Abstract
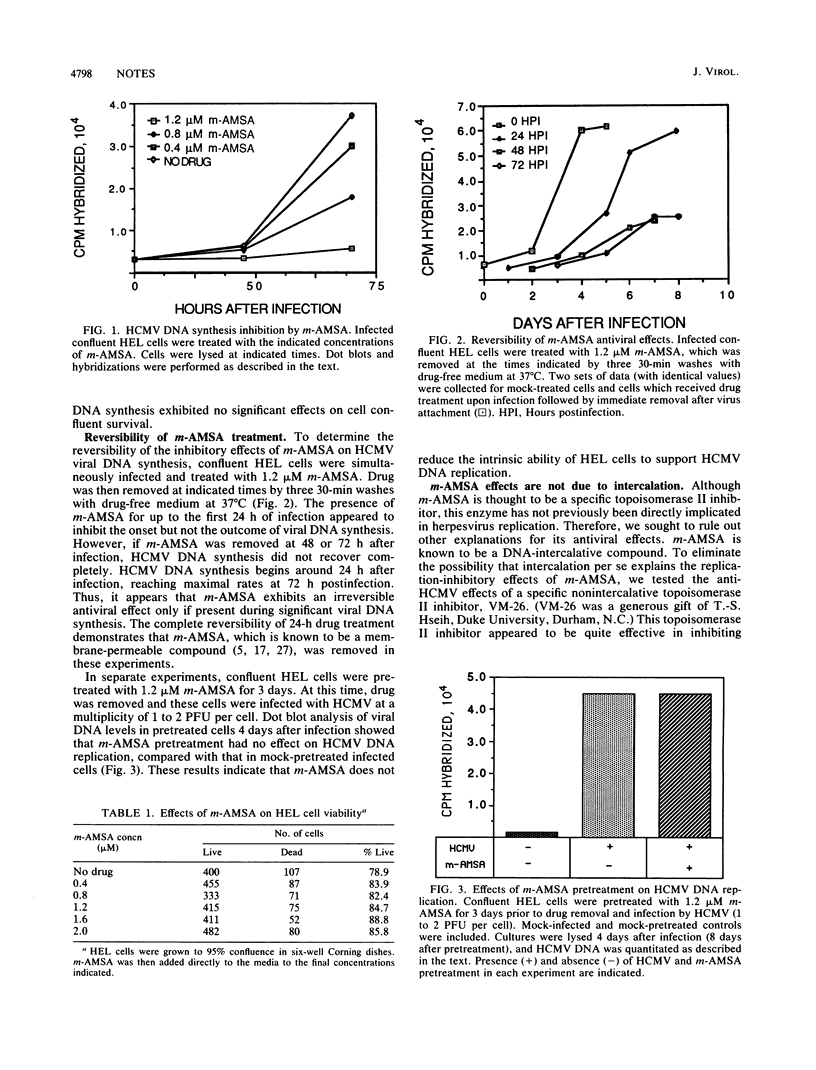
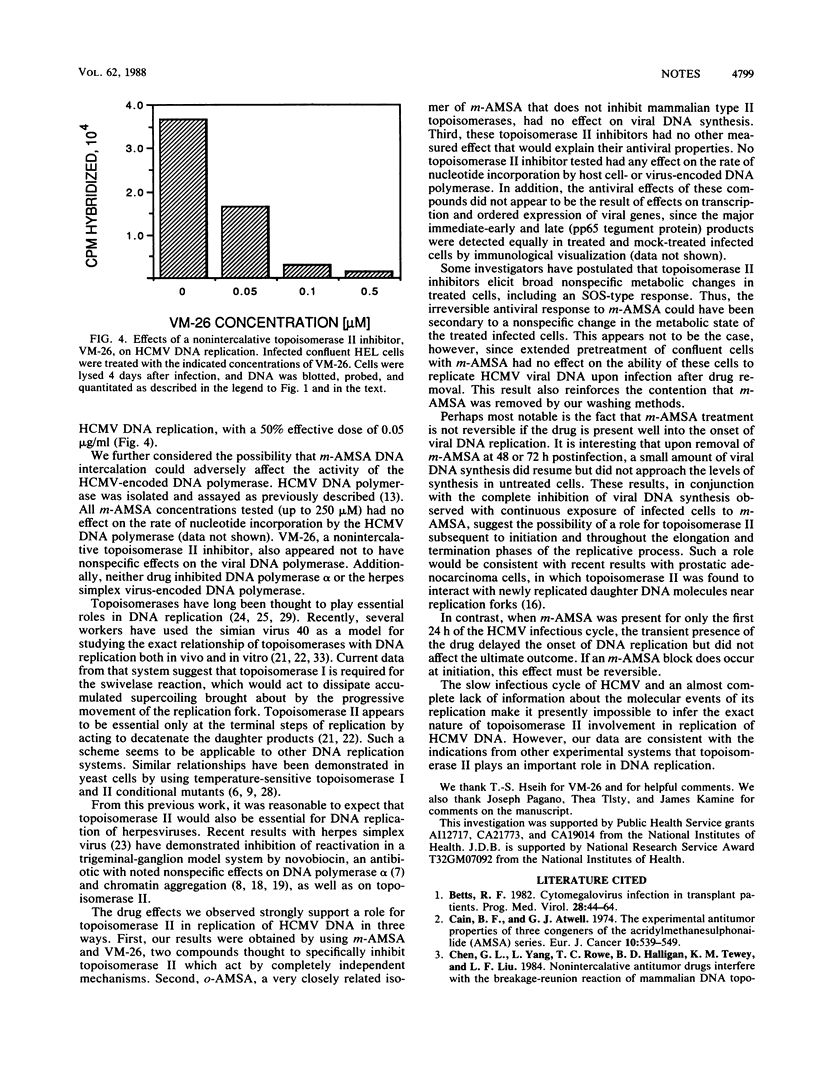
In this study, we show that human cytomegalovirus DNA synthesis is inhibited in infected confluent human embryonic lung cells treated with the DNA-intercalative topoisomerase II inhibitor 4-9'-(acridinylamino)methanesulfon-m-anisidide (m-AMSA). Similar inhibitory effects were observed with VM-26, a nonintercalative topoisomerase II inhibitor. This antiviral effect is not attributable to cytotoxic effects per se. Furthermore, m-AMSA appears to have a notably irreversible inhibitory effect on human cytomegalovirus DNA replication. No inhibition of viral DNA synthesis was observed with o-AMSA, a DNA-intercalative isomer of m-AMSA that does not inhibit topoisomerase II.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betts R. F. Cytomegalovirus infection in transplant patients. Prog Med Virol. 1982;28:44–64. [PubMed] [Google Scholar]
- Cain B. F., Atwell G. J. The experimental antitumour properties of three congeners of the acridylmethanesulphonanilide (AMSA) series. Eur J Cancer. 1974 Aug;10(8):539–549. doi: 10.1016/0014-2964(74)90079-6. [DOI] [PubMed] [Google Scholar]
- Chen G. L., Yang L., Rowe T. C., Halligan B. D., Tewey K. M., Liu L. F. Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem. 1984 Nov 10;259(21):13560–13566. [PubMed] [Google Scholar]
- Davis M. G., Mar E. C., Wu Y. M., Huang E. S. Mapping and expression of a human cytomegalovirus major viral protein. J Virol. 1984 Oct;52(1):129–135. doi: 10.1128/jvi.52.1.129-135.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaven L. L., Oka M. S., Tobey R. A. Cell-cycle-specific chromosome damage following treatment of cultured Chinese hamster cells with 4'-[(9-acridinyl)-amino]methanesulphon-m-anisidide-HCl. J Natl Cancer Inst. 1978 May;60(5):1155–1161. [PubMed] [Google Scholar]
- DiNardo S., Voelkel K., Sternglanz R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc Natl Acad Sci U S A. 1984 May;81(9):2616–2620. doi: 10.1073/pnas.81.9.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J. Novobiocin inhibition of simian virus 40 DNA replication. Nature. 1980 Jul 31;286(5772):529–531. doi: 10.1038/286529a0. [DOI] [PubMed] [Google Scholar]
- Felts S. J., Weil P. A., Chalkley R. Novobiocin inhibits interactions required for yeast TFIIIB sequestration during stable transcription complex formation in vitro. Nucleic Acids Res. 1987 Feb 25;15(4):1493–1506. doi: 10.1093/nar/15.4.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm C., Goto T., Wang J. C., Botstein D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell. 1985 Jun;41(2):553–563. doi: 10.1016/s0092-8674(85)80028-3. [DOI] [PubMed] [Google Scholar]
