamycin. Suspension cultures, which were fed every 3 d with agar overlay containing inhibitors, were incubated for 10–12 d at 41°C.
DISCUSSION
We have described here the effects of inhibitors of the Ras-MAPK and PI3K-mTOR pathways on transformation of CEF by v-Src. Inhibition of the Ras-MAPK pathway at the level of Ras or MEK was insufficient to block transformation. Similarly, inhibition of the PI3K-mTor pathway at the level of PI3K or mTor did not block transformation by v-Src. However, when both the Ras-MAPK and PI3K-mTor pathways were simultaneously inhibited, transformation by v-Src was blocked. We conclude that both the Ras-MAPK pathway and the PI3K-mTOR pathway contribute to transformation by v-Src, as illustrated in Figure 9. The inhibitors had the same effects when added to cells already transformed by wild-type Src as when added to tsSrc-expressing cells before a shift to the permissive temperature, indicating that these pathways are involved in both initiation and maintenance of the transformed state.
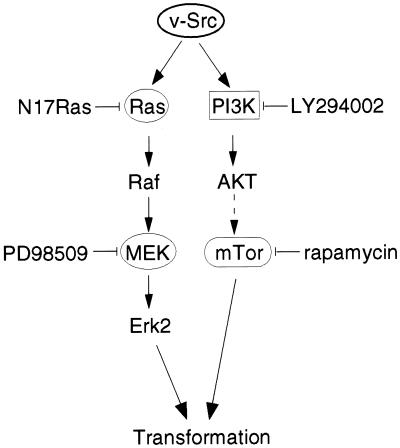
Figure 9.
Signaling pathways required for transformation by v-Src. v-Src activates both the Ras-MAPK pathway, which can be inhibited by coexpression of N17Ras or by PD98059, and, in parallel, the PI3K-Akt-mTOR pathway, which can be inhibited by LY294002 or rapamycin. Inhibition of both pathways is required to block transformation. Our data do not exclude the possibility that Src, Ras, and PI3K activate other pathways that are also necessary for transformation.
Although inhibition of the Ras-MAPK pathway did not block transformation, there was some reduction in several parameters of transformation. For example, there was a significant reduction in the level of deoxyglucose uptake in cells expressing N17Ras, although not in cells exposed to the MEK inhibitor PD98059. We previously observed a smaller effect of N17Ras expression on deoxyglucose uptake (Aftab et al., 1997); this difference may reflect differences in culture conditions. Similar effects were observed on inhibition of the PI3K-mTor pathway. Inhibition of PI3K by LY294002 or of mTor by rapamycin reduced the level of deoxyglucose uptake. Density-independent growth was blocked by rapamycin, probably because this agent is an effective inhibitor of protein synthesis. Thus, although activation of either pathway is sufficient to induce transformation detectable by a variety of assays, activation of both pathways leads to the more extensive transformation characteristic of v-Src.
Because expression of N17Ras fails to block Ras-mediated Raf activation by PKC (Marais et al., 1998), it might be argued that N17Ras expression is not sufficient to inhibit Ras signaling in cells expressing v-Src. This possibility appears to be excluded by the finding that N17Ras expression inhibits MAPK activation by v-Src (see above and Aftab et al., 1997). However, this finding does not exclude the possibility that some of the processes occurring in cells expressing N17Ras are dependent on the basal level of Ras function or a residual level of activation. In our experiments, N17Ras expression reduced MAPK activation to close to basal levels and sometimes to basal or subbasal levels (also see Aftab et al., 1997); the MEK inhibitor PD98059 reproducibly reduced MAPK activity to basal levels. This basal level of Ras-MAPK signaling may be required for transformation by v-Src; indeed some level of Ras function may be required for cell viability.
The results described here indicate that the PI3K-Akt-mTOR pathway can be activated by v-Src independently of Ras activation. Both Ras-dependent and Ras-independent activation of PI3K have been described previously (Rodriguez-Viciana et al., 1994). Again, the basal level of Ras function may be required for PI3K activation by v-Src. Consistent with this idea, Chan and Tsichlis (personal communication) have recently shown that Src and Ras can cooperate in the activation of PI3K and have suggested that Src may act not only upstream of Ras, by activating Ras GTP exchange factors, but in parallel with Ras, by regulating Ras-associated PI3K activity. It appears likely that Ras activates PI3K via interaction with the p110 catalytic subunit, whereas Src activates PI3K by a direct or indirect interaction with the p85 regulatory subunit.
