Abstract
The mechanisms responsible for the induction of matrix-degrading proteases during lung injury are ill defined. Macrophage-derived mediators are believed to play a role in regulating synthesis and turnover of extracellular matrix at sites of inflammation. We find a localized increase in the expression of the rat interstitial collagenase (MMP-13; collagenase-3) gene from fibroblastic cells directly adjacent to macrophages within silicotic rat lung granulomas. Conditioned medium from macrophages isolated from silicotic rat lungs was found to induce rat lung fibroblast interstitial collagenase gene expression. Conditioned medium from primary rat lung macrophages or J774 monocytic cells activated by particulates in vitro also induced interstitial collagenase gene expression. Tumor necrosis factor-α (TNF-α) alone did not induce interstitial collagenase expression in rat lung fibroblasts but did in rat skin fibroblasts, revealing tissue specificity in the regulation of this gene. The activity of the conditioned medium was found to be dependent on the combined effects of TNF-α and 12-lipoxygenase-derived arachidonic acid metabolites. The fibroblast response to this conditioned medium was dependent on de novo protein synthesis and involved the induction of nuclear activator protein-1 activity. These data reveal a novel requirement for macrophage-derived 12-lipoxygenase metabolites in lung fibroblast MMP induction and provide a mechanism for the induction of resident cell MMP gene expression during inflammatory lung processes.
INTRODUCTION
The cellular interactions and intracellular mechanisms responsible for remodeling associated with injury and inflammation are beginning to be elucidated. Macrophages are well established as critical effector cells in the response to injury, whether this response leads to resolution and reestablishment of normal tissue architecture and function or to the eventual development of fibrosis (Riches, 1996). A considerable body of work suggests that macrophages are recruited to sites of inflammation and mediate remodeling by locally producing factors that affect resident cell proliferation and extracellular matrix accumulation. Although increased production of macrophage-derived growth factors has been documented in many forms of pulmonary fibrosis (Bitterman et al., 1983; Martinet et al., 1987), the exact mechanisms of resident cell activation by macrophages are not completely understood. In fact, macrophages may be responsible for influencing phenotypic characteristics in fibroblasts other than (or in addition to) those directly resulting in increased interstitial matrix production and proliferation. For instance, some macrophage-derived products (e.g., prostaglandins) can inhibit cell proliferation and/or matrix synthesis.
Matrix remodeling in response to injury is one of the initial steps of wound healing. Although end-stage fibrotic pathology results from the excessive proliferation and extracellular matrix production of activated resident cells, these cells must first be induced to migrate to a developing lesion. Normal fibroblasts are embedded in a fibrillar collagen-rich matrix and enclosed by a basement membrane, whereas early lesion granulation tissue is rich in provisional matrix proteins and proteoglycans including hyaluronan. During acute lung injury, fibroblast invasion into granulation tissue is likely to involve the production of matrix-degrading proteases (Giannelli et al., 1997), permitting release of these cells from local sites within the tissue. Both inflammatory and resident cell-derived proteases are thought to contribute to these processes.
There are three mammalian interstitial collagenases (collagenase-1, -2, and -3) capable of initiating the degradation of interstitial collagens, each the product of a separate matrix metalloproteinase (MMP)1 gene. Collagenase-2 (MMP-8) is specifically produced and secreted by neutrophils, whereas collagenase-1 (MMP-1) and collagenase-3 (MMP-13) seem to be expressed by many different cell types including inflammatory cells, epithelial cells, and fibroblasts. In humans, the expression of collagenase-3 is highly restricted and seems to be particularly associated with inflammatory disease states such as arthritis (Mitchell et al., 1996). Rodents, however, lack the gene for collagenase-1, whose function appears to be compensated for by collagenase-3 alone. Although both increased and decreased total collagenase activities have been reported in pulmonary fibrosis (Gadek et al., 1979; Pardo et al., 1992; Blaisdell and Giri, 1995), it is likely that localized increases in this enzyme activity are necessary for the initial phases of resident cell activation and recruitment. For instance, in dermal wound repair, human collagenase-1 production by basal epithelial cells is involved in mediating the migration of these cells into the wound bed (Pilcher et al., 1997). In this manuscript we explore the induction of rodent collagenase-3 in lung fibroblasts by factors derived from particulate-activated macrophages during a model of inflammatory lung disease.
MATERIALS AND METHODS
Animals and Reagents
Normal adult (250 g) Sprague Dawley rats were purchased from Charles River Laboratories (Cambridge, MA). The murine monocytic cell line J774.1 was obtained from American Type Culture Collection (Rockville, MD). Silica (Min-U-Sil; 5 μm) was purchased from U.S. Silica (Berkeley Springs, WV), and zymosan, cycloheximide, indomethacin, pepsin, and phorbol 12-myristate 13-acetate (PMA) were from Sigma Chemical (St. Louis, MO). Murine tumor necrosis factor-α (TNF-α) and TNF-α- and interleukin-1β (IL-1β)-neutralizing antibodies were purchased from R&D Systems (Minneapolis, MN). The inhibitors nordihydroguaiaretic acid (NDGA), caffeic acid, and cinnamyl-3,4-dihydroxy-α-cyanocinnimate (CDC) were from Biomol (Plymouth Meeting, PA), and MK-886 was from Calbiochem (San Diego, CA).
In Situ Hybridization and Immunostaining
In situ hybridization was performed on lung sections from normal adult rats (250 g), rats receiving a single intratracheal instillation of 0.4 ml of sterile saline containing 16 mg of silica, or rats receiving a single intratracheal instillation of 0.4 ml of sterile saline as a vehicle control 7 d before being sacrificed. A detailed description of the induction of silicosis and the methods used for in situ hybridization have been published previously (Mariani et al., 1995, 1996). Antisense probes for rat interstitial collagenase (collagenase-3) were generated from the cDNA pUMRc’ase54 (Quinn et al., 1990). Immunostaining was performed with a Vectastain immunostaining kit (Vector Laboratories, Burlingame, CA) according to the manufacturer’s instructions. Antibody clone ED1 (Harlan, Indianapolis, IN) was used to identify monocyte and macrophage cells. Detection of TNF-α was performed with a commercially available antibody for murine TNF-α (R&D Systems). For dual in situ hybridization and immunohistochemistry, in situ hybridization was performed and immediately followed by complete immunostaining before exposure to photographic emulsion.
Isolation and Culture of Cells
Primary macrophages were isolated from healthy or silicotic adult rat lungs by bronchoalveolar lavage (BAL). Animals were killed with a lethal dose of sodium pentobarbital. The trachea was cannulated, and the pulmonary circulation was washed free of blood with saline (0.15 M NaCl). The lungs were removed from the thoracic cavity and lavaged repeatedly with a single 10 ml aliquot of normal saline. After lavage, the saline was collected and centrifuged at 500 × g for 5 min at 4°C. The pellet was resuspended in culture medium (Dulbecco’s modified Eagle’s medium supplemented with 10% calf serum, nonessential amino acids, l-glutamine, and antibiotics), and nucleated cells were counted with the use of a hemocytometer. Cells were plated on tissue culture-treated plastic dishes at a concentration of 0.5 × 106 cells/ml and allowed to adhere overnight. The next day, the culture dishes were washed to remove dead cells and refed with culture medium.
J774 cells were plated at a concentration of 0.5 × 106 cells/ml and allowed to adhere for 24 h. The next day J774 cells were washed with PBS and refed with culture medium. Primary adult rat lung fibroblasts (ALF) were isolated from normal adult rat lungs as described previously (Dunsmore et al., 1995). Neonatal rat lung fibroblasts (NRLF) (McGowan, 1992) and neonatal rat auricular chondrocytes (NRACs) (Pierce et al., 1992) were isolated and cultured as described previously. Adult and neonatal rat skin fibroblasts (ARSF and NRSF, respectively) were isolated by explant culture. All primary cells were analyzed for gene expression in early passage, confluent cultures.
Isolation and Application of Conditioned Medium
Twenty-four hours after plating primary macrophages or J774 cells as described above, cells were washed and refed with culture medium in the absence or presence of silica (0.1 mg/ml; 12.5 μg/cm2) or zymosan (4 mg/ml; 0.5 mg/cm2). Conditioned medium was harvested after 24 h and centrifuged for 10 min at 2000 × g to pellet particulates and cell debris. The supernatant was transferred to a sterile tube and stored at 4°C. The conditioned medium was filter sterilized through a 0.2 μm syringe filter (Gelman Sciences, Ann Arbor, MI) directly upon application to fibroblasts.
