Abstract
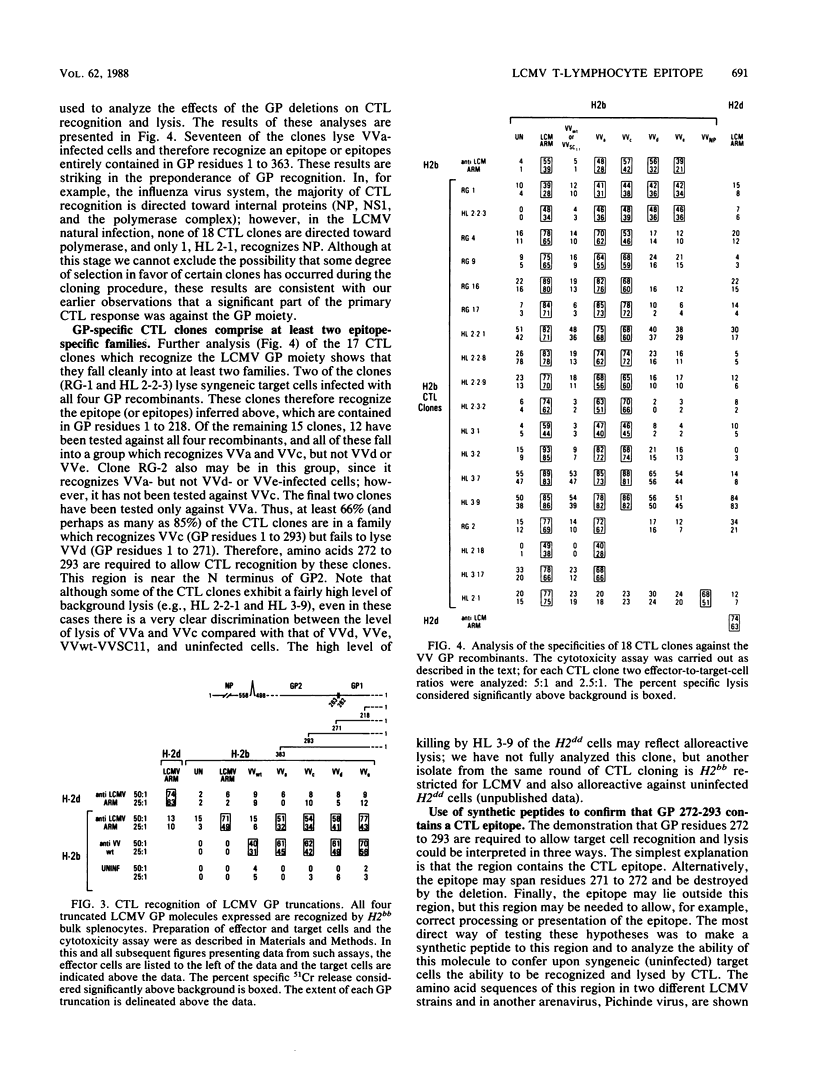
Analyses with segmental reassortants of lymphocytic choriomeningitis virus (LCMV) RNA have shown that cytotoxic T lymphocytes (CTL) are induced by and recognize proteins encoded by the viral short segment, which specifies two virus structural proteins, glycoprotein (GP) and nucleoprotein (NP). Expression of cDNA copies of these genes in vaccinia virus vectors demonstrates that C57BL/6 (H2bb) mice mount significant CTL responses to both GP and NP. We have used LCMV-specific H2bb-restricted CTL clones and a family of serial C-terminal truncations of the LCMV GP expressed in vaccinia virus to map the precise specificities of the anti-GP clones. Of the 18 CTL clones studied, 1 recognizes NP and the other 17 recognize GP. The reactivities of 14 of the 17 anti-GP CTL clones against the deleted GP molecules have been fully characterized, and two clear patterns of anti-GP activity have emerged, defining at least two CTL epitopes. The first epitope, recognized by only two of the clones, lies within GP residues 1 to 218. The second is recognized by all 12 of the remaining clones and was mapped, by using the GP deletions, to a 22-amino-acid region comprising GP residues 272 to 293. A synthetic peptide representing this area sensitized uninfected syngeneic target cells to lysis both by bulk CTL obtained from the spleen after a primary immunization and by appropriate CTL clones. Two sets of criteria are available which are said to identify potential T-cell epitopes, one based on primary amino acid sequence and the second based on protein secondary structure. Neither of these predictive schemes would have identified region 272 to 293 as a CTL recognition motif, indicating that such programs are of limited usefulness as presently conceived. Analysis of the CTL clones shows clearly that all three families (anti-NP and anti-GP 1 to 218 and 272 to 293) direct efficient cross-reactive killing against a variety of serologically distinct strains of LCMV.