- Huang E. S. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J Virol. 1975 Dec;16(6):1560–1565. doi: 10.1128/jvi.16.6.1560-1565.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macher A. M., Reichert C. M., Straus S. E., Longo D. L., Parrillo J., Lane H. C., Fauci A. S., Rook A. H., Manischewitz J. F., Quinnan G. V., Jr Death in the AIDS patient: role of cytomegalovirus. N Engl J Med. 1983 Dec 8;309(23):1454–1454. doi: 10.1056/NEJM198312083092312. [DOI] [PubMed] [Google Scholar]
- Mar E. C., Cheng Y. C., Huang E. S. Effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine on human cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 1983 Oct;24(4):518–521. doi: 10.1128/aac.24.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar E. C., Chiou J. F., Cheng Y. C., Huang E. S. Inhibition of cellular DNA polymerase alpha and human cytomegalovirus-induced DNA polymerase by the triphosphates of 9-(2-hydroxyethoxymethyl)guanine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine. J Virol. 1985 Mar;53(3):776–780. doi: 10.1128/jvi.53.3.776-780.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. D., Flournoy N., Thomas E. D. Nonbacterial pneumonia after allogeneic marrow transplantation: a review of ten years' experience. Rev Infect Dis. 1982 Nov-Dec;4(6):1119–1132. doi: 10.1093/clinids/4.6.1119. [DOI] [PubMed] [Google Scholar]
- Nelson W. G., Liu L. F., Coffey D. S. Newly replicated DNA is associated with DNA topoisomerase II in cultured rat prostatic adenocarcinoma cells. Nature. 1986 Jul 10;322(6075):187–189. doi: 10.1038/322187a0. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Kerrigan D., Schwartz R., Zwelling L. A. The formation and resealing of intercalator-induced DNA strand breaks in isolated L1210 cell nuclei. Biochem Biophys Res Commun. 1982 Jul 30;107(2):576–583. doi: 10.1016/0006-291x(82)91530-3. [DOI] [PubMed] [Google Scholar]
- Richter A., Strausfeld U., Knippers R. Effects of VM26 (teniposide), a specific inhibitor of type II DNA topoisomerase, on SV40 DNA replication in vivo. Nucleic Acids Res. 1987 Apr 24;15(8):3455–3468. doi: 10.1093/nar/15.8.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee D. F., Martin J. C., Verheyden J. P., Matthews T. R. Anti-herpesvirus activity of the acyclic nucleoside 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob Agents Chemother. 1983 May;23(5):676–682. doi: 10.1128/aac.23.