Abstract
Herpes simplex virus type 1 (HSV-1) DNA and RNA have been detected in peripheral nervous system (PNS) and central nervous system (CNS) tissues of latently infected mice. However, explant methods are successful in reactivating HSV-1 only from latently infected PNS tissues. In this report, latent herpesvirus infections in mouse PNS and CNS tissues were compared by in situ hybridization to determine whether the difference in reactivation was at the level of the virus or the host tissue. It was demonstrated that the HSV-1 transcripts present during latency in the mouse PNS and CNS originated from the same region of the genome, the repeats which bracket the long unique sequence. Therefore, the difference in reactivation with PNS and CNS tissues cannot be accounted for by differences in the extent of the HSV-1 genome transcribed during herpesvirus latency. Latent HSV-1 RNA was detected in the trigeminal ganglia (PNS) and the trigeminal system in the CNS from the mesencephalon to the spinal cord as well as other regions of the CNS not noted previously. Latent HSV-1 RNA was found predominantly in neurons but also in a small number of cells which could not be identified as neuronal cells. It is suggested that host differences in CNS and PNS tissues, rather than differences in latent virus transcription, may be important determinants in the HSV-1 reactivation process in explanted tissues.
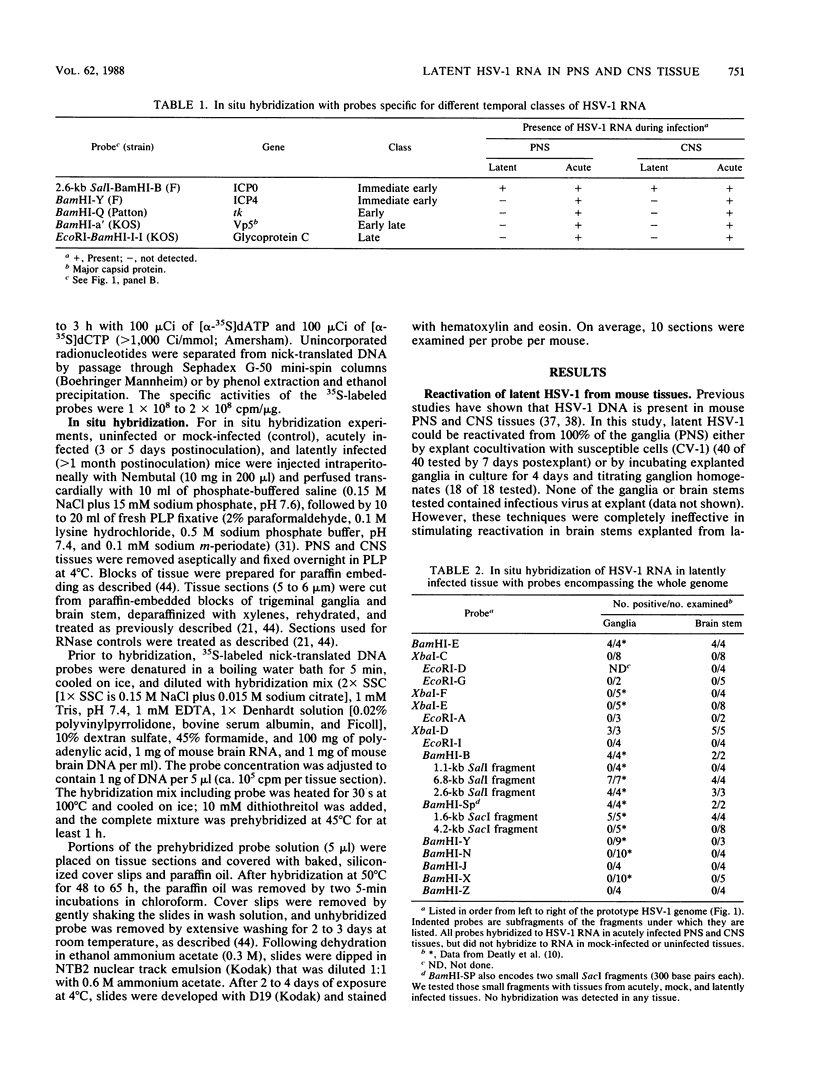
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R., Griffith J. F. Experimental herpes simplex encephalitis: early neuropathologic changes. J Neuropathol Exp Neurol. 1970 Jan;29(1):89–104. doi: 10.1097/00005072-197001000-00007. [DOI] [PubMed] [Google Scholar]
- Baringer J. R. Herpes simplex virus infection of nervous tissue in animals and man. Prog Med Virol. 1975;20:1–26. [PubMed] [Google Scholar]
- Benfey M., Aguayo A. J. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature. 1982 Mar 11;296(5853):150–152. doi: 10.1038/296150a0. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V., Wohlenberg C., Openshaw H., Rey-Mendez M., Puga A., Notkins A. L. Herpes simplex virus DNA sequences in the CNS of latently infected mice. Nature. 1980 Nov 20;288(5788):288–290. doi: 10.1038/288288a0. [DOI] [PubMed] [Google Scholar]
- Challberg M. D. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. B., McLauchlan J., McGeoch D. J. Orientation of herpes simplex virus type 1 immediate early mRNA's. Nucleic Acids Res. 1979 Sep 11;7(1):77–91. doi: 10.1093/nar/7.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Bastone V. B., Stevens J. G. Evidence that neurons harbor latent herpes simplex virus. Infect Immun. 1974 May;9(5):946–951. doi: 10.1128/iai.9.5.946-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Latent herpetic infections following experimental viraemia. J Gen Virol. 1976 Apr;31(1):75–80. doi: 10.1099/0022-1317-31-1-75. [DOI] [PubMed] [Google Scholar]
- Costa R. H., Devi B. G., Anderson K. P., Gaylord B. H., Wagner E. K. Characterization of a major late herpes simplex virus type 1 mRNA. J Virol. 1981 May;38(2):483–496. doi: 10.1128/jvi.38.2.483-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatly A. M., Spivack J. G., Lavi E., Fraser N. W. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987 May;84(10):3204–3208. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou S., Minson A. C., Field H. J., Anderson J. R., Wildy P. Detection of herpes simplex virus-specific DNA sequences in latently infected mice and in humans. J Virol. 1986 Feb;57(2):446–455. doi: 10.1128/jvi.57.2.446-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L. W., Madden M. J., Schiop-Stanley P., Vande Woude G. F. Cloning of herpes simplex type 1 DNA fragments in a bacteriophage lambda vector. Science. 1979 Feb 9;203(4380):541–544. doi: 10.1126/science.216076. [DOI] [PubMed] [Google Scholar]