The results described here suggest that the converse is also true, namely that the Ras-MEK-MAPK pathway can be activated by v-Src independent of PI3K activation. This contrasts with what has been observed in COS7 cells stimulated by fibronectin attachment, in which Raf activation is PI3K dependent (King et al., 1997). The strong signal emanating from v-Src may be sufficient to activate MEK and MAPK without any input from PI3K. Alternatively it is possible that a basal level of PI3K function may be required for Raf-MEK-MAPK signaling in CEF. Activation of Erk2 by mitogenic stimuli is repressed in cells transformed by v-Src (or v-Crk), and the level of activation of Erk2 in v-Src–transformed CEF is variable, depending on the conditions of infection (Greulich et al., 1996; Stofega et al., 1997; Penuel and Martin, unpublished observations). These observations are consistent with the hypothesis that MAPK-independent pathways are important for mitogenic signaling and transformation.
We did not detect significant levels of apoptosis in CEF expressing v-Src that were exposed to LY294002 in the absence or presence of coexpressed Ras or other inhibitors. This is perhaps surprising, in view of the fact that Akt and Ras are antiapoptotic (Kulik et al., 1997). Indeed we have observed extensive apoptosis in mammalian cells transformed by v-Src when Ras signaling and PI3K signaling are both blocked (Webb and Martin, unpublished results). We infer that the regulation of apoptosis in mammalian cells must differ in some way from the regulation of apoptosis in CEF.
One question raised by our findings is whether yet other pathways are required for transformation by v-Src. Although we have shown that either the Ras-MAPK or the PI3K-mTOR pathway is necessary for transformation, it is possible that either pathway induces transformation only when other pathways are also activated by v-Src. Recent evidence suggests that this may be the case. Two groups have demonstrated that dominant-negative Stat3 blocks transformation of mammalian cells by v-Src, implicating transcriptional regulation by STATs in transformation by v-Src (Bromberg et al., 1998; Turkson et al., 1998). In addition, overexpression of Raf is not sufficient to transform CEF (Li et al., 1996). It has recently been reported that both v-Src and Bcr-Abl induce the Ras-independent degradation of the Abi proteins, which are known to antagonize transformation (Dai et al., 1998). In platelet-derived growth factor–stimulated cells c-Src apears to induce Myc expression by an unknown Ras-independent pathway (Barone and Courtneidge, 1995), and this pathway might also be involved in transformation. Our finding that simultaneous blockade of the Ras-MAPK and PI3K-mTOR pathways does not completely block anchorage-independent growth is consistent with the idea that additional signaling pathways are involved in transformation by v-Src.
A long-standing question in this field is how the same phenotype—that of the transformed cell—can be induced by many distinct pathways, in this case the Ras-MAPK and PI3K-mTOR pathways. These two pathways may converge at a number of different sites within the cellular signaling network. For example, eIF4E may be regulated both by mTOR, via phosphorylation of 4E-BP1, and by MAPK, via the Mnk kinase (Waskiewicz et al., 1997). The expression of Myc, which appears to be required for c-Src–dependent DNA synthesis (Barone and Courtneidge, 1995) may be regulated at the transcriptional level by Raf (Kerkhoff et al., 1998) and at the translational level by the mTor pathway (West et al., 1998). Similarly, expression of cyclin D1, a key regulator of progression through G1, may be regulated at the transcriptional level by MAPK (Lavoie et al., 1996) and at level of nucleocytoplasmic transport by eIF4E (Rousseau et al., 1996b). A more complete understanding of the transformed phenotype will require identification of these regulatory nodes on which the signaling pathways that induce transformation converge.
ACKNOWLEDGMENTS
We thank T.O. Chan and P.M. Tsichlis for communicating results before publication and the members of the Martin laboratory for helpful discussions and critical reading of this manuscript. This work was supported by National Institutes of Health grant CA-17542 and by the facilities of the University of California Cancer Research Laboratory. E.P. was supported by National Institutes of Health training grant GM-07232.