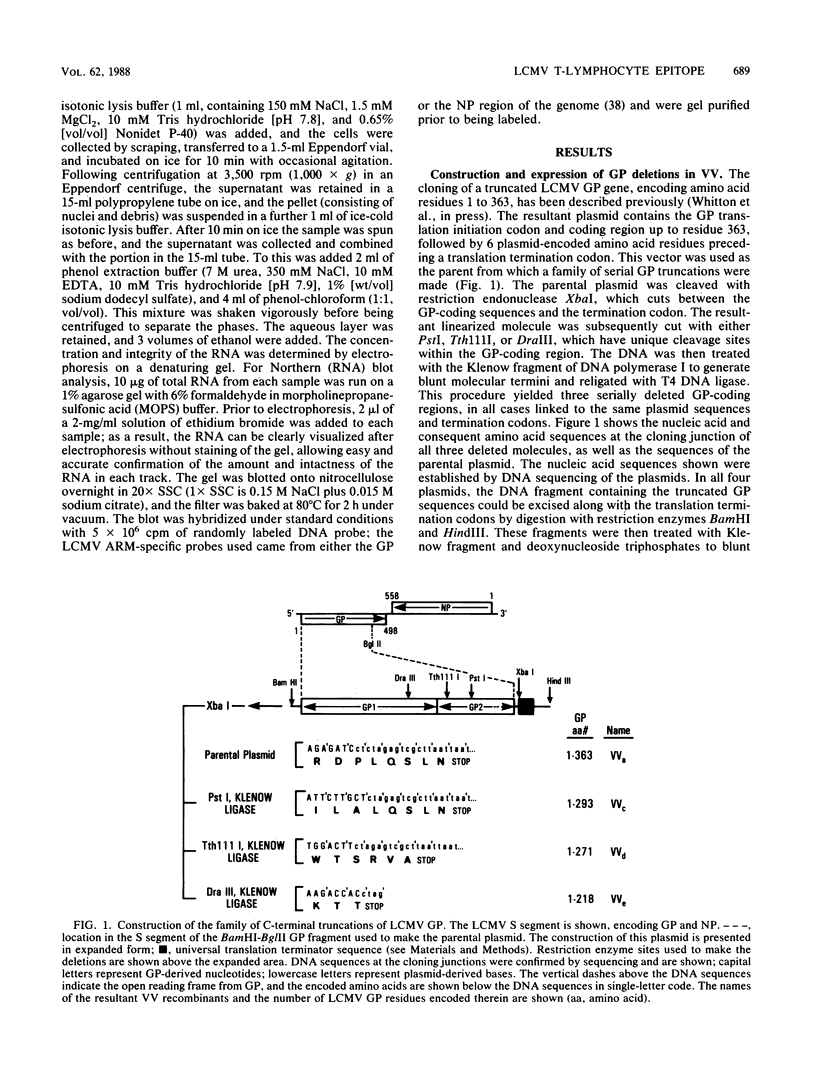
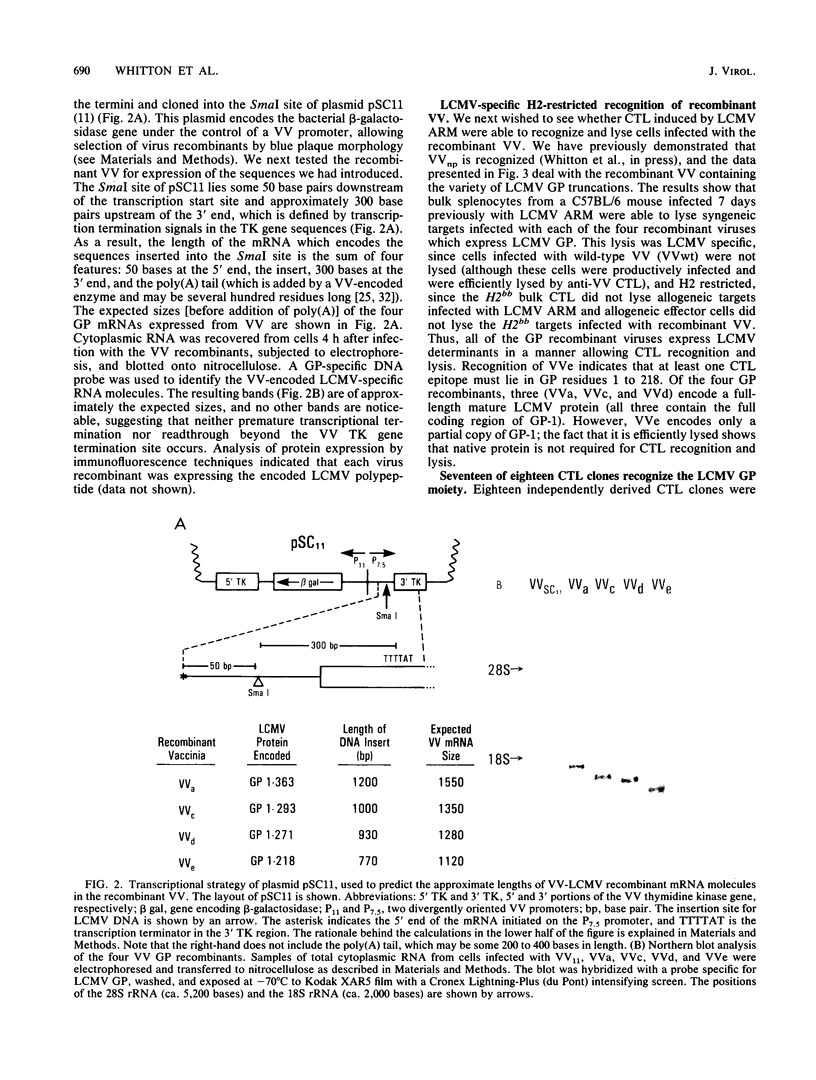
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., Byrne J. A., Oldstone M. B. Virus specificity of cytotoxic T lymphocytes generated during acute lymphocytic choriomeningitis virus infection: role of the H-2 region in determining cross-reactivity for different lymphocytic choriomeningitis virus strains. J Virol. 1984 Jul;51(1):34–41. doi: 10.1128/jvi.51.1.34-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham C. R., Openshaw P. J., Ball L. A., King A. M., Wertz G. W., Askonas B. A. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986 Dec 15;137(12):3973–3977. [PubMed] [Google Scholar]
- Bastin J., Rothbard J., Davey J., Jones I., Townsend A. Use of synthetic peptides of influenza nucleoprotein to define epitopes recognized by class I-restricted cytotoxic T lymphocytes. J Exp Med. 1987 Jun 1;165(6):1508–1523. doi: 10.1084/jem.165.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moss B. Anti-influenza virus cytotoxic T lymphocytes recognize the three viral polymerases and a nonstructural protein: responsiveness to individual viral antigens is major histocompatibility complex controlled. J Virol. 1987 Apr;61(4):1098–1102. doi: 10.1128/jvi.61.4.1098-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Auperin D. D. Arenavirus gene structure and organization. Curr Top Microbiol Immunol. 1987;133:5–17. doi: 10.1007/978-3-642-71683-6_2. [DOI] [PubMed] [Google Scholar]
- Braciale T. J., Henkel T. J., Lukacher A., Braciale V. L. Fine specificity and antigen receptor expression among influenza virus-specific cytolytic T lymphocyte clones. J Immunol. 1986 Aug 1;137(3):995–1002. [PubMed] [Google Scholar]
- Buchmeier M. J., Oldstone M. B. Protein structure of lymphocytic choriomeningitis virus: evidence for a cell-associated precursor of the virion glycopeptides. Virology. 1979 Nov;99(1):111–120. doi: 10.1016/0042-6822(79)90042-4. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Southern P. J., Parekh B. S., Wooddell M. K., Oldstone M. B. Site-specific antibodies define a cleavage site conserved among arenavirus GP-C glycoproteins. J Virol. 1987 Apr;61(4):982–985. doi: 10.1128/jvi.61.4.982-985.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Ahmed R., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus. I. Generation and recognition of virus strains and H-2b mutants. J Immunol. 1984 Jul;133(1):433–439. [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. A., Gilden D. H., Monjan A. A., Nathanson N. Lymphocytic choriomeningitis virus: pathogenesis of acute central nervous system disease. Fed Proc. 1971 Nov-Dec;30(6):1831–1841. [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983 Aug;64(Pt 8):1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- Finberg R., Benacerraf B. Induction, control and consequences of virus specific cytotoxic T cells. Immunol Rev. 1981;58:157–180. doi: 10.1111/j.1600-065x.1981.