Abstract
A total of 28 000 persons with fibrotic pulmonary lesions compatible with tuberculosis were followed for five years after receiving 12, 24, or 52 weeks of preventive treatment with isoniazid or placebo.
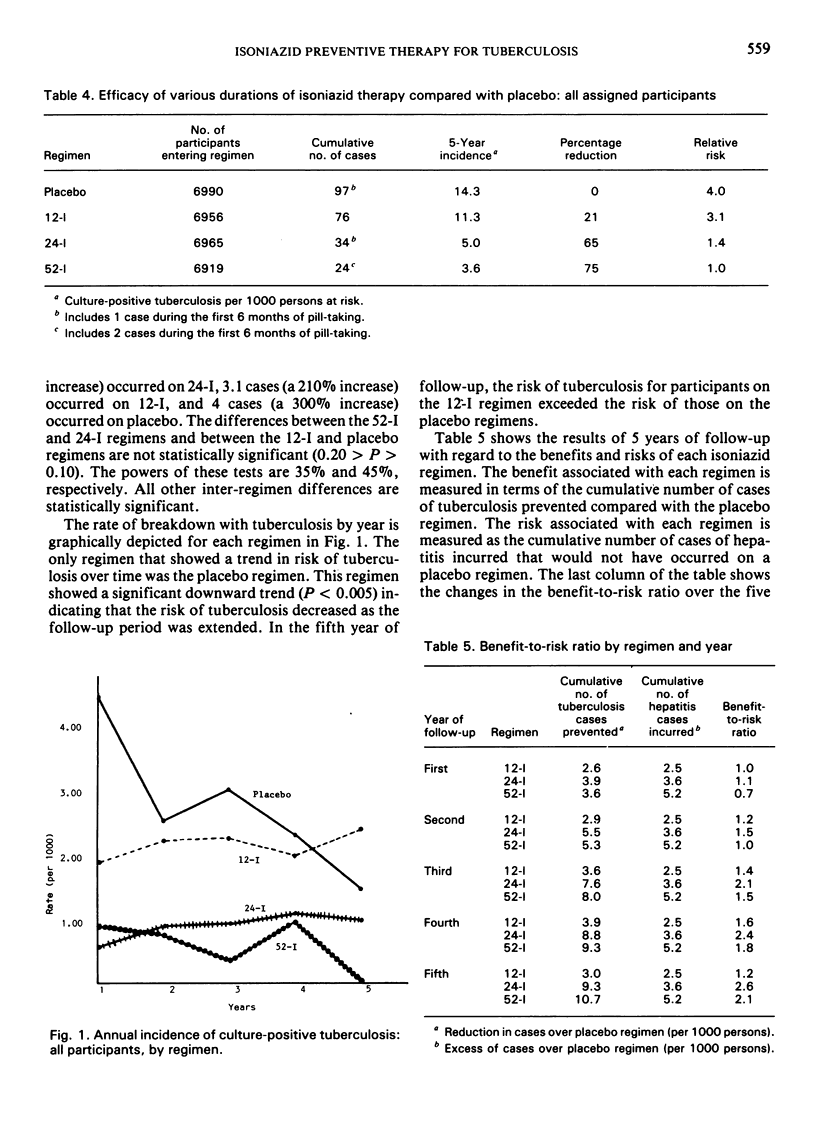
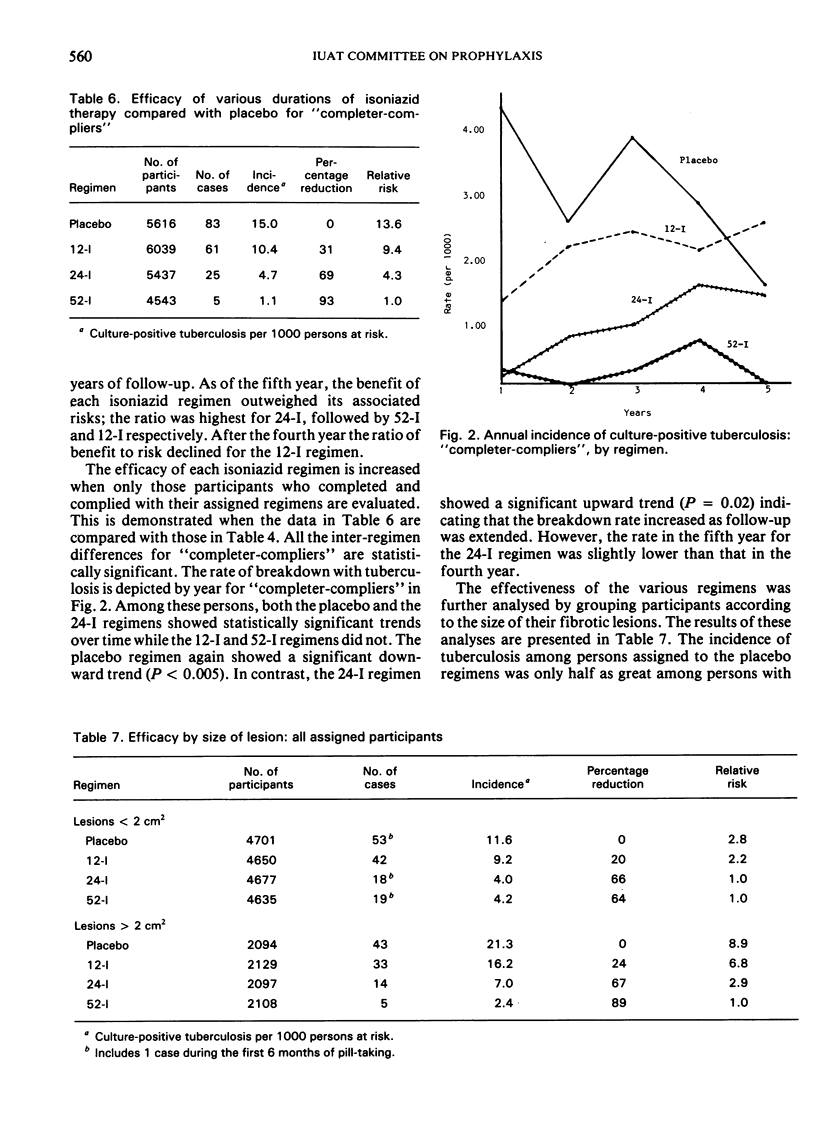
Compared with placebo, 12 weeks of isoniazid eliminated less than one-third, and 24 weeks eliminated