Abstract
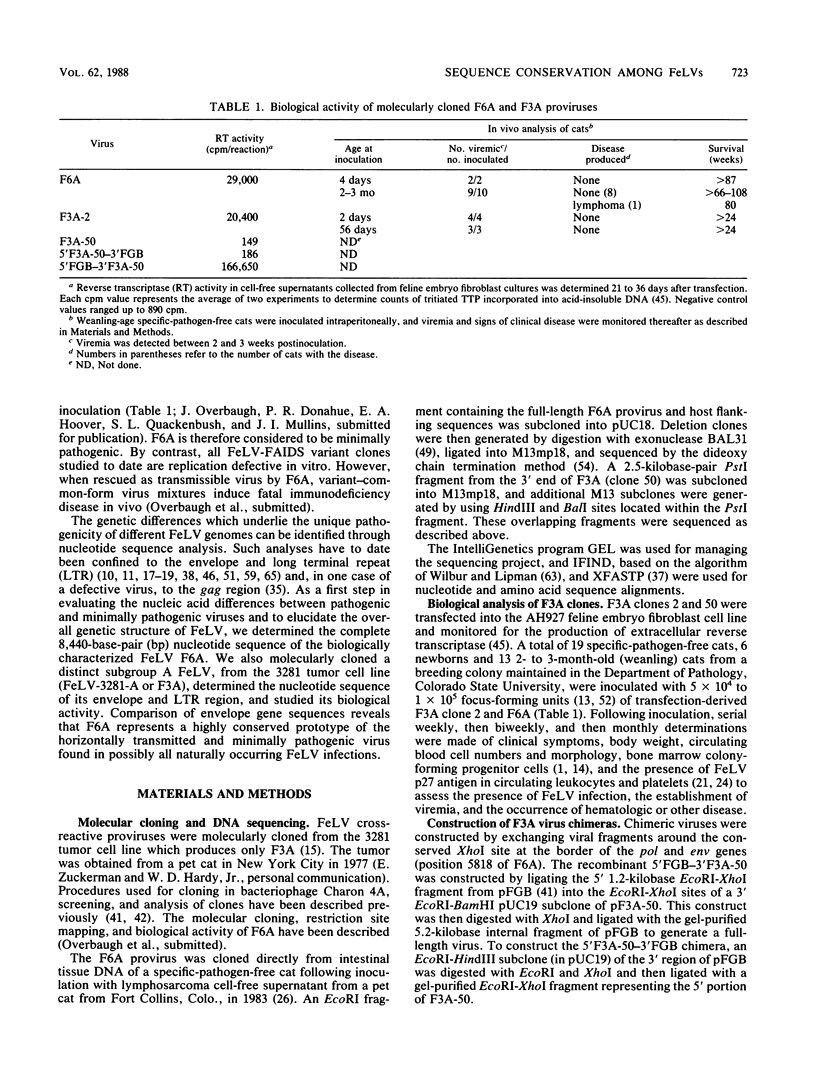
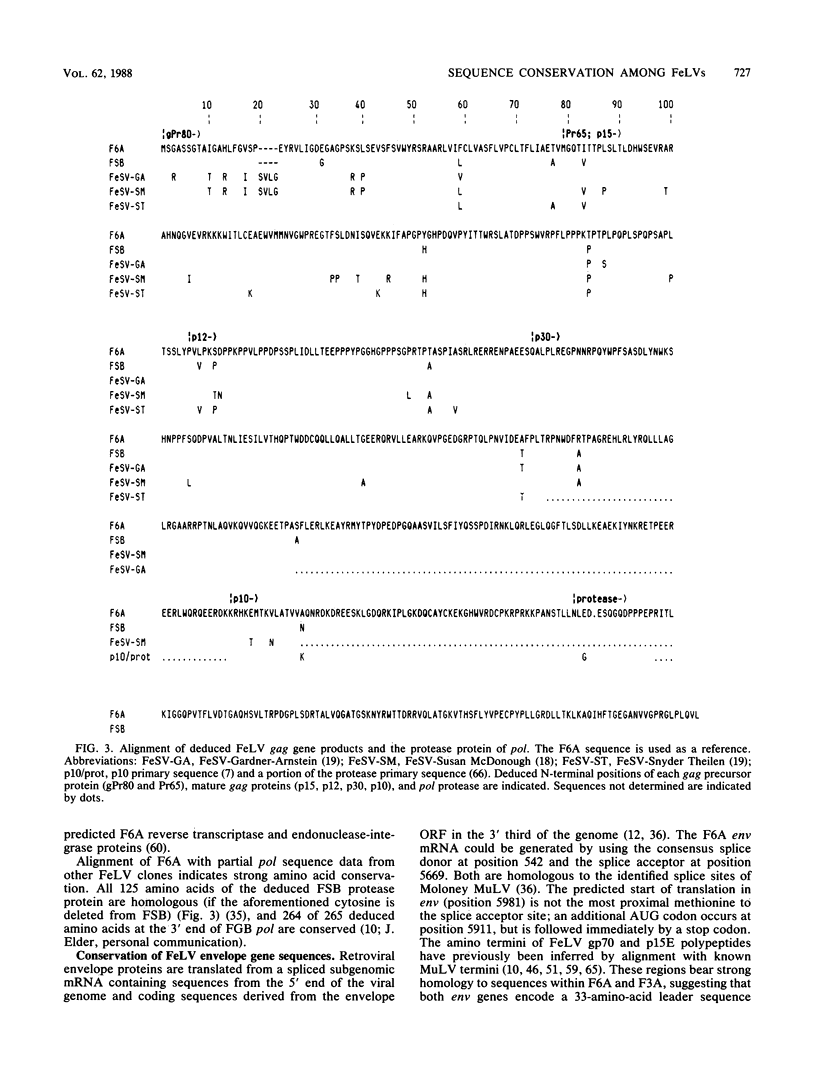
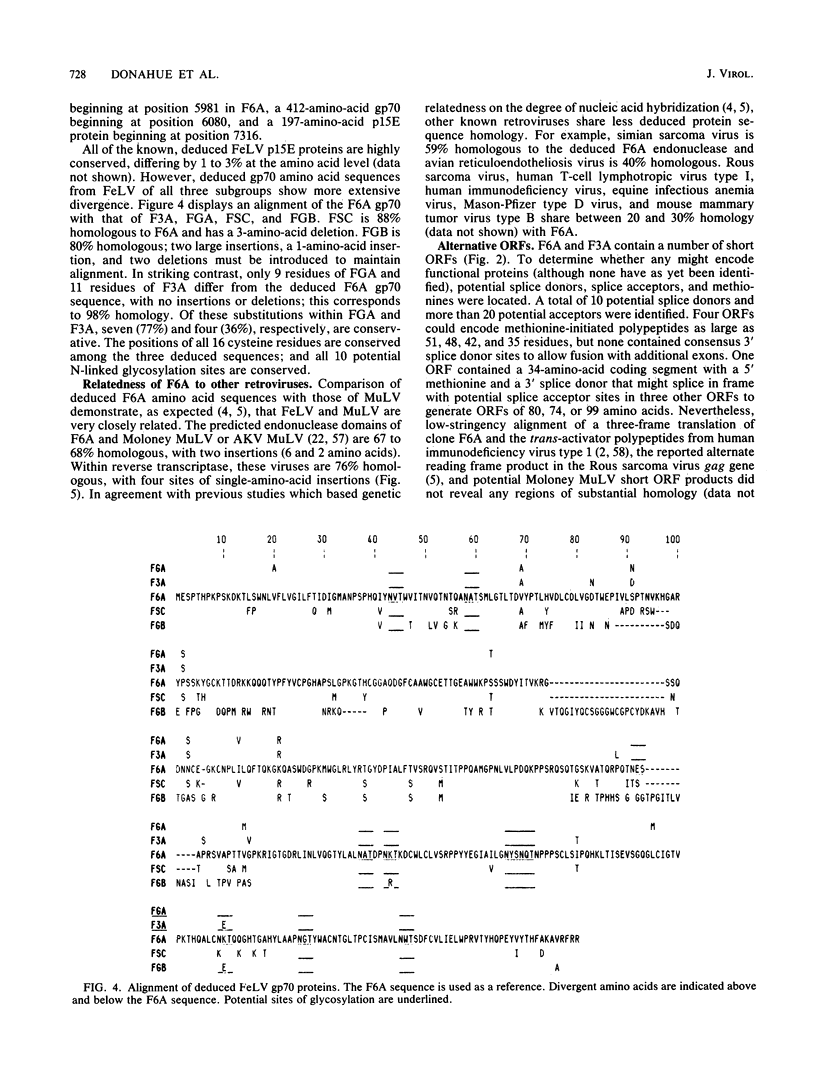
We report the first complete nucleotide sequence (8,440 base pairs) of a biologically active feline leukemia virus (FeLV), designated FeLV-61E (or F6A), and the molecular cloning, biological activity, and env-long terminal repeat (LTR) sequence of another FeLV isolate, FeLV-3281 (or F3A). F6A corresponds to the non-disease-specific common-form component of the immunodeficiency disease-inducing strain of FeLV, FeLV-FAIDS, and was isolated from tissue DNA of a cat following experimental transmission of naturally occurring feline acquired immunodeficiency syndrome. F3A clones were derived from a subgroup-A-virus-producing feline tumor cell line. Both are unusual relative to other molecularly cloned FeLVs studied to date in their ability to induce viremia in weanling (8-week-old) cats and in their failure to induce acute disease. The F6A provirus is organized into 5'-LTR-gag-pol-env-LTR-3' regions; the gag and pol open reading frames are separated by an amber codon, and env is in a different reading frame. The deduced extracellular glycoproteins of F6A, F3A, and the Glasgow-1 subgroup A isolate of FeLV (M. Stewart, M. Warnock, A. Wheeler, N. Wilkie, J. Mullins, D. Onions, and J. Neil, J. Virol. 58:825-834, 1986) are 98% homologous, despite having been isolated from naturally infected cats 6 to 13 years apart and from widely different geographic locations. As a group, their envelope gene sequences differ markedly from those of the disease-associated subgroup B and acutely pathogenic subgroup C viruses. Thus, F6A and F3A correspond to members of a highly conserved family and represent prototypes of the horizontally transmitted, minimally pathogenic FeLV present in all naturally occurring infections.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abkowitz J. L., Ott R. L., Nakamura J. M., Steinmann L., Fialkow P. J., Adamson J. W. Feline glucose-6-phosphate dehydrogenase cellular mosaicism. Application to the study of retrovirus-induced pure red cell aplasia. J Clin Invest. 1985 Jan;75(1):133–140. doi: 10.1172/JCI111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya S. K., Guo C., Josephs S. F., Wong-Staal F. Trans-activator gene of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Jul 5;229(4708):69–73. doi: 10.1126/science.2990040. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Sherr C. J., Todaro G. J. Evolution of type C viral genes: origin of feline leukemia virus. Science. 1975 Nov 28;190(4217):886–888. doi: 10.1126/science.52892. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of C-type viral genes: inheritance of exogenously acquired viral genes. Nature. 1974 Dec 6;252(5483):456–459. doi: 10.1038/252456a0. [DOI] [PubMed] [Google Scholar]
- Broome S., Gilbert W. Rous sarcoma virus encodes a transcriptional activator. Cell. 1985 Mar;40(3):537–546. doi: 10.1016/0092-8674(85)90202-8. [DOI] [PubMed] [Google Scholar]
- Copeland T. D., Gerard G. F., Hixson C. W., Oroszlan S. Amino- and carboxyl-terminal sequence of Moloney murine leukemia virus reverse transcriptase. Virology. 1985 Jun;143(2):676–679. doi: 10.1016/0042-6822(85)90411-8. [DOI] [PubMed] [Google Scholar]
- Copeland T. D., Morgan M. A., Oroszlan S. Complete amino acid sequence of the basic nucleic acid binding protein of feline leukemia virus. Virology. 1984 Feb;133(1):137–145. doi: 10.1016/0042-6822(84)90432-x. [DOI] [PubMed] [Google Scholar]
- Dorner A. J., Coffin J. M. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell. 1986 May 9;45(3):365–374. doi: 10.1016/0092-8674(86)90322-3. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Mullins J. I. Nucleotide sequence of the envelope gene of Gardner-Arnstein feline leukemia virus B reveals unique sequence homologies with a murine mink cell focus-forming virus. J Virol. 1983 Jun;46(3):871–880. doi: 10.1128/jvi.46.3.871-880.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficht T. A., Chang L. J., Stoltzfus C. M. Avian sarcoma virus gag and env gene structural protein precursors contain a common amino-terminal sequence. Proc Natl Acad Sci U S A. 1984 Jan;81(2):362–366. doi: 10.1073/pnas.81.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Blevins C. S., Nomura S. Simple, quantitative assay for both xenotropic murine leukemia and ecotropic feline leukemia viruses. J Virol. 1974 Jul;14(1):177–179. doi: 10.1128/jvi.14.1.177-179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase A. T. Pathogenesis of lentivirus infections. Nature. 1986 Jul 10;322(6075):130–136. doi: 10.1038/322130a0. [DOI] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Even J., Sherr C. J., Galibert F. Nucleotide sequences of feline sarcoma virus long terminal repeats and 5' leaders show extensive homology to those of other mammalian retroviruses. J Virol. 