Abbreviations used:
- BH
Bryan high-titer virus
- CEF
chicken embryo fibroblasts
- LY294002
2-[4-morpholinyl]-8-phenyl-4H-1-benzopyran-4-one
- MEK
MAPK kinase
- mTOR
mammalian target of rapamycin
- PDK
PI3K-dependent kinase
- PI3K
phosphatidylinositol-3-kinase
- ts
temperature-sensitive
REFERENCES
- Aftab DT, Kwan J, Martin GS. Ras-independent transformation by v-Src. Proc Natl Acad Sci USA. 1997;94:3028–3033. doi: 10.1073/pnas.94.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KE, Coadwell J, Stephens LR, Hawkins PT. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr Biol. 1998;8:684–691. doi: 10.1016/s0960-9822(98)70274-x. [DOI] [PubMed] [Google Scholar]
- Auger KR, Serunian LA, Soltoff SP, Libby P, Cantley LC. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989;57:167–75. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- August A, Sadra A, Dupont B, Hanafusa H. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the Pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci USA. 1997;94:11227–11232. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone MV, Courtneidge S. Myc but not fos rescue of PDGF signaling block caused by kinase inactive Src. Nature. 1995;378:509–512. doi: 10.1038/378509a0. [DOI] [PubMed] [Google Scholar]
- Beretta L, Gingras AC, Svitkin YV, Hall MN, Sonenberg N. Rapamycin blocks the phosphorylation of 4E-Bp1 and inhibits cap-dependent initiation of translation. EMBO J. 1996;15:658–664. [PMC free article] [PubMed] [Google Scholar]
- Bromberg JF, Horvath CM, Besser D, Lathem WW, Darnell JE. Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, Tsichlis PN, Cantley LC, Roberts TM, Vogt PK. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276:1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- Dai ZH, Quackenbush RC, Courtney KD, Grove M, Cortez D, Reuther GW, Pendergast AM. Oncogenic Abl and Src tyrosine kinases elicit the ubiquitin-dependent degradation of target proteins through a Ras-independent pathway. Genes & Dev. 1998;12:1415–1424. doi: 10.1101/gad.12.10.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue JE, Martin GS. Linker insertion-deletion mutagenesis of the v-src gene: isolation of host- and temperature-dependent mutants. J Virol. 1989;63:542–554. doi: 10.1128/jvi.63.2.542-554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeClue JE, Zhang K, Redford P, Vass WC, Lowy DR. Suppression of Src transformation by overexpression of full-length GTPase-activating protein (GAP) or of the GAP-C terminus. Mol Cell Biol. 1991;11:2819–2825. doi: 10.1128/mcb.11.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombrosky-Ferlan PM, Corey SJ. Yeast two-hybrid in vivo association of the Src kinase Lyn with the protooncogene product Cbl but not with the p85 subunit of PI 3-kinase. Oncogene. 1997;14:2019–2024. doi: 10.1038/sj.onc.1201031. [DOI] [PubMed] [Google Scholar]
- Dudley DT, Pang L, Decker SJ, Bridges AJ, Saltiel AR. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federspiel MJ, Hughes SH. Effects of the gag region on genome stability: avian retroviral vectors that contain sequences from the Bryan strain of Rous sarcoma virus. Virology. 1994;203:211–220. doi: 10.1006/viro.1994.1478. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Kennedy SG, Oleary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes & Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greulich H, Reichman C, Hanafusa H. Delay in serum stimulation of Erk activity caused by oncogenic transformation. Oncogene. 1996;12:1689–1695. [PubMed] [Google Scholar]
- Jain SK, Langdon WY, Varticovski L. Tyrosine phosphorylation of p120(cbl) in BCR/abl transformed hematopoietic cells mediates enhanced association with phosphatidylinositol 3-kinase. Oncogene. 1997;14:2217–2228. doi: 10.1038/sj.onc.1201049. [DOI] [PubMed] [Google Scholar]
- Kerkhoff E, Houben R, Loffler S, Troppmair J, Rapp UR. Regulation of c-myc expression by Ras/Raf signaling. Oncogene. 1998;16:211–216. doi: 10.1038/sj.onc.1201520. [DOI] [PubMed] [Google Scholar]
- King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klippel A, Kavanaugh WM, Pot D, Williams LT. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik G, Klippel A, Weber MJ. Antiapoptotic signaling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase, and Akt. Mol Cell Biol. 1997;17:1595–1606. doi: 10.1128/mcb.17.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie JN, Lallemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44(MAPK) and negatively by the p38/Hog(MAPK) pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- Lazariskaratzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5′ cap. Nature. 