Abstract
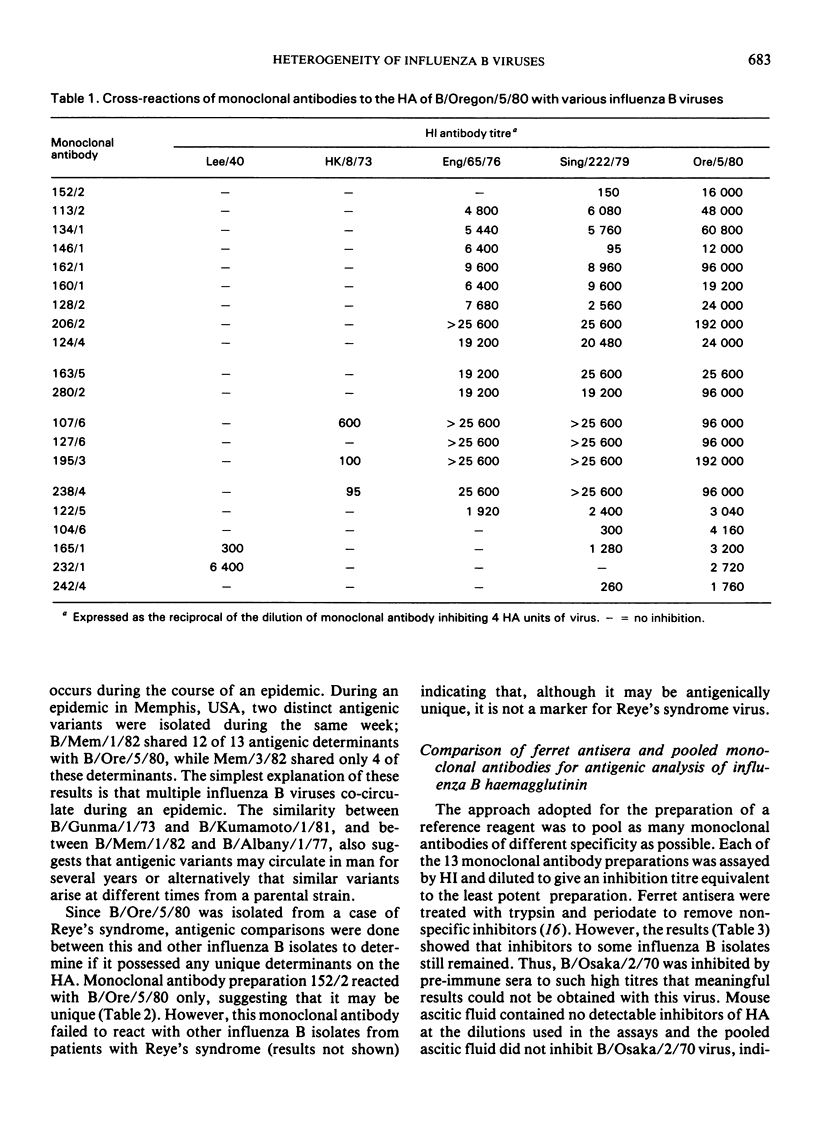
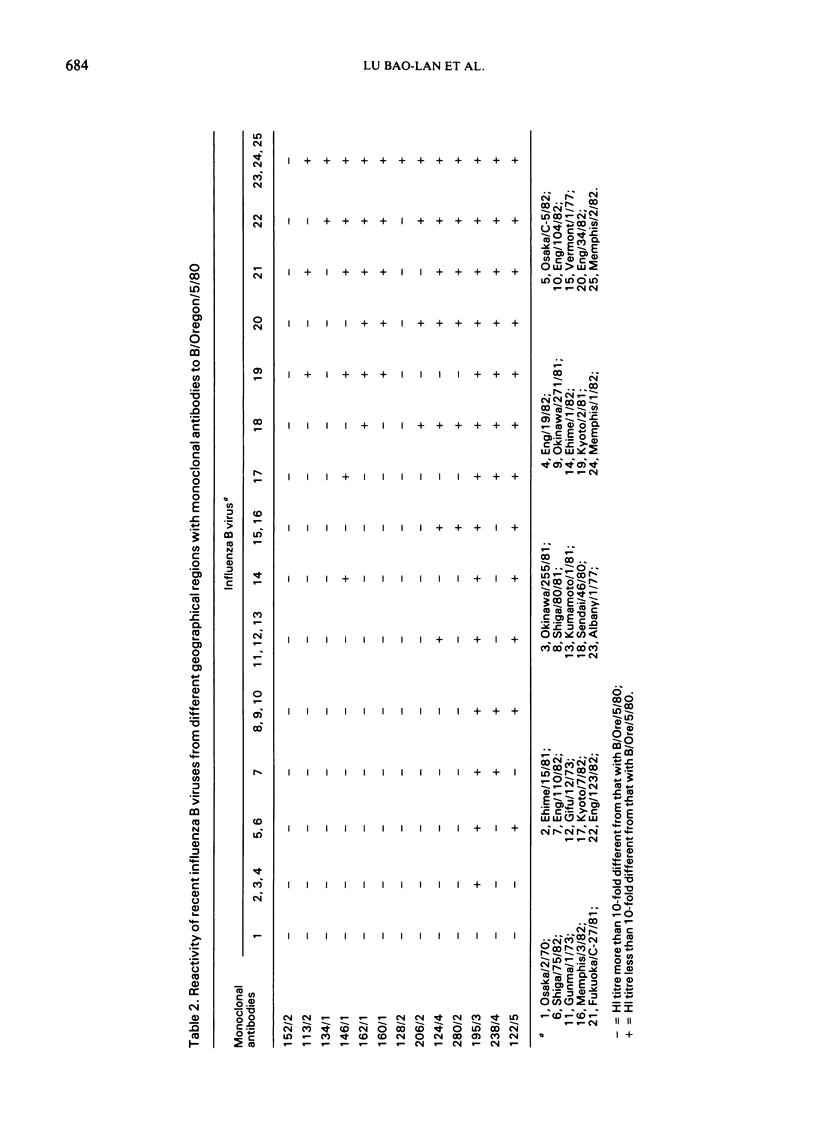
Antigenic analysis of influenza B strains isolated in 1981-82 from England, Japan, and the USA, using a panel of monoclonal antibodies to the haemagglutinin of B/Oregon/5/80, showed considerable heterogeneity among the isolates, the majority of which had distinct reactivity patterns. Antigenically similar viruses were isolated from England, Japan, and the USA, and heterogeneity was detected among isolates from each country. Further studies are needed to determine whether this marked heterogeneity reflects different cocirculating strains or antigenic drift during an epidemic. The monoclonal antibodies failed to detect any difference between influenza B isolates from patients with Reye's syndrome and those circulating in the community.
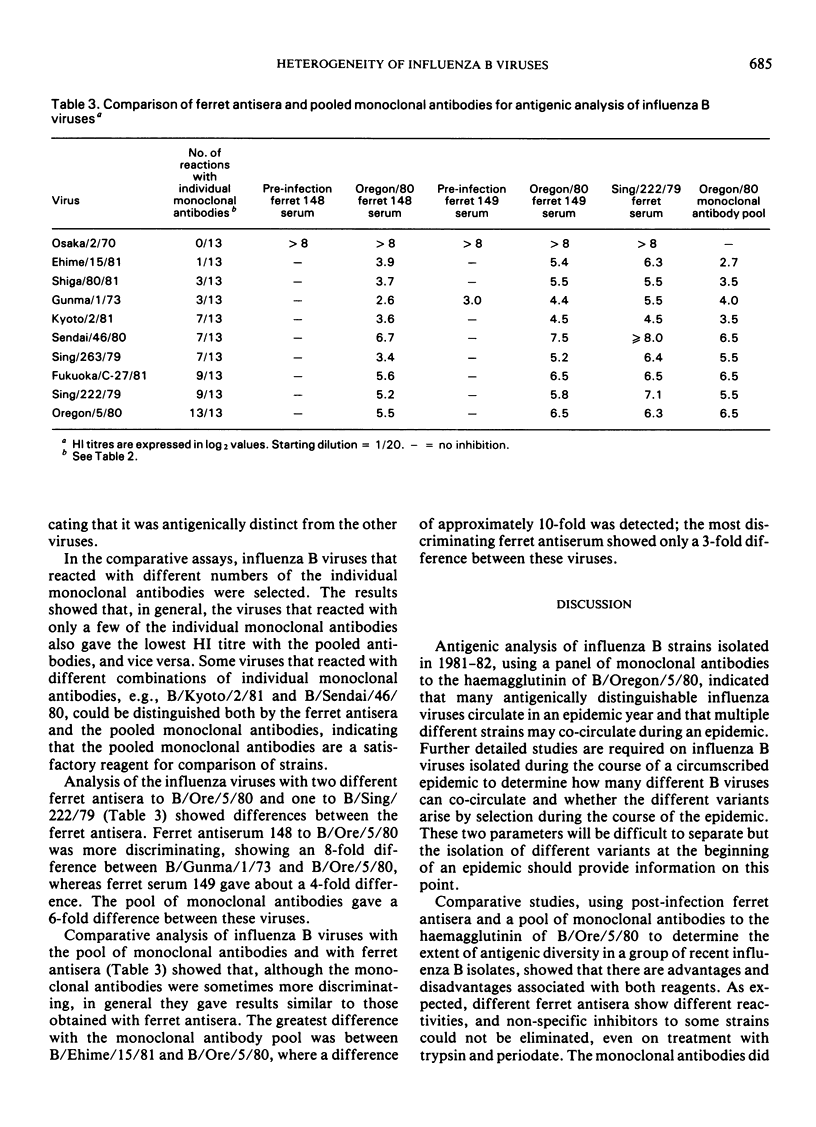
The monoclonal antibodies, each recognizing different determinants on the haemagglutinin of B/Oregon/5/80, were pooled and compared with ferret antisera to determine if the pool could be used as a reference reagent. The monoclonal antibody pool discriminated between isolates, contained no non-specific inhibitors of haemagglutination, and avoided the problems associated with differences between ferret antisera. In general, viruses that were shown to be antigenically different by ferret antisera were also different with the pooled monoclonal antibodies, but further studies are required to determine the optimal mixture of antibodies that will detect epidemiologically significant differences between strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chakraverty P. Antigenic relationship between influenza B viruses. Bull World Health Organ. 