1983 Jan;45(1):466–472. doi: 10.1128/jvi.45.1.466-472.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe A., Gobet M., Sherr C. J., Galibert F. Nucleotide sequence of the feline retroviral oncogene v-fms shows unexpected homology with oncogenes encoding tyrosine-specific protein kinases. Proc Natl Acad Sci U S A. 1984 Jan;81(1):85–89. doi: 10.1073/pnas.81.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe A., Laprevotte I., Galibert F., Fedele L. A., Sherr C. J. Nucleotide sequences of feline retroviral oncogenes (v-fes) provide evidence for a family of tyrosine-specific protein kinase genes. Cell. 1982 Oct;30(3):775–785. doi: 10.1016/0092-8674(82)90282-3. [DOI] [PubMed] [Google Scholar]
- Hardy W. D., Jr, Hirshaut Y., Hess P. Detection of the feline leukemia virus and other mammalian oncornaviruses by immunofluorescence. Bibl Haematol. 1973;39:778–799. doi: 10.1159/000427906. [DOI] [PubMed] [Google Scholar]
- Herr W. Nucleotide sequence of AKV murine leukemia virus. J Virol. 1984 Feb;49(2):471–478. doi: 10.1128/jvi.49.2.471-478.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover E. A., Kociba G. J., Hardy W. D., Jr, Yohn D. S. Erythroid hypoplasia in cats inoculated with feline leukemia virus. J Natl Cancer Inst. 1974 Nov;53(5):1271–1276. doi: 10.1093/jnci/53.5.1271. [DOI] [PubMed] [Google Scholar]
- Hoover E. A., Mathes L. E., Rojko J. L., Schaller J. P., Olsen R. G. Modifications of the immunofluorescence assay for feline leukemia virus group-specific antigens. Am J Vet Res. 1978 Dec;39(12):1877–1880. [PubMed] [Google Scholar]
- Hoover E. A., Mullins J. I., Quackenbush S. L., Gasper P. W. Experimental transmission and pathogenesis of immunodeficiency syndrome in cats. Blood. 1987 Dec;70(6):1880–1892. [PubMed] [Google Scholar]
- Jarrett O., Golder M. C., Toth S., Onions D. E., Stewart M. F. Interaction between feline leukaemia virus subgroups in the pathogenesis of erythroid hypoplasia. Int J Cancer. 1984 Aug 15;34(2):283–288. doi: 10.1002/ijc.2910340222. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Hardy W. D., Jr, Golder M. C., Hay D. The frequency of occurrence of feline leukaemia virus subgroups in cats. Int J Cancer. 1978 Mar 15;21(3):334–337. doi: 10.1002/ijc.2910210314. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Laird H. M., Hay D. Determinants of the host range of feline leukaemia viruses. J Gen Virol. 1973 Aug;20(2):169–175. doi: 10.1099/0022-1317-20-2-169. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Laird H. M., Hay D. Restricted host range of a feline leukaemia virus. Nature. 1972 Jul 28;238(5361):220–221. doi: 10.1038/238220a0. [DOI] [PubMed] [Google Scholar]
- Jarrett O., Russell P. H. Differential growth and transmission in cats of feline leukaemia viruses of subgroups A and B. Int J Cancer. 1978 Apr 15;21(4):466–472. doi: 10.1002/ijc.2910210411. [DOI] [PubMed] [Google Scholar]
- Johnson M. S., McClure M. A., Feng D. F., Gray J., Doolittle R. F. Computer analysis of retroviral pol genes: assignment of enzymatic functions to specific sequences and homologies with nonviral enzymes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7648–7652. doi: 10.1073/pnas.83.20.7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Stephenson J. R. Feline leukemia virus: biochemical and immunological characterization of gag gene-coded structural proteins. J Virol. 1977 Sep;23(3):599–607. doi: 10.1128/jvi.23.3.599-607.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprevotte I., Hampe A., Sherr C. J., Galibert F. Nucleotide sequence of the gag gene and gag-pol junction of feline leukemia virus. J Virol. 1984 Jun;50(3):884–894. doi: 10.1128/jvi.50.3.884-894.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo P. A., Prasad V., Tsichlis P. N. Splice acceptor site for the env message of Moloney murine leukemia virus. J Virol. 1987 Jun;61(6):2038–2041. doi: 10.1128/jvi.61.6.2038-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Modrow S., Hahn B. H., Shaw G. M., Gallo R. C., Wong-Staal F., Wolf H. Computer-assisted analysis of envelope protein sequences of seven human immunodeficiency virus isolates: prediction of antigenic epitopes in conserved and variable regions. J Virol. 1987 Feb;61(2):570–578. doi: 10.1128/jvi.61.2.570-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Burck K. B., Davidson N. Sequence arrangement and biological activity of cloned feline leukemia virus proviruses from a virus-productive human cell line. J Virol. 1981 May;38(2):688–703. doi: 10.1128/jvi.38.2.688-703.