two-thirds of the tuberculosis risk. Where preventive treatment is not currently practised, adopting a 24-week regimen could decrease the incidence of tuberculosis in such populations by 65%.
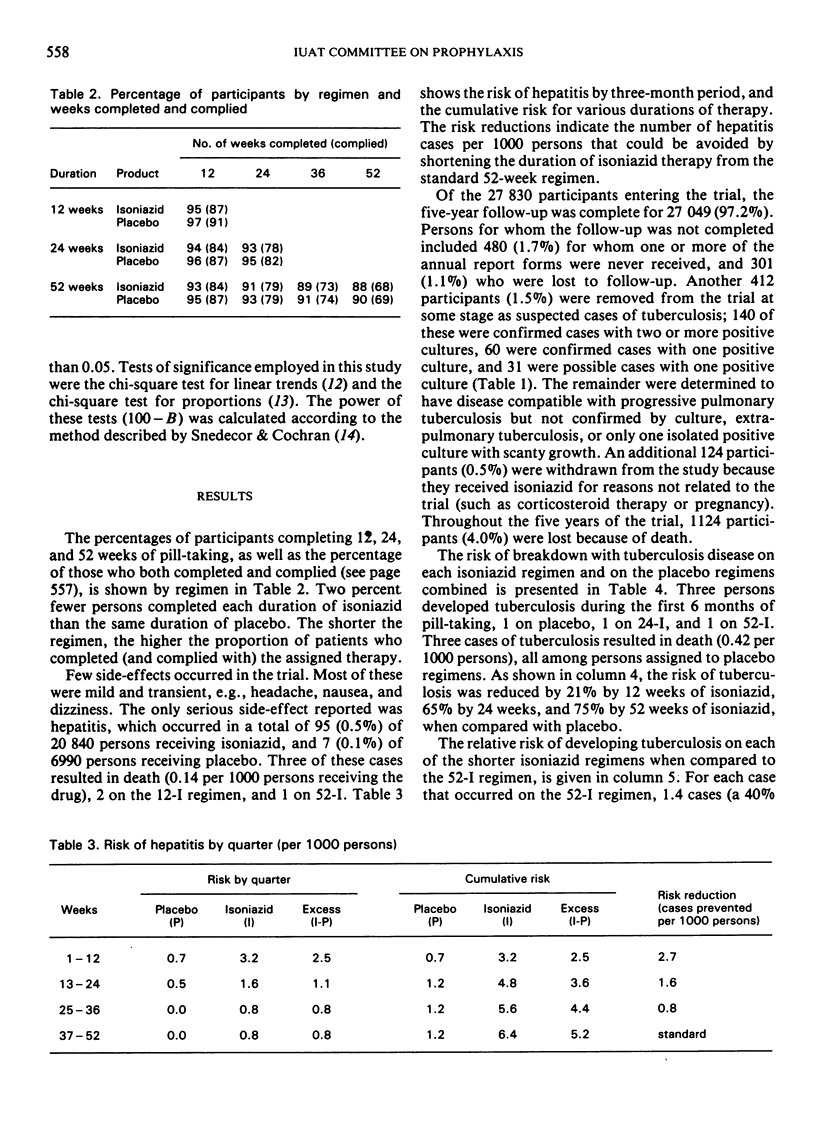
Hepatitis, the only serious side-effect encountered, occurred infrequently but was more common among isoniazid recipients (0.5%) than among placebo recipients (0.1%).