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- Li RH, Zhou RP, Duesberg P. Host range restrictions of oncogenes—Myc genes transform avian but not mammalian cells and Mht/Raf genes transform mammalian but not avian cells. Proc Natl Acad Sci USA. 1996;93:7522–7527. doi: 10.1073/pnas.93.15.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl EC, England LJ, DeClue JE, Martin GS. Host range mutants of v-src—alterations in kinase activity and substrate interactions. J Virol. 1992;66:4315–4324. doi: 10.1128/jvi.66.7.4315-4324.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M, Cantley LC. Lipid second messengers. Cell. 1994;77:329–334. doi: 10.1016/0092-8674(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Liu XQ, Marengere LEM, Koch CA, Pawson T. The v-Src SH3-domain binds phosphatidylinositol 3′-kinase. Mol Cell Biol. 1993;13:5225–5232. doi: 10.1128/mcb.13.9.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais R, Light Y, Mason C, Paterson H, Olson MF, Marshall CJ. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- Maroney AC, Qureshi SA, Foster DA, Brugge JS. Cloning and characterization of a thermolabile v-Src gene for use in reversible transformation of mammalian cells. Oncogene. 1992;7:1207–1214. [PubMed] [Google Scholar]
- Nori M, Vogel US, Gibbs JB, Weber MJ. Inhibition of v-Src-induced transformation by a GTPase-activating protein. Mol Cell Biol. 1991;11:2812–2818. doi: 10.1128/mcb.11.5.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham SM, Cox AD, Reynolds ER, Sizemore NS, Coffey RJ, Der CJ. Ras, but not Src, transformation of RIE-1 epithelial cells is dependent on activation of the mitogen-activated protein kinase cascade. Oncogene. 1998;16:2565–2573. doi: 10.1038/sj.onc.1201784. [DOI] [PubMed] [Google Scholar]
- Petropoulos CJ, Hughes SH. Replication-competent retrovirus vectors for the transfer and expression of gene cassettes in avian cells. J Virol. 1991;65:3728–3737. doi: 10.1128/jvi.65.7.3728-3737.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleiman CM, Hertz WM, Cambier JC. Activation of phosphatidylinositol-3′ kinase by Src-family kinase SH3 binding to the p85 subunit. Science. 1994;263:1609–1612. doi: 10.1126/science.8128248. [DOI] [PubMed] [Google Scholar]
- Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Rousseau D, Gingras AC, Pause A, Sonenberg N. The eIF4E-binding proteins 1 and 2 are negative regulators of cell growth. Oncogene. 1996a;13:2415–2420. [PubMed] [Google Scholar]
- Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci USA. 1996b;93:1065–1070. doi: 10.1073/pnas.93.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawver LK, Olson SA, White MK, Weber MJ. Degradation and biosynthesis of the glucose transporter protein in chicken embryo fibroblasts transformed by the src oncogene. Mol Cell Biol. 1987;7:2112–2118. doi: 10.1128/mcb.7.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, DeGudicibus SJ, Stacey DW. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986;320:540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey DW, Roudebush M, Day R, Mosser SD, Gibbs JB, Feig LA. Dominant inhibitory Ras mutants demonstrate the requirement for Ras activity in the action of tyrosine kinase oncogenes. Oncogene. 1991;6:2297–2304. [PubMed] [Google Scholar]
- Stofega MR, Yu CL, Wu J, Jove R. Activation of extracellular signal-regulated kinase (ERK) by mitogenic stimuli is repressed in v-Src-transformed cells. Cell Growth Differ. 1997;8:113–119. [PubMed] [Google Scholar]
- Turkson J, Bowman T, Garcia R, Caldenhoven E, DeGroot RP, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18:2545–2552. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonmanteuffel SR, Gingras AC, Ming XF, Sonenberg N, Thomas G. 4E-BP1 phosphorylation is mediated by the FRAP-p70S6k pathway and is independent of mitogen-activated protein kinase. Proc Natl Acad Sci USA. 1996;93:4076–4080. doi: 10.1073/pnas.93.9.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wages DS, Keefer J, Rall TB, Weber MJ. Mutations in the SH3 domain of the Src-oncogene which decrease association of phosphatidylinositol 3′-kinase activity with pp60v-Src and alter cellular morphology. J Virol. 1992;66:1866–1874. doi: 10.1128/jvi.66.4.1866-1874.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff P, Kang HY, Mylott D, Robbins PJ, White MK. Characterization of the avian Glut1 glucose transporter—differential regulation of Glut1 and Glut3 in chicken embryo fibroblasts. Mol Cell Biol. 1995;6:1575–1589. doi: 10.1091/mbc.6.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Stoneley M, Willis AE. Translational induction of the c-myc oncogene via activation of the FRAP/TOR signaling pathway. Oncogene. 1998;17:769–780. doi: 10.1038/sj.onc.1201990. [DOI] [PubMed] [Google Scholar]
- Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332:644–664. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]