- Fraser N. W., Lawrence W. C., Wroblewska Z., Gilden D. H., Koprowski H. Herpes simplex type 1 DNA in human brain tissue. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6461–6465. doi: 10.1073/pnas.78.10.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser N., Ziff E. RNA structures near poly(A) of adenovirus-2 late messenger RNAs. J Mol Biol. 1978 Sep 5;124(1):27–31. doi: 10.1016/0022-2836(78)90145-6. [DOI] [PubMed] [Google Scholar]
- Frink R. J., Anderson K. P., Wagner E. K. Herpes simplex virus type 1 HindIII fragment L encodes spliced and complementary mRNA species. J Virol. 1981 Aug;39(2):559–572. doi: 10.1128/jvi.39.2.559-572.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C., Shevchuk M., McDougall J. K. Detection of herpes simplex RNA in human sensory ganglia. Virology. 1979 May;95(1):265–268. doi: 10.1016/0042-6822(79)90429-x. [DOI] [PubMed] [Google Scholar]
- Goldin A. L., Sandri-Goldin R. M., Levine M., Glorioso J. C. Cloning of herpes simplex virus type 1 sequences representing the whole genome. J Virol. 1981 Apr;38(1):50–58. doi: 10.1128/jvi.38.1.50-58.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstein B. The nerve cell body response to axotomy. Exp Neurol. 1975 Sep;48(3 Pt 2):32–51. doi: 10.1016/0014-4886(75)90170-3. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. I. VIRUS PATHWAYS TO THE NERVOUS SYSTEM OF SUCKLING MICE DEMONSTRATED BY FLUORESCENT ANTIBODY STAINING. J Exp Med. 1964 Feb 1;119:343–356. doi: 10.1084/jem.119.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J. Effect of immune serum on the establishment of herpes simplex virus infection in trigeminal ganglia of hairless mice. J Gen Virol. 1980 Aug;49(2):401–405. doi: 10.1099/0022-1317-49-2-401. [DOI] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Latent herpes simplex virus in the central nervous system of rabbits and mice. J Exp Med. 1973 Sep 1;138(3):740–744. doi: 10.1084/jem.138.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo C. W. Localization of low abundance DNA sequences in tissue sections by in situ hybridization. J Cell Sci. 1986 Mar;81:143–162. doi: 10.1242/jcs.81.1.143. [DOI] [PubMed] [Google Scholar]
- MacLean A. R., Brown S. M. A herpes simplex virus type 1 variant which fails to synthesize immediate early polypeptide VmwIE63. J Gen Virol. 1987 May;68(Pt 5):1339–1350. doi: 10.1099/0022-1317-68-5-1339. [DOI] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Regulation of herpesvirus macromolecular synthesis: transcription-initiation sites and domains of alpha genes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7122–7126. doi: 10.1073/pnas.77.12.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- McLennan J. L., Darby G. Herpes simplex virus latency: the cellular location of virus in dorsal root ganglia and the fate of the infected cell following virus activation. J Gen Virol. 1980 Dec;51(Pt 2):233–243. doi: 10.1099/0022-1317-51-2-233. [DOI] [PubMed] [Google Scholar]