Confluent plates of ALFs at passage three were washed two times with PBS and refed with culture medium alone, or culture medium supplemented with 12.5–50% macrophage-conditioned medium. Treatment with conditioned medium was for 6–96 h, with media changes every 48 h. Replicate plates of ALFs were treated directly with silica (0.1 mg/ml; 12.5 μg/cm2) or zymosan (4 mg/ml; 0.5 mg/cm2) as controls. For serum-free experiments, all cells were grown as described above; then conditioning and treatment media used were free of serum. Primary cultures of NRLFs, NRACs, NRSFs, and ARSFs were treated with 50%-conditioned medium for 48 h and analyzed for gene expression. For each assay, at least three separate cultures of mesenchymal cells were treated as described and examined for extracellular matrix gene expression to ensure reproducibility.
To determine whether macrophage de novo protein synthesis was necessary for the production of the activity in the conditioned medium, we stimulated J774 cells with zymosan in the presence of 10 μg/ml cycloheximide. To determine whether fibroblast de novo protein synthesis was necessary for the response to conditioned medium, we treated ALFs with conditioned medium in the presence of 10 μg/ml cycloheximide.
Northern Blot Analysis
RNA isolation and Northern blot analysis was performed as previously described (Pierce et al., 1995). Briefly, total RNA was isolated from cultured cells by a modification of the guanidine-phenol method. Five micrograms of total RNA were denatured by incubating for 10 min at 68°C in 50% formamide, 1 M formaldehyde, and 50 ng/ml ethidium bromide and immediately separated in a 1% agarose gel containing 1 M formaldehyde. RNA was transferred to Hybond N+ (Amersham, Arlington Heights, IL). Rat collagenase-3 mRNA was detected with the use of purified insert DNA derived from the clone described above for in situ hybridization analysis. Clone pRGAPDH13 was used for the detection of GAPDH mRNA (Pierce et al., 1992). The murine TNF-α clone corresponding to nucleotides 398–705 (Fransen et al., 1985) was kindly provided by Dr. Sabine Werner (Max-Plank-Institute, Martinsried, Germany). The level of GAPDH mRNA was used as an internal control in each sample.
Partial Characterization of Conditioned Medium
In an effort to determine the nature of the macrophage-derived activity responsible for altered fibroblast extracellular matrix gene expression, zymosan-activated J774 serum-free conditioned medium was subjected to various treatments before addition to fibroblast cultures. Stability to transient acidification was determined by adjusting the pH of the conditioned medium to 2.5 with 0.5N HCl, incubating for 2 h at 37°C, and then neutralizing with 0.5N NaOH. Pepsin sensitivity was tested by following the same procedure and including 0.2 mg/ml pepsin in the 37°C incubation. Lipid solubility was determined by extracting the conditioned medium three successive times with two volumes of ethyl acetate. After each treatment, the conditioned medium was dialyzed against serum-free culture medium and filter sterilized before addition to fibroblasts.
Analysis of the Role of TNF-α in Conditioned Medium
Northern blot analysis for TNF-α mRNA was performed as described above on RNA isolated from J774 cells cultured for 24 h in the absence or presence of zymosan or treated with 50 ng/ml PMA. To investigate the effects of TNF-α on rat collagenase-3 gene expression, we treated primary cultures of ALFs with 20 ng/ml recombinant murine TNF-α for 48 h and harvested for Northern analysis as described above. The role of TNF-α in the zymosan-activated J774-conditioned medium was assessed by neutralization with a murine TNF-α-neutralizing antibody. Fifty percent zymosan-activated J774-conditioned medium was incubated with 10 μg/ml TNF-α antibody for 2 h at 37°C with gentle shaking. This neutralized conditioned medium was then assessed for the ability to alter ALF collagenase-3 gene expression as described. Treatment of conditioned medium with a murine IL-1β-neutralizing antibody (10 μg/ml) served as a control.
Analysis of Bioactive Lipids
Assessment of J774 cells for secretion of arachidonic acid metabolites was performed essentially as described by Wolf et al. (1997). J774 cells (1.5 × 105 cells in 2 ml of culture medium) were metabolically labeled with 50 μCi of [3H]arachidonic acid (Dupont NEN Research Products, Boston, MA) for 16 h, followed by repeated washing with serum-containing medium. Labeled J774 cells were fed with fresh medium with or without zymosan. After 1 or 24 h, medium and cell layers were harvested, and the percentage of radiolabel secreted was determined by liquid scintillation. For inhibitor studies, J774 cells were pretreated at 37°C for 30 min with the appropriate inhibitor and then refed with fresh inhibitor and zymosan. Replicate cultures of J774 cells were treated with zymosan alone to confirm production of the activity. Conditioned medium was then assessed for activity on ALFs as described above.
Electrophoretic Mobility Shift Assays
These assays were performed essentially as previously described (Doyle et al., 1997). Briefly, 5 μg of nuclear protein isolated from ALFs treated under the appropriate conditions was incubated with radiolabeled, double-stranded oligonucleotides corresponding to a consensus activator protein-1 (AP-1) site (5′-GATCAAAGCATGAGTCAGACACCT-3′) or a nuclear factor-κB (NFκB) site (5′-AGTTGAGGGGACTTTCCCAGGC-3′). Binding reactions were electrophoresed through 5% nondenaturing polyacrylamide gels at 4°C. Specificity of binding was confirmed by competition with 100-fold excess of unlabeled oligonucleotide. Identification of Fos proteins in the AP-1-binding complexes was performed with the use of antibody c-fos(K-25) from Santa Cruz Biotechnology (Santa Cruz, CA).
RESULTS
Collagenase-3 Is Expressed in Experimental Granulomatous Lung Disease
We have previously described alterations in the expression of the genes encoding the interstitial matrix proteins tropoelastin (Mariani et al., 1995) and α1(I) procollagen (Mariani et al., 1996) in a model of rat lung silicosis. We were interested in further defining alterations in extracellular matrix gene expression by granuloma fibroblasts in this model, primarily with respect to the remodeling of preexisting matrix.
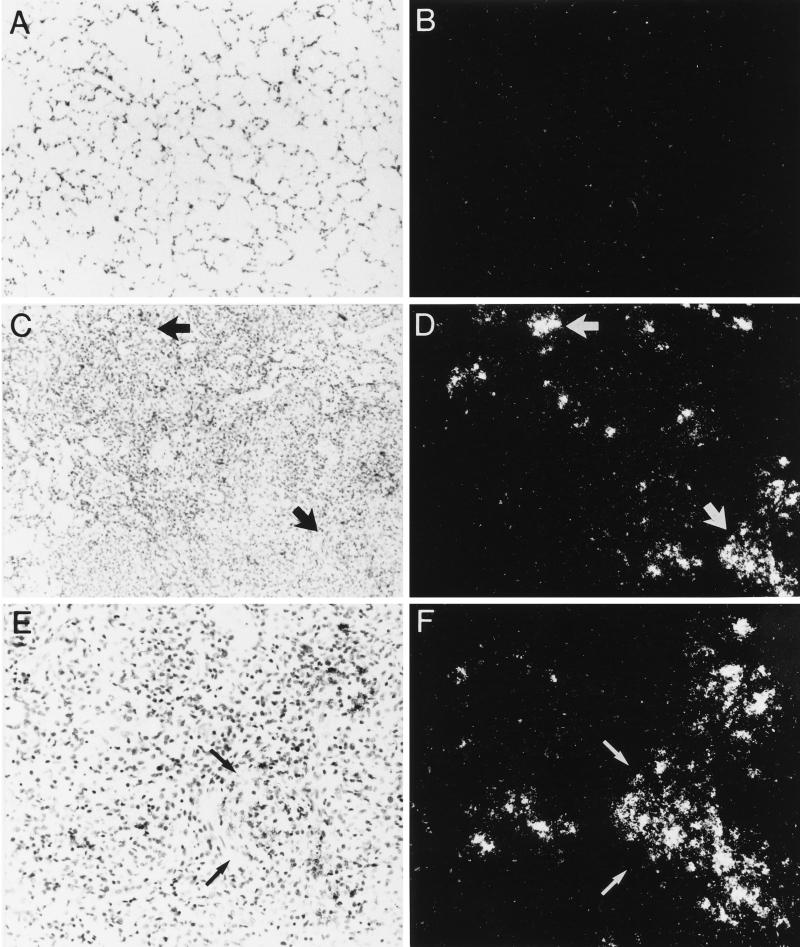
We investigated the expression of the gene encoding rat collagenase-3 (MMP-13) in control and silicotic rat lungs by in situ hybridization (Figure 1). Collagenase-3 gene expression was observed within localized populations of cells within granulomatous lesions of silicotic lungs at 7 d posttreatment (Figure 1, C–F). In lung sections from normal (Mariani and Pierce, unpublished data) and vehicle control (Figure 1, A and B) rats, no granulomatous lesions or collagenase-3 gene expression was observed. No specific signal was detected in any tissues studied using sense probe. Each in situ hybridization experiment was performed at least three times for each probe with sections from one normal rat lung, two vehicle control lungs, and three silicotic lungs.