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Gilden D. H., Cole G. A., Nathanson N. Immunopathogenesis of acute central nervous system disease produced by lymphocytic choriomeningitis virus. II. Adoptive immunization of virus carriers. J Exp Med. 1972 Apr 1;135(4):874–889. doi: 10.1084/jem.135.4.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M. F., Maloy W. L., Lunde M. N., Margalit H., Cornette J. L., Smith G. L., Moss B., Miller L. H., Berzofsky J. A. Construction of synthetic immunogen: use of new T-helper epitope on malaria circumsporozoite protein. Science. 1987 Feb 27;235(4792):1059–1062. doi: 10.1126/science.2434994. [DOI] [PubMed] [Google Scholar]
- Goverman J., Hunkapiller T., Hood L. A speculative view of the multicomponent nature of T cell antigen recognition. Cell. 1986 May 23;45(4):475–484. doi: 10.1016/0092-8674(86)90279-5. [DOI] [PubMed] [Google Scholar]
- Kees U., Krammer P. H. Most influenza A virus-specific memory cytotoxic T lymphocytes react with antigenic epitopes associated with internal virus determinants. J Exp Med. 1984 Feb 1;159(2):365–377. doi: 10.1084/jem.159.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstedt C. Interaction between antigenically different cells. Virus-induced cytotoxicity by immune lymphoid cells in vitro. Acta Pathol Microbiol Scand. 1969;75(1):139–152. [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mims C. A., Blanden R. V. Antiviral action of immune lymphocytes in mice infected with lymphocytic choriomeningitis virus. Infect Immun. 1972 Nov;6(5):695–698. doi: 10.1128/iai.6.5.695-698.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Tissue injury in lymphocytic choriomeningitis viral infection: virus-induced immunologically specific release of a cytotoxic factor from immune lymphoid cells. Virology. 1970 Dec;42(4):805–813. doi: 10.1016/0042-6822(70)90330-2. [DOI] [PubMed] [Google Scholar]
- Parekh B. S., Buchmeier M. J. Proteins of lymphocytic choriomeningitis virus: antigenic topography of the viral glycoproteins. Virology. 1986 Sep;153(2):168–178. doi: 10.1016/0042-6822(86)90020-6. [DOI] [PubMed] [Google Scholar]
- Puddington L., Bevan M. J., Rose J. K., Lefrançois L. N protein is the predominant antigen recognized by vesicular stomatitis virus-specific cytotoxic T cells. J Virol. 1986 Nov;60(2):708–717. doi: 10.1128/jvi.60.2.708-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere Y., Ahmed R., Southern P. J., Buchmeier M. J., Dutko F. J., Oldstone M. B. The S RNA segment of lymphocytic choriomeningitis virus codes for the nucleoprotein and glycoproteins 1 and 2. J Virol. 1985 Mar;53(3):966–968. doi: 10.1128/jvi.53.3.966-968.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riviere Y., Southern P. J., Ahmed R., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus. V. Recognition is restricted to gene products encoded by the viral S RNA segment. J Immunol. 1986 Jan;136(1):304–307. [PubMed] [Google Scholar]
