Abstract
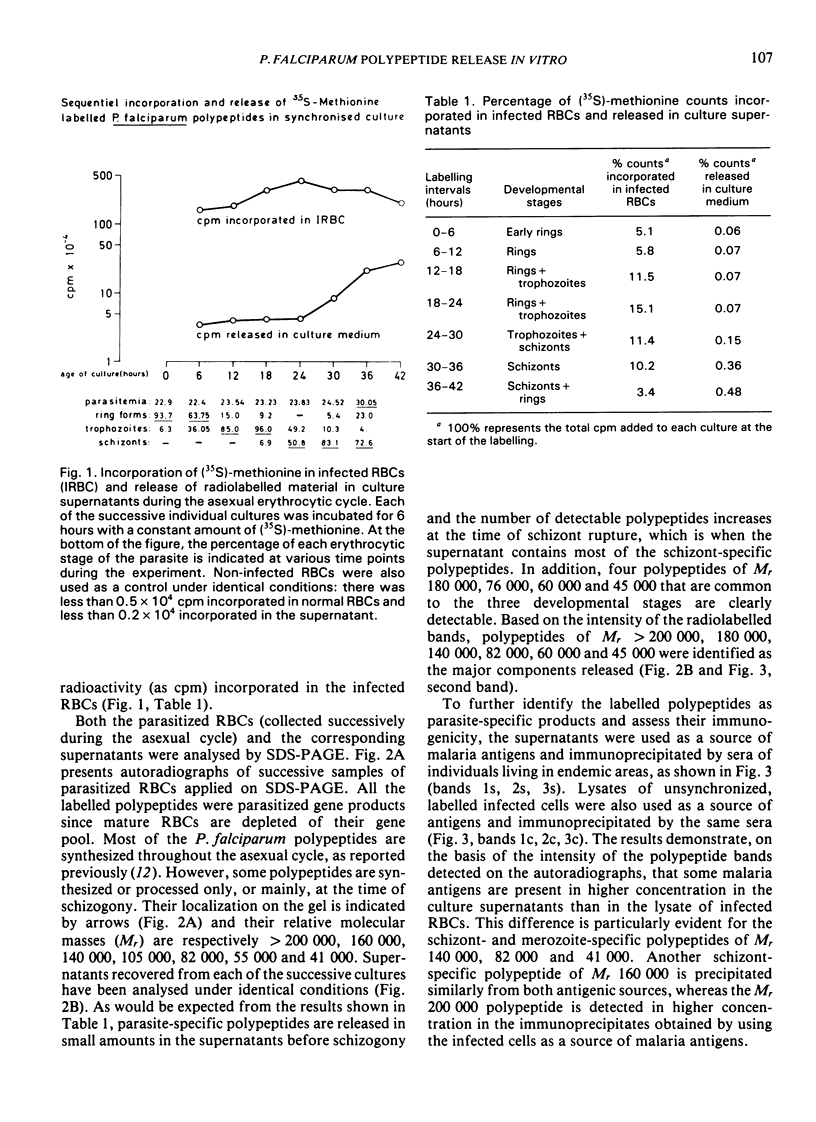
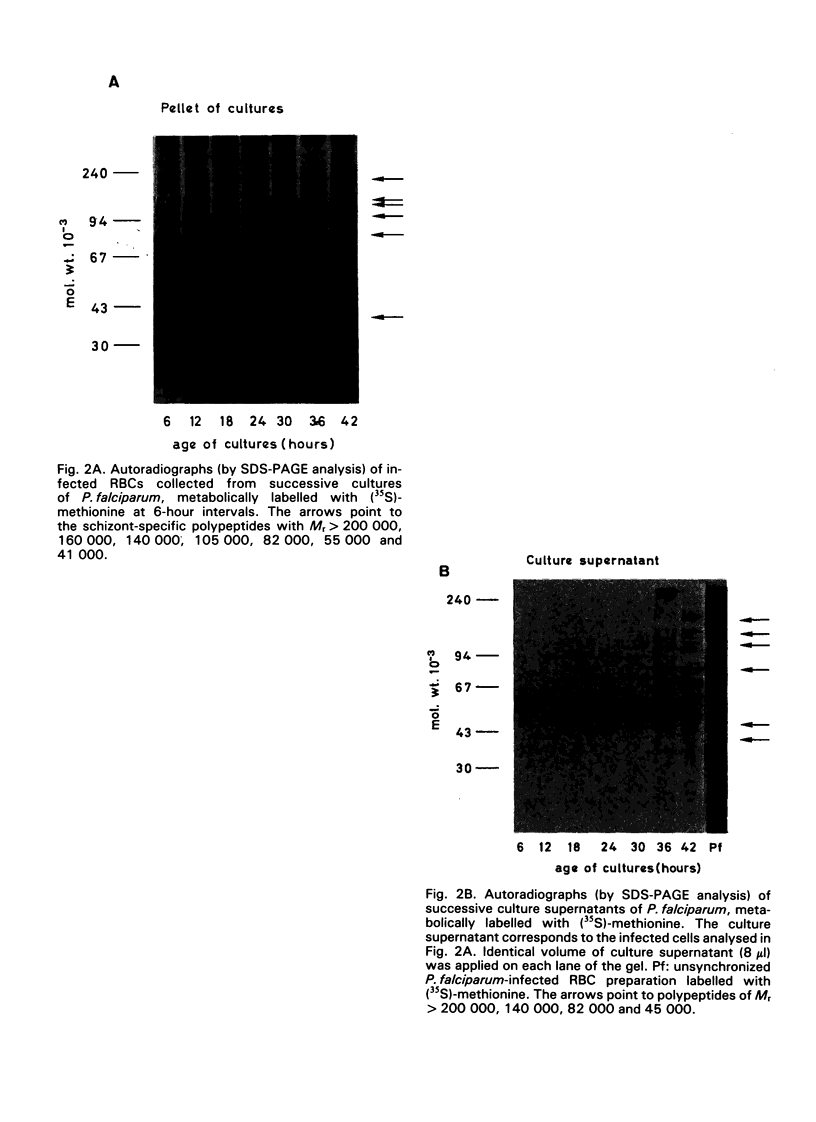
Synchronous cultures of Plasmodium falciparum were successively labelled with (35S)-methionine and both the supernatants and the pellets of infected red blood cells were collected. The release of TCA-precipitable material in the culture supernatants was low during the development of ring forms and trophozoites, increased during schizogony, and was maximum at the time of schizont rupture and merozoite reinvasion. Analysis of the supernatants by SDS — PAGE and autoradiography showed that both polypeptides common to the various developmental stages of the parasite and schizont/merozoite-specific polypeptides were released. Polypeptides of relative molecular mass 140 000, 82 000 and, to a lower degree, 41 000 were present in high amounts in the culture supernatants. These polypeptides have been shown to be the target of monoclonal antibodies that are able to inhibit the growth of P. falciparum cultures, and may be involved in protective immunity. The released polypeptides may also be used as target antigens in immunodiagnostic tests aiming at the detection of malaria infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikawa M., Miller L. H., Johnson J., Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol. 1978 Apr;77(1):72–82. doi: 10.1083/jcb.77.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature. 