1971;45(6):755–766. [PMC free article] [PubMed] [Google Scholar]
- Gerhard W., Webster R. G. Antigenic drift in influenza A viruses. I. Selection and characterization of antigenic variants of A/PR/8/34 (HON1) influenza virus with monoclonal antibodies. J Exp Med. 1978 Aug 1;148(2):383–392. doi: 10.1084/jem.148.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard W., Yewdell J., Frankel M. E., Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981 Apr 23;290(5808):713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- Kendal A. P., Noble G. R., Dowdle W. R. Swine influenza viruses isolated in 1976 from man and pig contain two coexisting subpopulations with antigenically distinguishable hemagglutinins. Virology. 1977 Oct 1;82(1):111–121. doi: 10.1016/0042-6822(77)90037-x. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Gerhard W., Croce C. M. Production of antibodies against influenza virus by somatic cell hybrids between mouse myeloma and primed spleen cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2985–2988. doi: 10.1073/pnas.74.7.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Air G. M., Webster R. G. Mechanism of antigenic drift in influenza virus. Amino acid sequence changes in an antigenically active region of Hong Kong (H3N2) influenza virus hemagglutinin. J Mol Biol. 1981 Jan 15;145(2):339–361. doi: 10.1016/0022-2836(81)90209-6. [DOI] [PubMed] [Google Scholar]