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Casey J. W., Nicolson M. O., Davidson N. Sequence organization of feline leukemia virus DNA in infected cells. Nucleic Acids Res. 1980 Aug 11;8(15):3287–3305. doi: 10.1093/nar/8.15.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. I., Chen C. S., Hoover E. A. Disease-specific and tissue-specific production of unintegrated feline leukaemia virus variant DNA in feline AIDS. Nature. 1986 Jan 23;319(6051):333–336. doi: 10.1038/319333a0. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Smart J. E., Hayman M. J., Jarrett O. Polypeptides of feline leukemia virus: a glycosylated gag-related protein is released into culture fluids. Virology. 1980 Aug;105(1):250–253. doi: 10.1016/0042-6822(80)90173-7. [DOI] [PubMed] [Google Scholar]
- Nicolson M. O., Hariri F., Krempin H. M., McAllister R. M., Gilden R. V. Infectious proviral DNA in human cells infected with transformation-defective type C viruses. Virology. 1976 Apr;70(2):301–312. doi: 10.1016/0042-6822(76)90273-7. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Williams M. E., Innis M. A. Nucleotide sequences of the envelope genes of two isolates of feline leukemia virus subgroup B. J Virol. 1984 Feb;49(2):629–632. doi: 10.1128/jvi.49.2.629-632.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Henderson L. E., Stephenson J. R., Copeland T. D., Long C. W., Ihle J. N., Gilden R. V. Amino- and carboxyl-terminal amino acid sequences of proteins coded by gag gene of murine leukemia virus. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1404–1408. doi: 10.1073/pnas.75.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho H. M., Gallo R. C. Characterization of reverse transcriptase from filine leukemia virus by radioimmunoassay. Virology. 1979 Nov;99(1):192–196. doi: 10.1016/0042-6822(79)90054-0. [DOI] [PubMed] [Google Scholar]
- Riedel N., Hoover E. A., Gasper P. W., Nicolson M. O., Mullins J. I. Molecular analysis and pathogenesis of the feline aplastic anemia retrovirus, feline leukemia virus C-Sarma. J Virol. 1986 Oct;60(1):242–250. doi: 10.1128/jvi.60.1.242-250.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. H., Jarrett O. An improved assay for feline leukaemia virus pseudotypes of murine sarcoma virus. J Gen Virol. 1976 Apr;31(1):139–143. doi: 10.1099/0022-1317-31-1-139. [DOI] [PubMed] [Google Scholar]