1981 Nov 26;294(5839):361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- Jerusalem C. Active immunization against malaria (Plasmodium berghei). I. Definition of antimalarial immunity. Z Tropenmed Parasitol. 1968 Jun;19(2):171–181. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kilejian A. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
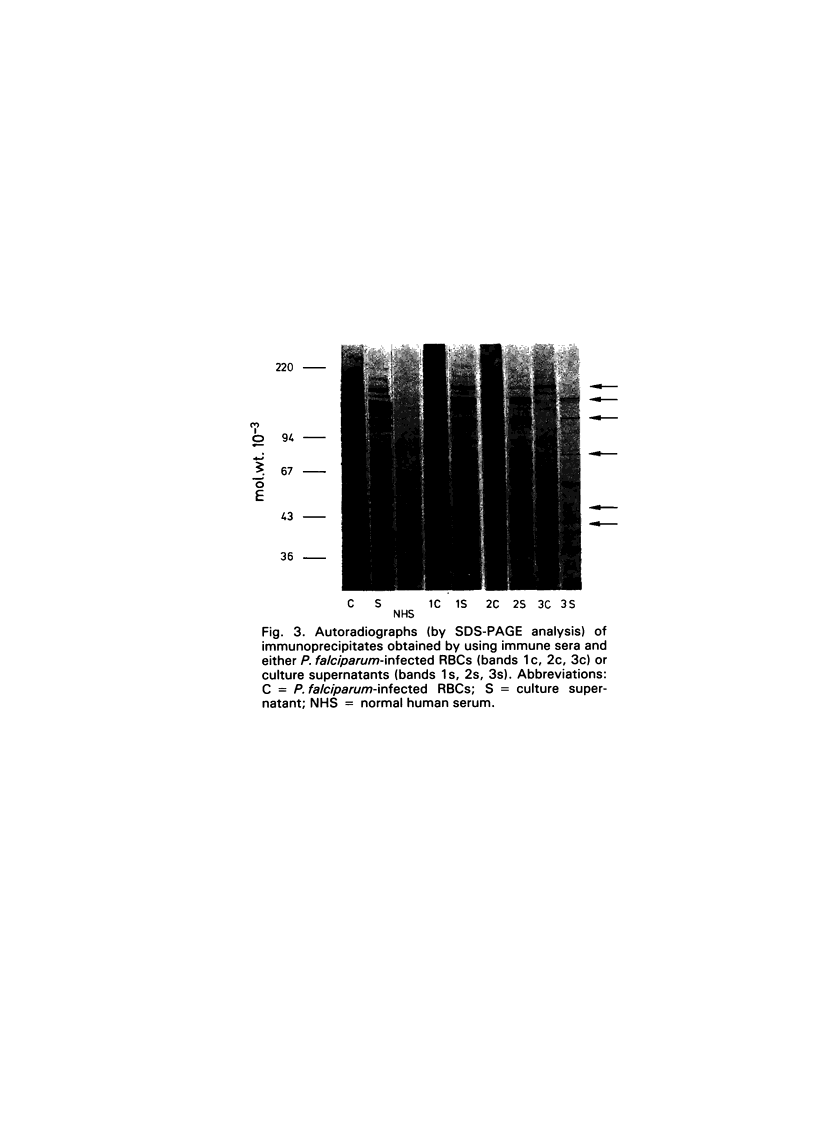
- Kilejian A. Stage-specific proteins and glycoproteins of plasmodium falciparum: identification of antigens unique to schizonts and merozoites. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3695–3699. doi: 10.1073/pnas.77.6.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- McColm A. A., Trigg P. I. Release of radio-isotope labelled antigens from Plasmodium knowlesi during merozoite re-invasion in vitro. Parasitology. 1980 Aug;81(1):199–-209. doi: 10.1017/s0031182000055153. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Dayal R. Immunity to asexual erythrocytic stages of Plasmodium falciparum: role of defined antigens in the humoral response. Immunol Rev. 1982;61:245–269. doi: 10.1111/j.1600-065x.1982.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Ramirez E., Lambert P. H., Miescher P. A. Inhibition of P. falciparum growth in human erythrocytes by monoclonal antibodies. Nature. 1981 Jan 22;289(5795):301–303. doi: 10.1038/289301a0. [DOI] [PubMed] [Google Scholar]