Figure 1.
Expression of collagenase-3 in experimental rat lung silicosis. Experimental lung silicosis was induced in adult rats, and lungs were harvested 7 d after treatment. Paired bright- and dark-field views of lung sections hybridized in situ for collagenase-3 mRNA are shown. (A and B) Lung sections from animals instilled with saline as a vehicle control. (C–F) Lung sections from animals instilled with silica. No in situ hybridization signal is detected in control lungs (A and B), whereas an intense signal for collagenase-3 mRNA (visible as white silver grains in the dark-field views) is detected in localized groups of cells within silicotic lung granulomas (C–F). Arrows indicate sites of concentrated gene expression. Original magnification is 100× for A–D and 200× for E and F.
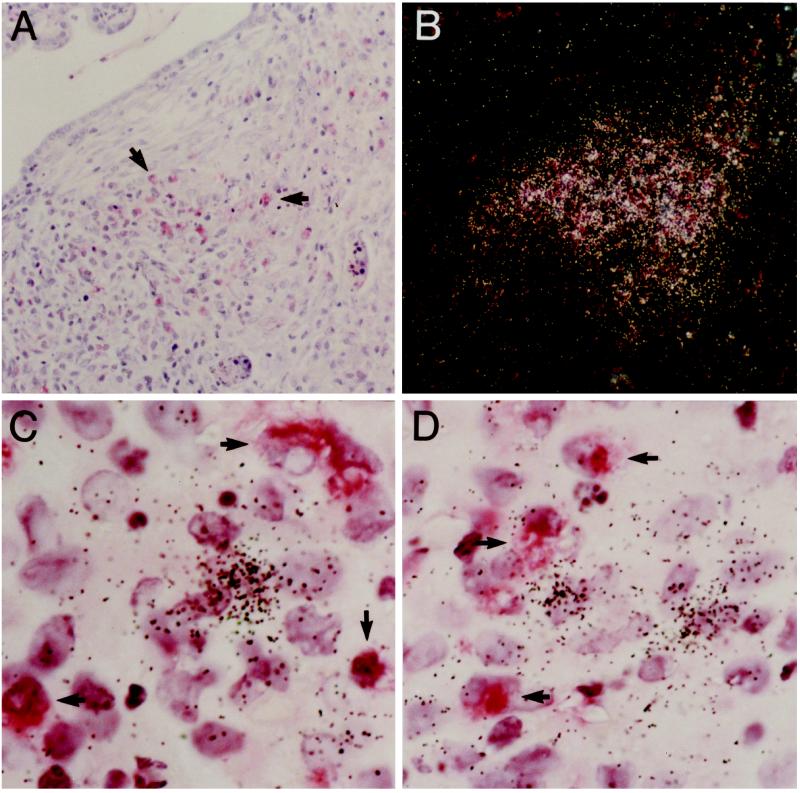
We next determined the spatial relationship between granuloma infiltrating macrophages and rat collagenase-3 gene expression. Immunohistochemistry and in situ hybridization of serial sections indicated that macrophages were present at sites of collagenase-3 gene expression but could not account for the entire population of collagenase-expressing cells (Figure 2, A and B). To define specifically the cell populations responsible for collagenase-3 gene expression within silicotic granulomas, we performed dual in situ hybridization and immunohistochemistry on individual sections from silicotic rat lungs (Figure 2, C and D). Macrophages (immunostaining for ED1) did not show a positive signal for collagenase-3 mRNA, while fibroblastic cells adjacent to these macrophages showed a strong hybridization signal for collagenase-3 mRNA. This indicates that granuloma fibroblasts are the primary source of collagenase-3 gene expression in the silicotic rat lung and suggests that these fibroblasts are responding to mediators locally produced and/or secreted by macrophages.
Figure 2.
Collagenase-3 gene expression in silicotic lungs predominates from fibroblastic cells adjacent to granuloma-infiltrating macrophages. Experimental lung silicosis was induced in adult rats as described in Materials and Methods, and lungs were harvested 7 d after treatment. (A) Immunohistochemistry for macrophages using the antibody clone ED1. (B) In situ hybridization for collagenase-3 mRNA. (C and D) Dual in situ hybridization and immunohistochemistry for collagenase-3 and macrophages. Immunohistochemistry for macrophages using the antibody clone ED1 (A) and in situ hybridization for collagenase-3 (B) on serial tissue sections reveal that granuloma-infiltrating macrophages (arrows) are abundant at sites of collagenase-3 gene expression (white grains). Dual in situ hybridization and immunohistochemistry for collagenase-3 and macrophages (C and D) show that the hybridization signal for collagenase-3 mRNA (black grains) is predominantly associated with fibroblastic cells adjacent to macrophages (arrows) and not from the macrophages themselves. Original magnification is 100× for A and B and 1000× for C and D.
Primary Lung Macrophage-conditioned Medium Induces Lung Fibroblast Collagenase-3 Gene Expression
To investigate the potential role of macrophage-derived mediators in the induction of rat collagenase-3 in lung fibroblasts, we isolated macrophages by BAL from the lungs of silicotic rats sacrificed 7 d after intratracheal instillation of silica. These cells were allowed to condition culture medium for 24 h. This conditioned medium was assessed for the ability to modify collagenase-3 gene expression in primary lung fibroblasts. Northern blot analysis indicated that untreated primary cultures of ALFs did not express detectable levels of collagenase-3 (Figure 3A, lane 1). Conditioned medium from silicotic rat lung macrophages strongly induced collagenase-3 mRNA expression in ALF cultures (Figure 3A, lane 2). These effects were consistent in two separate isolates of primary macrophages from silicotic rat lungs.
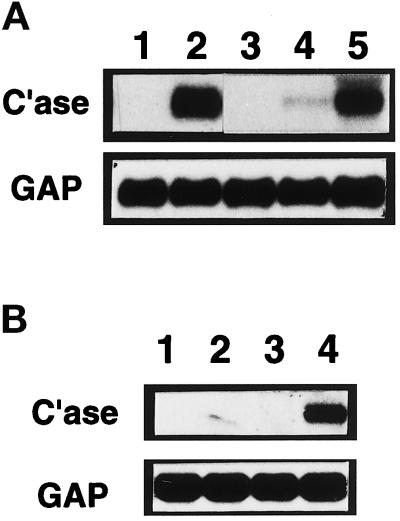
Figure 3.
Silicotic rat lung macrophages, particulate-stimulated normal rat lung macrophages, and particulate-stimulated J774 cells induce lung fibroblast collagenase-3 gene expression. (A) Rat lung macrophages were isolated by BAL from silicotic rat lungs 7 d after intratracheal instillation of silica and allowed to condition medium for 24 h. Similarly, macrophages were isolated by BAL from normal, healthy rat lungs and allowed to condition medium for 24 h when unstimulated or in the presence of silica (0.1 mg/ml) or zymosan (4 mg/ml). Primary cultures of ALFs were untreated (lane 1), treated with 50% silicotic rat lung macrophage-conditioned medium (CM) (lane 2), treated with 50% unstimulated normal macrophage-CM (lane 3), treated with 50% silica-stimulated normal macrophage-CM (lane 4), or treated with 50% zymosan-stimulated normal macrophage-CM (lane 5); cultures were harvested after 48 h for Northern blot analysis of collagenase-3 (C’ase) and glyceraldehyde-3-phosphate dehydrogenase (GAP) mRNAs. (B) Murine monocytic J774 cells were allowed to condition medium for 24 h when unstimulated or in the presence of silica (0.1 mg/ml) or zymosan (4 mg/ml). Primary cultures of ALFs untreated (lane 1) or treated with 50% unstimulated J774-CM (lane 2), silica-treated J774-CM (lane 3), or zymosan-treated J774-CM (lane 4) were harvested after 48 h for Northern blot analysis as above.
