Abstract
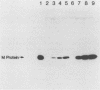
Initial attempts to clone the matrix (M) gene of vesicular stomatitis virus (VSV) in a vaccinia virus expression vector failed, apparently because the expressed M protein, and particularly a carboxy-terminus-distal two-thirds fragment, was lethal for the virus recombinant. Therefore, a transient eucaryotic expression system was used in which a cDNA clone of the VSV M protein mRNA was inserted into a region of plasmid pTF7 flanked by the promoter and terminator sequences for the T7 bacteriophage RNA polymerase. When CV-1 cells infected with recombinant vaccinia virus vTF1-6,2 expressing the T7 RNA polymerase were transfected with pTF7-M3, the cells produced considerable amounts of M protein reactive by Western blot (immunoblot) analysis with monoclonal antibodies directed to VSV M protein. Evidence for biological activity of the plasmid-expressed wild-type M protein was provided by marker rescue of the M gene temperature-sensitive mutant tsO23(III) at the restrictive temperature. Somewhat higher levels of M protein expression were obtained in CV-1 cells coinfected with a vaccinia virus-M gene recombinant under control of the T7 polymerase promoter along with T7 polymerase-expressing vaccinia virus vTF1-6,2.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barenholz Y., Moore N. F., Wagner R. R. Enveloped viruses as model membrane systems: microviscosity of vesicular stomatitis virus and host cell membranes. Biochemistry. 1976 Aug 10;15(16):3563–3570. doi: 10.1021/bi00661a026. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Repik P., Obijeski J. F., Moore N. F., Wagner R. R. Restitution of infectivity to spikeless vesicular stomatitis virus by solubilized viral components. J Virol. 1975 Jul;16(1):75–84. doi: 10.1128/jvi.16.1.75-84.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Cochran M. A., Mackett M., Moss B. Eukaryotic transient expression system dependent on transcription factors and regulatory DNA sequences of vaccinia virus. Proc Natl Acad Sci U S A. 1985 Jan;82(1):19–23. doi: 10.1073/pnas.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P., Thornton G. B., Luk D., Banerjee A. K. Purified matrix protein of vesicular stomatitis virus blocks viral transcription in vitro. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7137–7141. doi: 10.1073/pnas.79.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Earl P. L., Moss B. Use of a hybrid vaccinia virus-T7 RNA polymerase system for expression of target genes. Mol Cell Biol. 1987 Jul;7(7):2538–2544. doi: 10.1128/mcb.7.7.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Yilma T., Rose J. K., Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985 Jan 25;227(4685):433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Tobin G. J., McGowan J. J., Brown J. C. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. doi: 10.1128/jvi.41.3.1055-1062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden J. R., Pal R., Wagner R. R. Mapping regions of the matrix protein of vesicular stomatitis virus which bind to ribonucleocapsids, liposomes, and monoclonal antibodies. J Virol. 1986 Jun;58(3):860–868. doi: 10.1128/jvi.58.3.860-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wagner R. R. Regulation of viral transcription by the matrix protein of vesicular stomatitis virus probed by monoclonal antibodies and temperature-sensitive mutants. J Virol. 1985 Nov;56(2):386–394. doi: 10.1128/jvi.56.2.386-394.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal R., Grinnell B. W., Snyder R. M., Wiener J. R., Volk W. A., Wagner R. R. Monoclonal antibodies to the M protein of vesicular stomatitis virus (Indiana serotype) and to a cDNA M gene expression product. J Virol. 1985 Aug;55(2):298–306. doi: 10.1128/jvi.55.2.298-306.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. In vitro synthesis of triphosphate-initiated N-gene mRNA oligonucleotides is regulated by the matrix protein of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):897–904. doi: 10.1128/jvi.42.3.897-904.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Expression from cloned cDNA of cell-surface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell. 1982 Oct;30(3):753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloemer R. H., Wagner R. R. Association of vesicular stomatitis virus glycoprotein with virion membrane: characterization of the lipophilic tail fragment. J Virol. 1975 Aug;16(2):237–240. doi: 10.1128/jvi.16.2.237-240.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T., Lenard J. Interaction of wild-type and mutant M protein vesicular stomatitis virus with nucleocapsids in vitro. Biochemistry. 1981 Mar 3;20(5):1349–1354. doi: 10.1021/bi00508a048. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Wagner R. R. Localization of membrane-associated proteins in vesicular stomatitis virus by use of hydrophobic membrane probes and cross-linking reagents. J Virol. 1980 Oct;36(1):93–102. doi: 10.1128/jvi.36.1.93-102.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]