Abstract
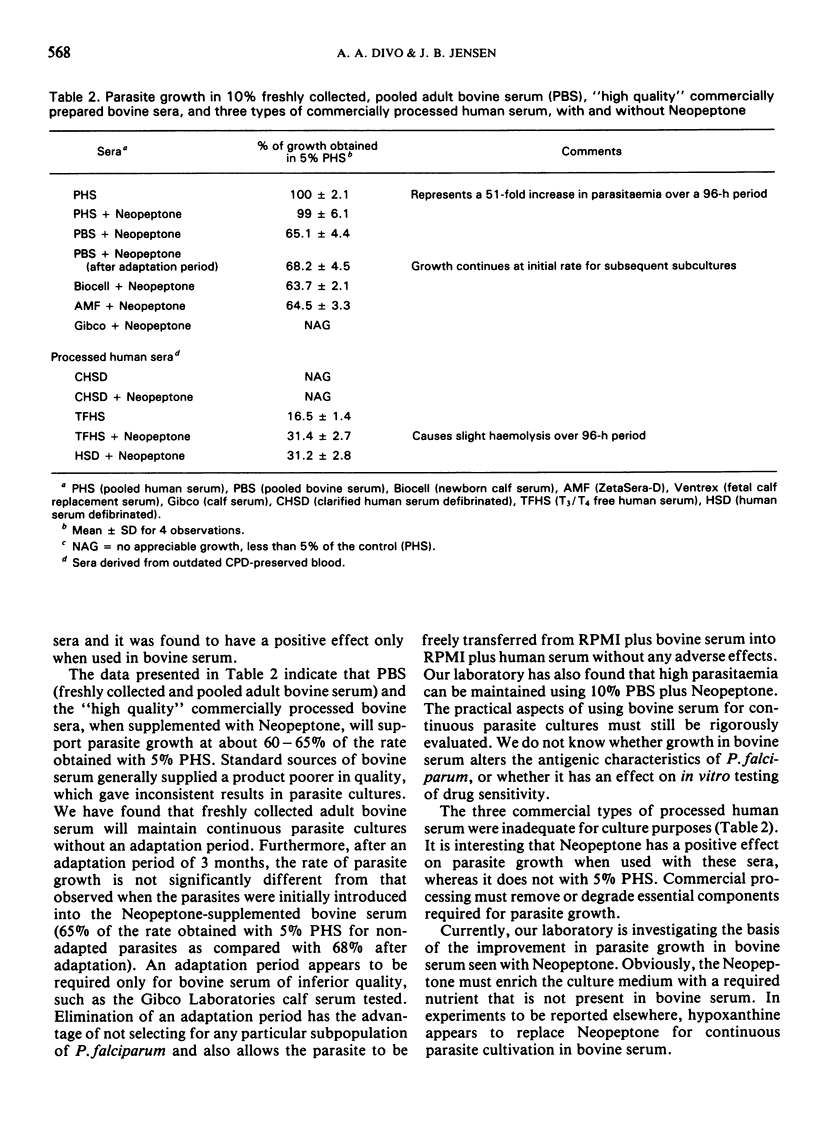
Pooling a number of freshly collected lots of human serum eliminated the variability observed between individual serum samples, and reduced the amount of human serum required for optimum parasite growth in continuous culture. In the present experiments the addition of 50 ml of pooled human serum per litre of RPMI (5% serum) resulted in optimum growth. Batches of RPMI 1640 supplemented with freshly collected and pooled lots of bovine, porcine, goat, equine, or ovine sera, as well as commercially available fetal-and young-calf sera, were tested and compared with 5% pooled human serum. Various combinations of animal sera with and without Neopeptone were also examined as supplements to the basic culture medium. As an alternative to human serum, only bovine serum supplemented with Neopeptone could support continuous parasite growth, but at significantly reduced levels. Continuous parasite growth was obtained by transferring parasites directly from 5% human serum into medium plus freshly collected, Neopeptone-supplemented, pooled bovine serum, without any need for an adaptation period.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone C. W., Mantel N., Caruso T. D., Jr, Kazam E., Stevenson R. E. Quality control studies on fetal bovine serum used in tissue culture. In Vitro. 1971 Nov-Dec;7(3):174–189. doi: 10.1007/BF02617963. [DOI] [PubMed] [Google Scholar]
- Ifediba T., Vanderberg J. P. Peptones and calf serum as a replacement for human serum in the cultivation of Plasmodium falciparum. J Parasitol. 1980 Apr;66(2):236–239. [PubMed] [Google Scholar]
- Jensen J. B., Trager W. Plasmodium falciparum in culture: establishment of additional strains. Am J Trop Med Hyg. 1978 Jul;27(4):743–746. doi: 10.4269/ajtmh.1978.27.743. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Trager W. Plasmodium falciparum in culture: use of outdated erthrocytes and description of the candle jar method. J Parasitol. 1977 Oct;63(5):883–886. [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]