To model in vitro the activity of the macrophages isolated from silicotic rat lungs, we isolated primary macrophages by BAL from normal, healthy adult rats and stimulated these cells with the particulates silica (0.1 mg/ml; 12.5 μg/cm2) or zymosan (4 mg/ml; 0.5 mg/cm2). BAL cells were assessed before and after conditioning medium and found to be >95% macrophages as determined by immunocytochemistry using antibody clone ED1 (Mariani and Pierce, unpublished data). Phagocytosis of particulate material by intact cells was also readily apparent.
Conditioned medium from unstimulated cultures of primary normal rat lung macrophages did not affect rat collagenase-3 gene expression in ALFs (Figure 3A, lane 3). Conditioned medium from macrophages stimulated with silica in vitro resulted in a modest induction of ALF collagenase-3 gene expression (Figure 3A, lane 4). Conditioned medium from macrophages stimulated with zymosan induced a greater level of ALF collagenase-3 gene expression (Figure 3A, lane 5). These effects were consistent in four separate isolates of primary macrophages from healthy rat lungs.
Particulate-Stimulated J774 Cell-Conditioned Medium Induces Fibroblast Collagenase-3 Gene Expression
Next, the murine macrophage-like cell line J774 was assessed for the ability to release factors that would induce ALF rat collagenase-3 gene expression in a manner similar to that observed for primary rat lung macrophages (Figure 3B). As observed for primary macrophages, conditioned medium from unstimulated J774 cells had no effect on ALF gene expression (Figure 3A, lanes 1 and 2). Conditioned medium from silica-stimulated J774 cells also did not affect collagenase-3 gene expression from ALFs (Figure 3B, lane 3). Conditioned medium from zymosan-treated J774 cells, however, strongly induced ALF collagenase-3 gene expression (Figure 3B, lane 4). This response was identical to that observed for both silicotic lung macrophages and primary macrophages activated by silica or zymosan in vitro. These effects were consistently seen in eight separate isolates of J774 cell-conditioned medium and for eight separate isolates of primary ALFs.
Although all particulates were removed from conditioned medium samples in these studies by filter sterilization, we tested the ability of direct treatment with particulates to induce fibroblast rat collagenase-3 expression. Direct treatment of ALFs with either silica or zymosan did not induce collagenase-3 gene expression (Mariani and Pierce, unpublished data). This emphasizes the necessity for macrophages to induce the fibroblast collagenase gene expression observed in silicotic rat lungs in vivo.
Silicotic rat lung macrophage-conditioned medium, in vitro zymosan-stimulated primary rat lung macrophage, and zymosan-stimulated J774-conditioned medium also repressed α1(I) procollagen and tropoelastin expression and increased fibronectin expression in ALFs (Mariani and Pierce, unpublished data). These findings indicate that the conditioned medium does not cause a general stimulatory effect on ALF gene expression. These data also strongly suggest that the conditioned media from the different macrophage sources are functionally equivalent.
Initial Characterization of Fibroblast Collagenase-3-Inducing Activity
To characterize the macrophage-derived activity responsible for the alteration of ALF rat collagenase-3 gene expression, we pretreated zymosan-stimulated J774-conditioned medium under conditions that would remove or inactivate potential mediators (Figure 4). These experiments were performed in serum-free conditions; the lack of serum in the assay did not affect the fibroblast collagenase-inducing activity of the zymosan-activated J774-conditioned medium (Figure 4, lanes 1 and 2). Treatment of the conditioned medium with pepsin (under acidic pH) completely abrogated its ability to modify ALF collagenase-3 gene expression (Figure 4, lane 3). Acidification alone had no effect on the activity of the conditioned medium (Figure 4, lane 4). Lipid extraction of the conditioned medium also abrogated the ability of the conditioned medium to induce ALF collagenase-3 (Figure 4, lane 5). These data indicate a multifactoral nature for the macrophage-derived activity, with both protease-sensitive and lipid-extractable factors necessary.
Figure 4.
Macrophage-derived activity is pepsin-labile and lipid-extractable. Murine monocytic J774 cells were allowed to condition serum-free medium for 24 h in the presence of zymosan (4 mg/ml). Primary cultures of ALFs were untreated (lane 1), were treated with 50% zymosan-stimulated J774-conditioned medium (CM) that had previously been dialyzed (lane 2), pepsin digested (lane 3), transiently acidified (lane 4), or lipid extracted (lane 5), or were treated with 50% zymosan-stimulated J774-CM obtained in the presence of 10 μg/ml cycloheximide (lane 6). After 48 h, cells were harvested for Northern blot analysis of collagenase-3 (C’ase) and glyceraldehyde-3-phosphate dehydrogenase (GAP) mRNAs.
To address the need for macrophage de novo protein synthesis in this system, J774 cells were activated with zymosan in the presence of cycloheximide. Conditioned medium from J774 cells treated with zymosan in the presence of 10 μg/ml cycloheximide failed to modify ALF rat collagenase-3 gene expression (Figure 4, lane 6). This confirms that a protein factor is required for the J774-mediated alteration in fibroblast collagenase-3 gene expression and/or indicates that new protein synthesis is necessary to produce and/or secrete the factor(s).
Lipid Cofactor Is a 12-Lipoxygenase Metabolite of Arachidonic Acid
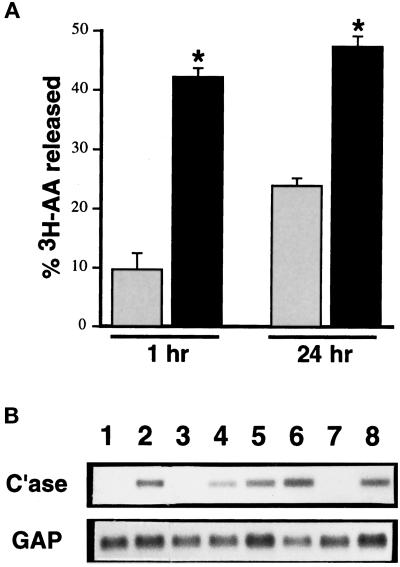
To determine whether the lipid-soluble mediator of altered fibroblast gene expression could be macrophage-derived arachidonic acid metabolites, we assessed the release of [3H]arachidonic acid from unstimulated and zymosan-stimulated J774 cells (Figure 5A). Unstimulated cells released 9.7% ± 2.7% of total [3H]arachidonic acid metabolites into the conditioned medium at 1 h and 23.8% ± 1.3% at 24 h. Zymosan-stimulated J774 cells secreted significantly more arachidonic acid at both 1 h (42.0 ± 1.6%) and 24 h (47.5 ± 1.7%).
Figure 5.
Macrophage-mediated lung fibroblast collagenase-3 induction is dependent on macrophage 12-lipoxygenase activity. (A) Murine monocytic J774 cells were labeled with 50 μCi of [3H]arachidonic acid (AA) for 16 h. After thorough washing, the percentage of incorporated 3H released (mean ± SD) from labeled J774 cells into the conditioned medium (CM) in the absence (shaded bars) and presence (solid bars) of zymosan (4 mg/ml) was determined by liquid scintillation after 1 or 24 h (*p < 0.01 by Student’s t test). (B) Primary cultures of ALFs were untreated (lane 1) or were treated with 50% zymosan-stimulated J774-CM obtained in the absence (lane 2) or presence of 100 μM NDGA (lane 3), 10 μM NDGA (lane 4), 25 μM MK-886 (lane 5), 50 μM caffeic acid (lane 6), 50 μM CDC (lane 7), or 1 μg/ml indomethacin (lane 8). After 24 h, cells were harvested for Northern blot analysis of collagenase-3 (C’ase) and glyceraldehyde-3-phosphate dehydrogenase (GAP) mRNAs.