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapka R. M., Powelson M. A., Strayer J. M. Swiveling and decatenation of replicating simian virus 40 genomes in vivo. Mol Cell Biol. 1988 Feb;8(2):515–521. doi: 10.1128/mcb.8.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapka R. M. Topoisomerase inhibitors can selectively interfere with different stages of simian virus 40 DNA replication. Mol Cell Biol. 1986 Dec;6(12):4221–4227. doi: 10.1128/mcb.6.12.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., O'Boyle D. R., 2nd, Fraser N. W. Novobiocin and coumermycin A1 inhibit viral replication and the reactivation of herpes simplex virus type 1 from the trigeminal ganglia of latently infected mice. J Virol. 1987 Oct;61(10):3288–3291. doi: 10.1128/jvi.61.10.3288-3291.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Arrest of segregation leads to accumulation of highly intertwined catenated dimers: dissection of the final stages of SV40 DNA replication. Cell. 1981 Sep;25(3):659–669. doi: 10.1016/0092-8674(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Deaven L. L., Oka M. S. Kinetic response of cultured Chinese hamster cells to treatment with 4'-[(9-acridinyl)-amino]methanesulphon-m-anisidide-HCl. J Natl Cancer Inst. 1978 May;60(5):1147–1153. doi: 10.1093/jnci/60.5.1147. [DOI] [PubMed] [Google Scholar]
- Uemura T., Yanagida M. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J. 1984 Aug;3(8):1737–1744. doi: 10.1002/j.1460-2075.1984.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren B., Rudén U., Gadler H., Oberg B., Eriksson B. Activity of the cytomegalovirus genome in the presence of PPi analogs. J Virol. 1985 Dec;56(3):996–1001. doi: 10.1128/jvi.56.3.996-1001.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- West C., Stratford I. J., Barrass N., Smith E. A comparison of adriamycin and mAMSA in vitro: cell lethality and SCE studies. Br J Cancer. 1981 Dec;44(6):798–809. doi: 10.1038/bjc.1981.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Wold M. S., Li J. J., Kelly T. J., Liu L. F. Roles of DNA topoisomerases in simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1987 Feb;84(4):950–954. doi: 10.1073/pnas.84.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwelling L. A., Michaels S., Erickson L. C., Ungerleider R. S., Nichols M., Kohn K. W. Protein-associated deoxyribonucleic acid strand breaks in L1210 cells treated with the deoxyribonucleic acid intercalating agents 4'-(9-acridinylamino) methanesulfon-m-anisidide and adriamycin. Biochemistry. 1981 Nov 10;20(23):6553–6563. doi: 10.1021/bi00526a006. [DOI] [PubMed] [Google Scholar]