- Plummer G., Hollingsworth D. C., Phuangsab A., Bowling C. P. Chronic infections by herpes simplex viruses and by the horse and cat herpesviruses. Infect Immun. 1970 Apr;1(4):351–355. doi: 10.1128/iai.1.4.351-355.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Conley A. J., Mocarski E. S., Roizman B. Cloning of reiterated and nonreiterated herpes simplex virus 1 sequences as BamHI fragments. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4201–4205. doi: 10.1073/pnas.77.7.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A., Notkins A. L. Continued expression of a poly(A)+ transcript of herpes simplex virus type 1 in trigeminal ganglia of latently infected mice. J Virol. 1987 May;61(5):1700–1703. doi: 10.1128/jvi.61.5.1700-1703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A., Rosenthal J. D., Openshaw H., Notkins A. L. Herpes simplex virus DNA and mRNA sequences in acutely and chronically infected trigeminal ganglia of mice. Virology. 1978 Aug;89(1):102–111. doi: 10.1016/0042-6822(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983 Apr 7;302(5908):523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Latent herpes simplex virus type 1 DNA contains two copies of the virion DNA joint region. J Virol. 1985 Sep;55(3):849–852. doi: 10.1128/jvi.55.3.849-852.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Fraser N. W. Detection of herpes simplex virus type 1 transcripts during latent infection in mice. J Virol. 1987 Dec;61(12):3841–3847. doi: 10.1128/jvi.61.12.3841-3847.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., O'Boyle D. R., 2nd, Fraser N. W. Novobiocin and coumermycin A1 inhibit viral replication and the reactivation of herpes simplex virus type 1 from the trigeminal ganglia of latently infected mice. J Virol. 1987 Oct;61(10):3288–3291. doi: 10.1128/jvi.61.10.3288-3291.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Prusoff W. H., Tritton T. R. Dissociation of the inhibitory effects of 2-deoxy-D-glucose on Vero cell growth and the replication of herpes simplex virus. Antimicrob Agents Chemother. 1982 Aug;22(2):284–288. doi: 10.1128/aac.22.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Wagner E. K., Devi-Rao G. B., Cook M. L., Feldman L. T. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987 Feb 27;235(4792):1056–1059. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Stroop W. G., Rock D. L., Fraser N. W. Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest. 1984 Jul;51(1):27–38. [PubMed] [Google Scholar]
- Tenser R. B., Dawson M., Ressel S. J., Dunstan M. E. Detection of herpes simplex virus mRNA in latently infected trigeminal ganglion neurons by in situ hybridization. Ann Neurol. 1982 Mar;11(3):285–291. doi: 10.1002/ana.410110309. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Hsiung G. D. Pathogenesis of latent herpes simplex virus infection of the trigeminal ganglion in guinea pigs: effects of age, passive immunization, and hydrocortisone. Infect Immun. 1977 Apr;16(1):69–74. doi: 10.1128/iai.16.1.69-74.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullo A. B., Shimeld C., Blyth W. A., Hill T. J., Easty D. L. Spread of virus and distribution of latent infection following ocular herpes simplex in the non-immune and immune mouse. J Gen Virol. 1982 Nov;63(Pt 1):95–101. doi: 10.1099/0022-1317-63-1-95. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Notkins A. L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974 Jun 14;184(4142):1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Brown S. M., Wroblewska Z., Gilden D., Koprowski H., Subak-Sharpe J. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med. 1978 May 11;298(19):1068–1069. doi: 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Devlin M., Gilden D. H., Wroblewska Z., Brown S. M., Subak-Sharpe J., Koprowski H. Isolation of Herpes simplex virus from human trigeminal ganglia, including ganglia from one patient with multiple sclerosis. Lancet. 1977 Sep 24;2(8039):637–639. doi: 10.1016/s0140-6736(77)92501-6. [DOI] [PubMed] [Google Scholar]