Cellular arachidonic acid metabolism can be mediated by many different enzymes, including various cyclooxygenases and lipoxygenases. Therefore, we tested the ability of J774 cells to produce collagenase-3-inducing conditioned medium in the presence of inhibitors of these pathways. Zymosan-activated J774 cells were allowed to condition medium in the presence of various cyclooxygenase and lipoxygenase inhibitors, and this conditioned medium was subsequently tested for activity in ALFs (Figure 5B). Conditioned medium derived from zymosan-stimulated J774 cells cotreated with the broad-substrate lipoxygenase inhibitor NDGA at high concentrations (100 μM) had no activity (Figure 5B, lane 3), while conditioned medium produced from cells treated with low concentrations of NDGA (1–10 μM) retained (slightly reduced) collagenase-inducing activity (Figure 5B, lane 4). These concentrations did not affect J774 TNF-α gene expression (Mariani and Pierce, unpublished data). Because NDGA completely inhibits 12/15-lipoxygenase only at high concentrations, whereas it inhibits 5-lipoxygenase at low concentrations, these data suggested that a 12/15-lipoxygenase, but not a 5-lipoxygenase, was responsible for the production of the activity. In agreement with the inability of low concentrations of NDGA to inhibit the production of the activity, the 5-lipoxygenase inhibitors MK-886 (1–25 μM) and caffeic acid (1–50 μM) did not inhibit the ability of zymosan-stimulated J774 cells to produce the collagenase-inducing activity (Figure 5B, lanes 5 and 6). Conditioned medium derived from zymosan-stimulated J774 cells treated with the 12-lipoxygenase inhibitor CDC (50 μM) had no activity (Figure 5B, lane 7). Murine monocytes and macrophages are not believed to contain a specific 15-lipoxygenase enzyme (Funk, 1996), and we were unable to detect any 15-lipoxygenase enzyme in unstimulated or zymosan-stimulated J774 cells by Western blot (Mariani and Pierce, unpublished data). Cotreatment of J774 cells with zymosan in the presence of 1 μg/ml indomethacin failed to abrogate the ability of the conditioned medium to induce ALF collagenase-3 gene expression (Figure 5B, lane 8). Taken together, these data strongly implicate a 12-lipoxygenase metabolite as the bioactive lipid component of J774-conditioned medium and indicate that other lipoxygenase products (e.g., leukotrienes and 15-HETEs) and cyclooxygenase products (e.g., prostaglandins) are not essential.
TNF-α Is Necessary but Insufficient for the Macrophage-mediated Induction of Lung Fibroblast Collagenase-3 Gene Expression
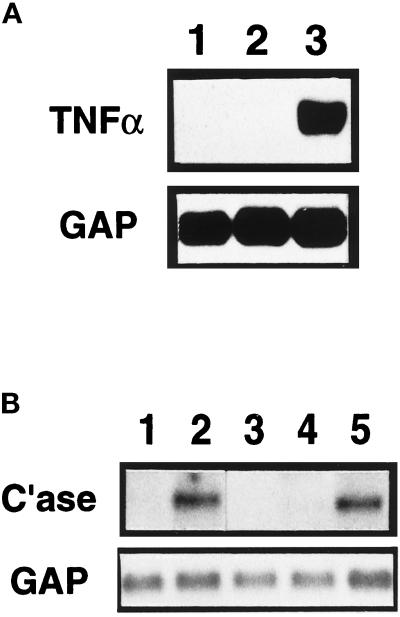
TNF-α has previously been implicated in the pathogenesis of numerous pulmonary disorders and is a potent stimulator of MMP expression (Brenner et al., 1989). We therefore investigated whether exposure to particulates influenced the production of TNF-α in J774 cells (Figure 6A). Northern blot analysis showed that J774 cells grown in normal culture medium do not express detectable levels of mRNA for TNF-α (Figure 6A, lane 1). Upon activation with zymosan, J774 cells produce high levels of mRNA for TNF-α (Figure 6A, lane 3). Stimulation of J774 cells with 50 ng/ml PMA did not result in the production of the fibroblast collagenase-inducing activity (Mariani and Pierce, unpublished data) and did not induce TNF-α expression in J774 cells (Figure 6A, lane 2), further suggesting a necessity for TNF-α in the induction of ALF rat collagenase-3 gene expression. Western blot analysis of conditioned medium from and immunocytochemistry of unstimulated and zymosan-stimulated J774 cells supported these findings (Mariani and Pierce, unpublished data).
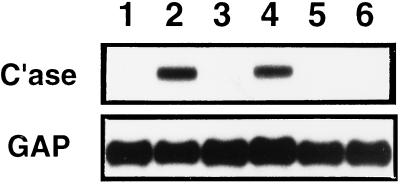
Figure 6.
Macrophage-derived TNF-α contributes to fibroblast collagenase-3 induction. (A) Murine monocytic J774 cells were cultured for 24 h alone (lane 1) or in the presence of 50 ng/ml phorbol-myristate acetate (lane 2) or 4 mg/ml zymosan (lane 3) and harvested for Northern blot analysis of TNF-α mRNA. (B) Murine monocytic J774 cells were allowed to condition medium for 24 h in the presence of zymosan (4 mg/ml). Primary cultures of ALFs were untreated (lane 1) or were treated with 50% zymosan-treated J774-conditioned medium (CM) alone (lane 2), with 20 ng/ml recombinant murine TNF-α alone (lane 3), or with 50% zymosan-treated J774-CM pretreated with 10 μg/ml TNF-α-neutralizing antibody (lane 4) or 10 μg/ml IL-1β-neutralizing antibody (lane 5). After 48 h, cells were harvested for Northern blot analysis of collagenase-3 (C’ase) and glyceraldehyde-3-phosphate dehydrogenase (GAP) mRNAs.
We next tested the ability of recombinant murine TNF-α to replicate the induction of rat collagenase-3 gene expression in ALFs mediated by activated macrophage-conditioned medium. TNF-α alone was unable to induce collagenase-3 gene expression in ALFs (Figure 6B, lane 3). This differs significantly from various other cell types in which TNF-α has been shown to be sufficient for induction of human collagenase-1 (MMP-1) expression. These findings are consistent, however, with the requirement for both pepsin-labile and lipid-extractable factors for the activity of the conditioned medium. To neutralize TNF-α, we incubated zymosan-stimulated J774-conditioned medium with neutralizing antibodies to TNF-α for 2 h at 37°C. Neutralization of TNF-α activity resulted in abrogation of the ability of the conditioned medium to mediate rat collagenase-3 induction in lung fibroblasts (Figure 6B, lane 4). In contrast, treatment of the conditioned medium with a murine IL-1β-neutralizing antibody had no effect on the activity of the conditioned medium (Figure 6B, lane 5). These data indicate that TNF-α is necessary but insufficient to cause the characterized macrophage-mediated induction of ALF collagenase-3 gene expression. Cooperation with a secreted lipid-soluble mediator is required.
Macrophage-derived Activity Induces Collagenase-3 in Various Cell Types
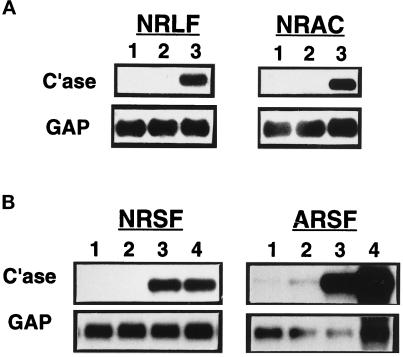
TNF-α is known to be a potent inducer of human collagenase-1 (MMP-1) in most cell types. However, we find that TNF-α alone does not induce rat collagenase-3 gene expression in ALFs. To determine whether this may be due to developmental, tissue-specific, and/or gene-specific differences, we investigated the ability of zymosan-stimulated J774-conditioned medium to induce collagenase-3 gene expression in numerous primary rat cell types. Zymosan-activated J774 cell-conditioned medium strongly induced collagenase-3 gene expression in primary cultures of NRLFs and NRACs (Figure 7A) as well as in primary cultures of NRSFs and ARSFs (Figure 7B). This conditioned medium also strongly induced collagenase-1 gene expression in human skin fibroblasts and human lung fibroblasts (Mariani and Pierce, unpublished data).
Figure 7.
Effects of macrophage-conditioned medium or TNF-α on collagenase-3 gene expression in multiple mesenchymal cell types. Murine monocytic J774 cells were allowed to condition medium for 24 h when unstimulated or in the presence of zymosan (4 mg/ml). Mesenchymal cells were untreated (lane 1) or treated with 50% unstimulated J774-conditioned medium (CM) (lane 2), 50% zymosan-activated J774-CM (lane 3), or 20 ng/ml recombinant murine TNF-α (lane 4). Treated cells were harvested after 48 h for Northern blot analysis of collagenase-3 (C’ase) and glyceraldehyde-3-phosphate dehydrogenase (GAP) mRNAs. (A) Representative Northern from primary cultures of NRLFs and NRACs. (B) Representative Northern from primary cultures of NRSFs and ARSFs.