Fifty-two weeks of isoniazid prevented the most tuberculosis, but 24 weeks prevented more tuberculosis cases per case of hepatitis caused. Where preventive treatment is currently practised for 52 weeks, adopting a 24-week regimen would decrease hepatitis by one-third and increase tuberculosis by 40%.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush O. B., Jr, Sugimoto M., Fujii Y., Brown F. A., Jr Isoniazid prophylaxis in contacts of persons with known tuberculosis. Second report. Am Rev Respir Dis. 1965 Nov;92(5):732–740. doi: 10.1164/arrd.1965.92.5.732. [DOI] [PubMed] [Google Scholar]
- COMSTOCK G. W. Isoniazid prophylaxis in an undeveloped area. Am Rev Respir Dis. 1962 Dec;86:810–822. doi: 10.1164/arrd.1962.86.6.810. [DOI] [PubMed] [Google Scholar]
- Comstock G. W., Baum C., Snider D. E., Jr Isoniazid prophylaxis among Alaskan Eskimos: a final report of the bethel isoniazid studies. Am Rev Respir Dis. 1979 May;119(5):827–830. doi: 10.1164/arrd.1979.119.5.827. [DOI] [PubMed] [Google Scholar]
- Comstock G. W., Ferebee S. H., Hammes L. M. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis. 1967 Jun;95(6):935–943. doi: 10.1164/arrd.1967.95.6.935. [DOI] [PubMed] [Google Scholar]
- Egsmose T., Ang'awa J. O., Poti S. J. The use of isoniazid among household contacts of open cases of pulmonary tuberculosis. Bull World Health Organ. 1965;33(3):419–433. [PMC free article] [PubMed] [Google Scholar]
- KATZ J., KUNOFSKY S., DAMIJONAITIS V., LAFLEUR A., CARON T. EFFECT OF ISONIAZID UPON THE REACTIVATION OF INACTIVE TUBERCULOSIS; FINAL REPORT. Am Rev Respir Dis. 1965 Mar;91:345–350. doi: 10.1164/arrd.1965.91.3.345. [DOI] [PubMed] [Google Scholar]
- KATZ J., KUNOFSKY S., DAMIJONAITIS V., LAFLEUR A., CARON T. Effect of isoniazid upon the reactivation of inactive tuberculosis. Preliminary report. Am Rev Respir Dis. 1962 Jul;86:8–15. doi: 10.1164/arrd.1962.86.1.8. [DOI] [PubMed] [Google Scholar]
- Krebs A. The IUAT trial on isoniazid preventive treatment in persons with fibrotic lung lesions. Bull Int Union Tuberc. 1976;51(1):193–201. [PubMed] [Google Scholar]
- NIELSCH W., GIEFER L. [Photometric determination of isonicotinic acid hydrazide (INH), INH derivatives and their metabolites in biological specimens. Part 2]. Arzneimittelforschung. 1959 Nov;9:700–707. [PubMed] [Google Scholar]
- Riska N. Hepatitis cases in isoniazid treated groups and in a control group. Bull Int Union Tuberc. 1976;51(1):203–208. [PubMed] [Google Scholar]
- Woolpert S. F., Dankova D., Krebs A., Vadasz I. Enquête de l'UICT sur la durée du traitement prophylactique des sujets porteurs de lésions fibreuses - premiers résultats. Introduction générale. Bull Int Union Tuberc. 1974;49(1):302–305. [PubMed] [Google Scholar]