- Russell P. H., Jarrett O. The specificity of neutralizing antibodies to feline leukaemia viruses. Int J Cancer. 1978 Jun 15;21(6):768–778. doi: 10.1002/ijc.2910210615. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma P. S., Log T. Subgroup classification of feline leukemia and sarcoma viruses by viral interference and neutralization tests. Virology. 1973 Jul;54(1):160–169. doi: 10.1016/0042-6822(73)90125-6. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Sodroski J. G., Rosen C. A., Haseltine W. A. Trans-acting transcriptional activation of the long terminal repeat of human T lymphotropic viruses in infected cells. Science. 1984 Jul 27;225(4660):381–385. doi: 10.1126/science.6330891. [DOI] [PubMed] [Google Scholar]
- Stewart M. A., Warnock M., Wheeler A., Wilkie N., Mullins J. I., Onions D. E., Neil J. C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. J Virol. 1986 Jun;58(3):825–834. doi: 10.1128/jvi.58.3.825-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanese N., Roth M. J., Goff S. P. Analysis of retroviral pol gene products with antisera raised against fusion proteins produced in Escherichia coli. J Virol. 1986 Aug;59(2):328–340. doi: 10.1128/jvi.59.2.328-340.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Rutledge R. A., Dias S., Folks T., Theodore T., Buckler C. E., Martin M. A. Identification of conserved and divergent domains within the envelope gene of the acquired immunodeficiency syndrome retrovirus. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5038–5042. doi: 10.1073/pnas.83.14.5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wünsch M., Schulz A. S., Koch W., Friedrich R., Hunsmann G. Sequence analysis of Gardner-Arnstein feline leukaemia virus envelope gene reveals common structural properties of mammalian retroviral envelope genes. EMBO J. 1983;2(12):2239–2246. doi: 10.1002/j.1460-2075.1983.tb01729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Murine leukemia virus protease is encoded by the gag-pol gene and is synthesized through suppression of an amber termination codon. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1618–1622. doi: 10.1073/pnas.82.6.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaka Y., Katoh I., Copeland T. D., Oroszlan S. Translational readthrough of an amber termination codon during synthesis of feline leukemia virus protease. J Virol. 1985 Sep;55(3):870–873. doi: 10.1128/jvi.55.3.870-873.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