Previous reports have indicated that some fibroblasts express interstitial collagenase(s) under standard tissue culture conditions, without stimulation by agonists. Under our culture conditions, we find that for rat collagenase-3, this is tissue and developmentally restricted. Neither ALFs, NRLFs, nor NRSFs expressed detectable levels of collagenase-3 mRNA without stimulation. ARSFs, however, did express detectable (although very low) collagenase-3 mRNA levels before stimulation (Figure 7B). Zymosan-activated J774 cell-conditioned medium also greatly induced collagenase-3 gene expression in ARSFs as well as in NRSFs (Figure 7B). Surprisingly, TNF-α alone was able to induce collagenase-3 gene expression in rat skin fibroblasts but not lung fibroblasts, suggesting tissue-specific differences in the regulation of fibroblast collagenase-3 production. These data also indicate that macrophage-derived factors are capable of inducing rat collagenase-3 production in cells derived from multiple tissues and at different stages of development.
Kinetics of Macrophage-mediated Induction in Lung Fibroblast Collagenase-3 Gene Expression
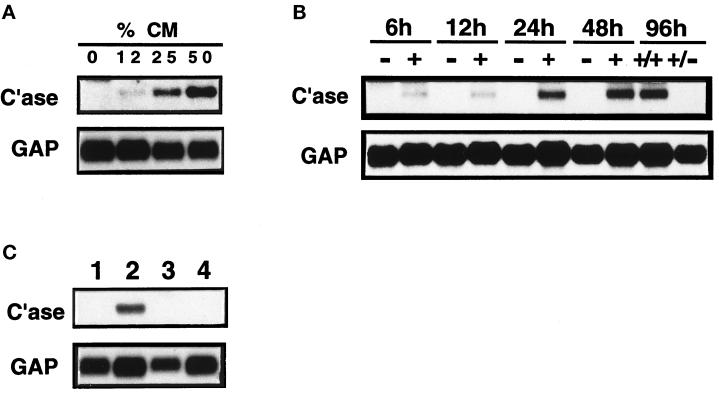
To determine the concentration and duration of exposure to macrophage-conditioned medium required for this response, we treated ALFs with conditioned medium at varying doses or for varying times (Figure 8, A and B). The minimum dose of conditioned medium necessary for induction of rat collagenase-3 was 25%, and this response was augmented at 50%. A time-course experiment showed that collagenase-3 gene expression was induced after 6 h of exposure to conditioned medium, and expression persisted for up to 96 h of exposure. We tested whether the response was dependent on continuous exposure of ALFs to the conditioned medium or resulted in a stable, phenotypic change in lung fibroblast phenotype. ALFs were treated with conditioned medium for 48 h and then washed and refed with conditioned medium (Figure 8B, 96 h +/+) or nonconditioned medium (Figure 8B, 96 h +/−) for another 48 h. This washing led to a reversion of collagenase-3 gene expression to levels seen in untreated cells. This indicates the macrophage-conditioned medium does not produce a stable phenotypic change in ALFs.
Figure 8.
Kinetics of macrophage-mediated induction of fibroblast collagenase-3. Murine monocytic J774 cells were allowed to condition medium for 24 h in the presence of zymosan (4 mg/ml). (A) Primary cultures of ALFs were treated with 0–50% zymosan-treated J774-conditioned medium (CM) for 48 h and harvested for Northern blot analysis of collagenase-3 (C’ase) and glyceraldehyde-3-phosphate dehydrogenase (GAP) mRNAs. (B) Primary cultures of ALFs were untreated (−) or treated with 50% zymosan-treated J774-CM (+) for 6–96 h and harvested for Northern blot analysis as above. Medium was changed after 48 h; therefore, 96 h cultures received 48 h of 50% CM followed by either another 48 h of 50% CM (+/+) or 48 h of nonconditioned medium (+/−). (C) Primary cultures of ALFs were untreated (lane 1) or were treated with 50% zymosan-treated J774-CM alone (lane 2) or with 50% zymosan-treated J774-CM in the presence of 10 μg/ml cycloheximide (lane 3). Fibroblasts were also treated with cycloheximide in the absence of CM (lane 4) as a control.
Fibroblasts Play an Active Role in Macrophage-mediated Induction of Collagenase-3 Gene Expression
To determine whether the response of lung fibroblasts to macrophage-conditioned medium was direct (passive) or was dependent on fibroblast de novo protein synthesis, we assessed the ability of fibroblasts to respond in the presence of the protein synthesis inhibitor cycloheximide. ALF cultures were treated as previously described with 50% zymosan-activated J774-conditioned medium in the absence or presence of 10 μg/ml cycloheximide (Figure 8C). Cells treated with cycloheximide failed to respond to the conditioned medium by inducing rat collagenase-3 gene expression (Figure 8C, lane 3). Direct treatment of fibroblasts with cycloheximide had no effect on steady-state levels of collagenase-3 mRNA (Figure 8C, lane 4). These data indicate that the fibroblasts play an active role in the response to the conditioned medium.
Collagenase-3 Expression Corresponds with Superinduction of Nuclear AP-1 Activity
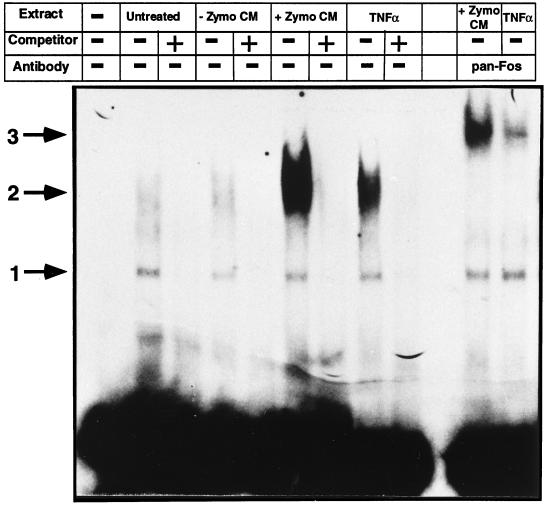
TNF-α signal transduction leads to increased nuclear binding activity for AP-1, which is necessary for maximal human collagenase-1 production. To characterize changes in nuclear AP-1 activity upon stimulation with activated macrophage-conditioned medium, we performed electrophoretic mobility shift assays using consensus oligonucleotides for AP-1 (Figure 9) and NFκB. Untreated ALFs contained low but detectable levels of nuclear binding activity for AP-1. This was detected as two complexes, one high-mobility complex (Figure 9, arrow 1) and a more diffuse, low-mobility complex (arrow 2). The complex 1 binding activity was constant under all conditions studied. The complex 2 activity was unchanged in ALFs treated with unstimulated J774-conditioned medium but was greatly enhanced upon treatment with zymosan-stimulated J774-conditioned medium. Treatment with a maximal dose of TNF-α (20 ng/ml) also enhanced nuclear complex 2 binding activity but to a lesser extent than did treatment with zymosan-stimulated J774-conditioned medium. All binding activity was competed by 100-fold excess of unlabeled probe. Similar quantitative changes were obtained for nuclear NFκB binding activity (Mariani and Pierce, unpublished data). These data confirm activation of the TNF-α signal transduction pathway by zymosan-stimulated J774-conditioned medium. Furthermore, they suggest that the lipid cofactor may cooperate with TNF-α to superinduce nuclear AP-1 (and NFκB) activity and thereby initiate ALF rat collagenase-3 gene expression.
Figure 9.
Macrophage-mediated induction of fibroblast collagenase-3 involves maximal induction of nuclear AP-1 binding activity. Primary cultures of ALFs were untreated or treated with 50% unstimulated J774-conditioned medium (CM) (−Zymo CM), 50% zymosan-stimulated J774-CM (+Zymo CM), or 20 ng/ml TNF-α and were harvested after 24 h for electrophoretic mobility shift assays as previously described. Five micrograms of total nuclear protein isolated from cells treated as indicated were incubated with radiolabeled probe for a consensus AP-1 site in the absence or presence of pan-Fos antibodies. Arrows indicate specific protein–DNA complexes able to be competed with excess unlabeled probe.
Previous studies have indicated a necessity for Fos proteins, particularly c-Fos, in the induction of human collagenase-1 and -3 gene expression (Angel et al., 1987; Hu et al., 1994; Borden et al., 1996; Doyle et al., 1997). Using a pan-Fos antibody, we were able to detect these proteins in our nuclear extracts from both zymosan-stimulated J774-conditioned medium–treated and TNF-α-treated ALFs (Figure 9). Treatment of nuclear extract-bound AP-1 oligonucleotides with anti-pan-Fos antibodies resulted in a supershift of all detectable complex 2 to form complex 3 (Figure 9, arrow 3), indicating all complex 2 binding activity contains Fos proteins. Complex 3 showed a quantitative distribution similar to that for complex 2. Complex 1 was unaffected by treatment with this antibody. This data supports the conclusion by Borden et al. (1996) that differential regulation of collagenase-3 compared with collagenase-1 is not mediated by qualitative changes in Fos proteins but suggests quantitative changes are involved.