- Ruitenberg E. J., Steerenberg P. A., Brosi B. J., Buys J. Reliability of the enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of Trichinella spiralis infections in conventionally raised pigs. J Immunol Methods. 1976;10(1):67–83. doi: 10.1016/0022-1759(76)90008-9. [DOI] [PubMed] [Google Scholar]
- Schild G. C., Pereira M. S., Chakraverty P., Coleman M. T., Dowdle W. R., Chang W. K. Antigenic variants of influenza B virus. Br Med J. 1973 Oct 20;4(5885):127–131. doi: 10.1136/bmj.4.5885.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Berton M. T. Analysis of antigenic drift in the haemagglutinin molecule of influenza B virus with monoclonal antibodies. J Gen Virol. 1981 Jun;54(Pt 2):243–251. doi: 10.1099/0022-1317-54-2-243. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Kendal A. P., Gerhard W. Analysis of antigenic drift in recently isolated influenza A (H1N1) viruses using monoclonal antibody preparations. Virology. 1979 Jul 15;96(1):258–264. doi: 10.1016/0042-6822(79)90189-2. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G. Determination of the number of nonoverlapping antigenic areas on Hong Kong (H3N2) influenza virus hemagglutinin with monoclonal antibodies and the selection of variants with potential epidemiological significance. Virology. 1980 Jul 15;104(1):139–148. doi: 10.1016/0042-6822(80)90372-4. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]