- Rohrmann G., Yuen L., Moss B. Transcription of vaccinia virus early genes by enzymes isolated from vaccinia virions terminates downstream of a regulatory sequence. Cell. 1986 Sep 26;46(7):1029–1035. doi: 10.1016/0092-8674(86)90702-6. [DOI] [PubMed] [Google Scholar]
- Romanowski V., Matsuura Y., Bishop D. H. Complete sequence of the S RNA of lymphocytic choriomeningitis virus (WE strain) compared to that of Pichinde arenavirus. Virus Res. 1985 Sep;3(2):101–114. doi: 10.1016/0168-1702(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. Antigen presentation: fugue in T-lymphocyte recognition. Nature. 1987 Apr 23;326(6115):738–739. doi: 10.1038/326738a0. [DOI] [PubMed] [Google Scholar]
- Singh M. K., Fuller-Pace F. V., Buchmeier M. J., Southern P. J. Analysis of the genomic L RNA segment from lymphocytic choriomeningitis virus. Virology. 1987 Dec;161(2):448–456. doi: 10.1016/0042-6822(87)90138-3. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Bishop D. H. Sequence comparison among arenaviruses. Curr Top Microbiol Immunol. 1987;133:19–39. doi: 10.1007/978-3-642-71683-6_3. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Blount P., Oldstone M. B. Analysis of persistent virus infections by in situ hybridization to whole-mouse sections. Nature. 1984 Dec 6;312(5994):555–558. doi: 10.1038/312555a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Singh M. K., Riviere Y., Jacoby D. R., Buchmeier M. J., Oldstone M. B. Molecular characterization of the genomic S RNA segment from lymphocytic choriomeningitis virus. Virology. 1987 Mar;157(1):145–155. doi: 10.1016/0042-6822(87)90323-0. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Bastin J., Gould K., Brownlee G. G. Cytotoxic T lymphocytes recognize influenza haemagglutinin that lacks a signal sequence. Nature. 1986 Dec 11;324(6097):575–577. doi: 10.1038/324575a0. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Gotch F. M., Davey J. Cytotoxic T cells recognize fragments of the influenza nucleoprotein. Cell. 1985 Sep;42(2):457–467. doi: 10.1016/0092-8674(85)90103-5. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Allen P. M. The basis for the immunoregulatory role of macrophages and other accessory cells. Science. 1987 May 1;236(4801):551–557. doi: 10.1126/science.2437650. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Mackett M., Lefrancois L., Lyles D. S., Moss B. Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986 Jun 1;163(6):1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974 Apr 19;248(5450):701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]