DISCUSSION
Induction of interstitial collagenase expression is a hallmark of both acute and chronic inflammatory processes, whether or not these states ultimately lead to fibrosis. In this study, we investigated the role of mediators elaborated by particulate-activated macrophages in the induction of collagenase expression in lung fibroblasts. In a model of acute inflammatory lung disease resulting from the intratracheal instillation of silica into rat lungs, we find abundant rat collagenase-3 gene expression within early silicotic granulomas. The collagenase-1 gene is not found in rodents, and its activity is presumed to be compensated for by collagenase-3. Most, if not all, collagenase-3 gene expression in this model is derived from fibroblastic cells within granulomatous lesions that are in close proximity to macrophages. We find that macrophages from the lungs of silicotic rats and macrophages or J774 cells activated by particulates in vitro elaborate factors that induce fibroblast collagenase-3 gene expression. Unlike other models in which TNF-α is sufficient to induce MMP expression, this induction is dependent on the combined effects of TNF-α- and 12-lipoxygenase-derived metabolites.
We have presented an in vitro model system for the analysis of the mechanisms responsible for macrophage-mediated induction of rat collagenase-3 gene expression in lung fibroblasts. Stimulation of primary macrophages or the macrophage-like J774 cell line by silica in vitro did not perfectly model in vivo stimulation of alveolar macrophages by silica, as evidenced by the reduced ability to elaborate factors that stimulate fibroblast collagenase expression. However, treatment of J774 cells by zymosan resulted in the production of factors that display all the characteristics of the activity derived from primary lung macrophages activated by particulates in vitro and, more importantly, from macrophages isolated from the lungs of silicotic rats. Zymosan is a highly phagocytic particulate derivative of yeast cell wall that induces growth factor (including TNF-α) production in macrophages (Claudio et al., 1995). It is unclear why primary alveolar macrophages or the J774 cell line did not respond to silica in vitro by producing the collagenase-3-stimulating activity. Previous studies have shown, however, that this cell line will not produce appreciable TNF-α in response to silica (Claudio et al., 1995). It is likely that the complete activation of macrophages in vivo involves costimulation with silica and an undefined mediator (soluble or structural) not present in our tissue culture system. Significantly, the response of increased collagenase-3 expression by the fibroblasts is dependent on continuous exposure to the macrophage mediators (Figure 8B) and on fibroblast secondary mechanisms (Figure 8C).
The role of TNF-α was directly assessed in this model, because it is a macrophage-derived cytokine sufficient to induce human collagenase-1 expression (Brenner et al., 1989). TNF-α expression was induced in particulate-activated macrophages, but recombinant TNF-α alone did not induce rat collagenase-3 expression in rat lung fibroblasts. However, neutralization of TNF-α in activated macrophage-conditioned medium resulted in abrogation of the induction in ALF rat collagenase-3 gene expression. This is in agreement with the proposed activity of the protein factor(s) in zymosan-stimulated macrophage-conditioned medium and reveals that TNF-α is necessary but insufficient for the macrophage-mediated induction of ALF rat collagenase-3. These results agree with the critical role shown for TNF-α in the development of silicosis (Piguet et al., 1990). These data also reveal a possible mechanism for TNF-α in macrophage-mediated fibroblast activation in lung injury but support the hypothesis that this cytokine is not exclusively involved.
Although TNF-α alone can induce interstitial collagenase in many mesenchymal cell types, the insufficiency of this cytokine to induce rat collagenase-3 in rat lung fibroblasts may be due to tissue-specific differences as well as to differences in the regulation of the genes encoding collagenase-1 and -3. TNF-α treatment alone was not sufficient to induce collagenase-3 induction (or maximal AP-1 activation) in rodent lung fibroblasts but was sufficient for induction of collagenase-3 in rodent skin fibroblasts. Others have reported a highly tissue-specific regulation for this gene (Mitchell et al., 1996). Maximal induction of interstitial collagenase gene expression (collagenase-1 or -3) involves nuclear localization of factors that bind AP-1 sites, predominantly c-fos (Nakabeppu et al., 1988; Borden et al., 1996). Interestingly, we find that the zymosan-activated macrophage-conditioned medium induction of ALF rat collagenase-3 expression corresponds with maximal nuclear AP-1 binding activity, whereas TNF-α alone does not induce maximal AP-1 activity or collagenase-3 expression. It is possible that the lung uses novel mechanisms for the local production of interstitial collagenase by resident cells.
Arachidonic acid metabolism has long been appreciated as a component of inflammatory diseases of many organs, including the lung, because of the induction of metabolites associated with the disease process. Cyclooxygenase (e.g. prostaglandins) and lipoxygenase (e.g. leukotrienes) products can affect multiple aspects of the disease process, including cell recruitment, cell proliferation, and cellular function (Goetzl et al., 1995). Increases in 5- or 15-lipoxygenase have been reported in patients with idiopathic pulmonary fibrosis (Wilborn et al., 1996) or asthma (Bradding et al., 1995), respectively. Prostaglandins (Clohisy et al., 1994) and leukotrienes (Medina et al., 1994; Rajah et al., 1997) have previously been shown to induce MMP production. Our data reveal that closely related arachidonic acid metabolites derived from 12-lipoxygenase can induce rat collagenase-3 gene expression. Although 15-lipoxygenase is prominent in human pulmonary macrophages, its function seems to be compensated for by leukocyte 12-lipoxygenase in rodents (Funk, 1996). Our data strongly suggest that the bioactive lipid activity in conditioned medium from zymosan-stimulated J774 cells is derived from 12-lipoxygenase. We speculate that TNF-α in cooperation with secreted lipoxygenase-derived arachidonic acid metabolites is responsible for the induction of fibroblast collagenase-3 gene expression that occurs in experimental silicosis. An analogous role could be performed by 15-lipoxygenase activity in the human lung, which has previously been reported to be increased in human pulmonary disease.
Our data show that activated macrophages are spatially and temporally associated with the induction of matrix-degrading metalloproteinase activity in resident fibroblasts and elaborate mediators sufficient for this induction. Fibroblast migration into granulation tissue is an early event in wound repair, regardless of whether the result is restoration of normal tissue architecture or fibrosis. This recruitment is likely mediated by numerous molecules including growth factors, chemotactic cytokines, and matrix components. However, release from the fibrillar collagen-rich matrix surrounding these cells would seem to be absolutely necessary for cell migration to initiate. An analogous situation exists in tumor evolution, where matrix degradation is necessary for tumor cell invasion and metastasis. In fact, tumor cell invasiveness can be modulated by eicosanoid-mediated collagenase expression (Leppert et al., 1995; Liu et al., 1996; Reich and Martin, 1996). Expression of interstitial collagenase by fibroblasts within granulomatous lesions may be required for their migration within developing lesions. Alternatively, this induction of rat collagenase-3 expression may represent a futile attempt by certain populations of fibroblasts to abate the fibrotic process.
ACKNOWLEDGMENTS
We thank Dr. David Koh for assistance in silica instillation and Drs. Meltem Arikan, Sarah Dunsmore, Qinglang Li, and Xueming Ye for assistance in the isolation of primary cells used in this study. We also thank Dr. William Parks for many helpful discussions and Dr. Colin Funk for assistance concerning arachidonic acid metabolism. This work was supported by National Institutes of Health grants HL-54049, HL-29594, and HL-09179.
Footnotes
Abbreviations used: ALF, adult rat lung fibroblast; AP-1, activator protein-1; ARSF, adult rat skin fibroblast; BAL, bronchoalveolar lavage; CDC, cinnamyl-3,4-dihydroxy-α-cyanocinnimate; IL-1β, interleukin-1β; MMP, matrix metalloproteinase; NDGA, nordihydroguaiaretic acid; NFκB, nuclear factor κB; NRAC, neonatal rat auricular chondrocyte; NRLF, neonatal rat lung fibroblast; NRSF, neonatal rat skin fibroblast; PBS, phosphate-buffered saline; PMA, phorbol 12-myristate 13-acetate; TNF-α, tumor necrosis factor-α.