- Potocnjak P., Yoshida N., Nussenzweig R. S., Nussenzweig V. Monovalent fragments (Fab) of monoclonal antibodies to a sporozoite surface antigen (Pb44) protect mice against malarial infection. J Exp Med. 1980 Jun 1;151(6):1504–1513. doi: 10.1084/jem.151.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R. T., Langreth S. G., Trager W. Isolation of stages of the human parasite Plasmodium falciparum from culture and from animal blood. Bull World Health Organ. 1979;57 (Suppl 1):53–61. [PMC free article] [PubMed] [Google Scholar]
- Smith R. D., James M. A., Ristic M., Aikawa M., Vega y Murguia C. A. Bovine babesiosis: protection of cattle with culture-derived soluble Babesia bovis antigen. Science. 1981 Apr 17;212(4492):335–338. doi: 10.1126/science.7209532. [DOI] [PubMed] [Google Scholar]
- Todorovic R., Ferris D., Ristic M. Immunogenic properties of serum antigens from chickens acutely infected with Plasmodium gallinaceum. Ann Trop Med Parasitol. 1967 Jun;61(2):117–124. doi: 10.1080/00034983.1967.11686467. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Wilson R. J., Bartholomew R. K. The release of antigens by Plasmodium falciparum. Parasitology. 1975 Oct;71(2):183–192. doi: 10.1017/s0031182000046631. [DOI] [PubMed] [Google Scholar]
- Wilson R. J., McGregor I. A., Wilson M. E. The stability and fractionation of malarial antigens from the blood of Africans infected with Plasmodium falciparum. Int J Parasitol. 1973 Jul;3(4):511–520. doi: 10.1016/0020-7519(73)90048-9. [DOI] [PubMed] [Google Scholar]
- Wilson R. J. The production of antigens by Plasmodium falciparum in vitro. Int J Parasitol. 1974 Oct;4(5):537–547. doi: 10.1016/0020-7519(74)90072-1. [DOI] [PubMed] [Google Scholar]