REFERENCES
- Angel P, Baumann I, Stein B, Delius H, Rahmsdorf H-J, Herrlich P. 12-O-tetradecanoyl-phorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5′-flanking region. Mol Cell Biol. 1987;7:2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitterman PB, Adelberg S, Crystal RG. Mechanisms of pulmonary fibrosis: spontaneous release of the alveolar macrophage-derived growth factor in the interstitial lung disorders. J Clin Invest. 1983;72:1801–1813. doi: 10.1172/JCI111140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaisdell RJ, Giri SN. Mechanism of antifibrotic effect of taurine and niacin in the multidose bleomycin-hamster model of lung fibrosis: inhibition of lysyl oxidase and collagenase. J Biochem Toxicol. 1995;10:203–210. doi: 10.1002/jbt.2570100404. [DOI] [PubMed] [Google Scholar]
- Borden P, Solymar D, Sucharczuk A, Lindman B, Cannon P, Heller RA. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J Biol Chem. 1996;271:23577–23581. doi: 10.1074/jbc.271.38.23577. [DOI] [PubMed] [Google Scholar]
- Bradding P, Redington AE, Djukanovic R, Conrad DJ, Holgate ST. 15-Lipoxygenase immunoreactivity in normal and asthmatic airways. Am J Respir Crit Care Med. 1995;151:1201–1204. doi: 10.1164/ajrccm/151.4.1201. [DOI] [PubMed] [Google Scholar]
- Brenner DA, O’Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989;337:661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- Claudio E, Segade F, Wrobel K, Ramos S, Lazo P. Activation of murine macrophages by silica particles in vitro is a process independent of silica-induced cell death. Am J Respir Cell Mol Biol. 1995;13:547–554. doi: 10.1165/ajrcmb.13.5.7576690. [DOI] [PubMed] [Google Scholar]
- Clohisy JC, Connolly TJ, Bergman KD, Quinn CO, Partridge NC. Prostanoid-induced expression of matrix metalloproteinase-1 messenger ribonucleic acid in rat osteosarcoma cells. Endocrinology. 1994;135:1447–1454. doi: 10.1210/endo.135.4.7925106. [DOI] [PubMed] [Google Scholar]
- Doyle GAR, Pierce RA, Parks WC. Transcriptional induction of collagenase-1 in differentiated monocyte-like (U937) cells is regulated by AP-1 and an upstream C/BBP-β site. J Biol Chem. 1997;272:11840–11849. doi: 10.1074/jbc.272.18.11840. [DOI] [PubMed] [Google Scholar]
- Dunsmore SE, Osborne CL, Goodman RA, Rannels DE. Composition of the extracellular matrix of type II pulmonary epithelial cells in primary culture. Am J Physiol. 1995;269:L754–L765. doi: 10.1152/ajplung.1995.269.6.L754. [DOI] [PubMed] [Google Scholar]
- Fransen L, et al. Molecular cloning of mouse tumour necrosis factor cDNA and its eukaryotic expression. Nucleic Acids Res. 1985;13:4417–4429. doi: 10.1093/nar/13.12.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CD. The molecular biology of mammalian lipoxygenases and the quest for eicosanoid functions using lipoxygenase-deficient mice. Biochim Biophys Acta. 1996;1304:65–84. doi: 10.1016/s0005-2760(96)00107-5. [DOI] [PubMed] [Google Scholar]
- Gadek JE, Kelman JA, Fells G, Weinberger SE, Horwitz AL, Reynolds HY, Fulmer JD, Crystal RG. Collagenase in the lower respiratory tract of patients with idiopathic pulmonary fibrosis. N Engl J Med. 1979;301:737–742. doi: 10.1056/NEJM197910043011401. [DOI] [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloproteinase-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, An SZ, Smith WL. Specificity of expression and effects of eicosanoid mediators in normal physiology and human diseases. FASEB J. 1995;9:1051–1058. doi: 10.1096/fasebj.9.11.7649404. [DOI] [PubMed] [Google Scholar]
- Hu E, Mueller E, Oliviero S, Papaioannou VE, Johnson R, Spiegelman BM. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994;13:3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert D, Hauser SL, Kishiyama JL, An SZ, Zeng L, Goetzl EJ. Stimulation of matrix metalloproteinase-dependent migration of T cells by eicosanoids. FASEB J. 1995;9:1473–1481. doi: 10.1096/fasebj.9.14.7589989. [DOI] [PubMed] [Google Scholar]
- Liu XH, Connolly JM, Rose DP. Eicosanoids as mediators of linoleic acid-stimulated invasion and type IV collagenase production by a metastatic human breast cancer cell line. Clin Exp Metastasis. 1996;14:145–152. doi: 10.1007/BF00121211. [DOI] [PubMed] [Google Scholar]
- Mariani TJ, Crouch E, Roby JD, Starcher B, Pierce RA. Increased elastin production in experimental granulomatous lung disease. Am J Pathol. 1995;147:988–1000. [PMC free article] [PubMed] [Google Scholar]
- Mariani TJ, Roby JD, Mecham RP, Parks WC, Crouch E, Pierce RA. Localization of type I procollagen gene expression in silica-induced granulomatous lung disease and implication of transforming growth factor-β as a mediator of fibrosis. Am J Pathol. 1996;148:151–164. [PMC free article] [PubMed] [Google Scholar]
- Martinet Y, Rom WM, Grotendorst GR, Martin GR, Crystal RG. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987;317:202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- McGowan SE. Influences of endogenous and exogenous TGF-β on elastin in rat lung fibroblasts and aortic smooth muscle cells. Am J Physiol. 1992;263:L257–L263. doi: 10.1152/ajplung.1992.263.2.L257. [DOI] [PubMed] [Google Scholar]
- Medina L, Perez-Ramos J, Ramirez R, Selman M, Pardo A. Leukotriene E4 upregulates collagenase expression and synthesis in human lung fibroblasts. Biochim Biophys Acta. 1994;1224:168–174. doi: 10.1016/0167-4889(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y, Ryder K, Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988;55:907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Pardo A, Selman M, Ramirez R, Ramos C, Montano M, Stricklin G, Raghu G. Production of collagenase and tissue inhibitor of metalloproteinases by fibroblasts derived from normal and fibrotic human lungs. Chest. 1992;102:1085–1089. doi: 10.1378/chest.102.4.1085. [DOI] [PubMed] [Google Scholar]
- Pierce RA, Kolodziej ME, Parks WC. 1,25 Dihydroxyvitamin D3 represses tropoelastin expression by a posttranscriptional mechanism. J Biol Chem. 1992;267:11593–11599. [PubMed] [Google Scholar]
- Pierce RA, Mariencheck W, Sandefur S, Crouch EC, Parks WC. Glucocorticoids upregulate tropoelastin expression during late stages of fetal lung development. Am J Physiol. 1995;268:L491–L500. doi: 10.1152/ajplung.1995.268.3.L491. [DOI] [PubMed] [Google Scholar]
- Piguet PF, Collart MA, Grau GE, Sappino A-P, Vassalli P. Requirements of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990;344:245–247. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137:1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CO, Scott DK, Brinckerhoff CE, Matrisian LM, Jeffrey JJ, Partridge NC. Rat collagenase: cloning, amino acid sequence comparison and parathyroid hormone regulation in osteoblastic cells. J Biol Chem. 1990;265:13521–13527. [PubMed] [Google Scholar]
- Rajah R, Nunn SE, Herrick DJ, Grunstein MM, Cohen P. Leukotriene D4 induces MMP-1, which functions as an IGFBP protease in human airway smooth muscle cells. Am J Physiol. 1997;15:L1014–L1022. doi: 10.1152/ajplung.1996.271.6.L1014. [DOI] [PubMed] [Google Scholar]
- Reich R, Martin GR. Identification of arachidonic acid pathways required for the invasive and metastatic activity of malignant tumor cells. Prostaglandins. 1996;51:1–17. doi: 10.1016/0090-6980(95)00154-9. [DOI] [PubMed] [Google Scholar]
- Riches DWH. The Molecular and Cellular Biology of Wound Repair. R.A.F. Clark, New York: Plenum Press; 1996. Macrophage involvement in wound repair, remodeling, and fibrosis; pp. 95–141. [Google Scholar]
- Wilborn J, Bailie M, Coffey M, Burdick M, Strieter R, Peters-Goldin M. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1996;97:1827–1836. doi: 10.1172/JCI118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MJ, Wang J, Turk J, Gross RW. Depletion of intracellular calcium stores activates smooth muscle cell calcium-independent phospholipase A2. J Biol Chem. 1997;272:1522–1526. doi: 10.1074/jbc.272.3.1522. [DOI] [PubMed] [Google Scholar]