Abstract
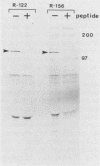
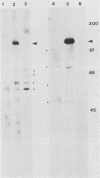
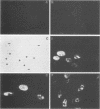
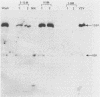
We have identified, in varicella-zoster virus (VZV)-infected cells, the product of the gene predicted to code for the VZV analog of the herpes simplex virus major DNA-binding protein. The open reading frame of the VZV gene has the potential to code for a protein with a predicted molecular weight of 132,000 (a 132K protein). To detect the protein, a 12-amino-acid oligopeptide corresponding to the carboxyl terminus of the putative open reading frame was synthesized and used to prepare antisera in rabbits. The resulting antibodies reacted specifically in Western immunoblot analysis and immunoprecipitation with a single 130K polypeptide found in VZV-infected cells. The specific reactivity of the antisera with the 130K polypeptide was inhibited by the addition of synthetic peptide. Immunofluorescence studies with the antisera as probe for the 130K polypeptide suggested that this peptide is located predominantly within the nuclei of infected cells. Analysis of proteins that bind to single-stranded DNA immobilized on cellulose matrices indicated that 30 to 50% of the 130K polypeptide is capable of interacting with single-stranded DNA and that this interaction is overcome with 0.5 M NaCl. Thus, we have prepared a specific polyclonal antiserum that identifies a VZV DNA-binding protein whose properties are similar to those of the herpes simplex virus ICP8 (Vmw130) DNA-binding protein.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Barry J., Bedinger P., Formosa T., Jongeneel C. V., Kreuzer K. N. Studies on DNA replication in the bacteriophage T4 in vitro system. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):655–668. doi: 10.1101/sqb.1983.047.01.077. [DOI] [PubMed] [Google Scholar]
- Anders D. G., Irmiere A., Gibson W. Identification and characterization of a major early cytomegalovirus DNA-binding protein. J Virol. 1986 May;58(2):253–262. doi: 10.1128/jvi.58.2.253-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss G. J., Marsden H. S., Hay J. Herpes simplex virus proteins: DNA-binding proteins in infected cells and in the virus structure. Virology. 1975 Nov;68(1):124–134. doi: 10.1016/0042-6822(75)90154-3. [DOI] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Davison A. J., McGeoch D. J. Evolutionary comparisons of the S segments in the genomes of herpes simplex virus type 1 and varicella-zoster virus. J Gen Virol. 1986 Apr;67(Pt 4):597–611. doi: 10.1099/0022-1317-67-4-597. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Waters D. J., Edson C. M. Identification of the products of a varicella-zoster virus glycoprotein gene. J Gen Virol. 1985 Oct;66(Pt 10):2237–2242. doi: 10.1099/0022-1317-66-10-2237. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Wilkie N. M. Location and orientation of homologous sequences in the genomes of five herpesviruses. J Gen Virol. 1983 Sep;64(Pt 9):1927–1942. doi: 10.1099/0022-1317-64-9-1927. [DOI] [PubMed] [Google Scholar]
- De Clercq E. Specific targets for antiviral drugs. Biochem J. 1982 Jul 1;205(1):1–13. doi: 10.1042/bj2050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B. The biochemistry and mechanism of action of acyclovir. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):9–17. doi: 10.1093/jac/12.suppl_b.9. [DOI] [PubMed] [Google Scholar]
- Felser J. M., Straus S. E., Ostrove J. M. Varicella-zoster virus complements herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1987 Jan;61(1):225–228. doi: 10.1128/jvi.61.1.225-228.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T., Hay J. Properties of herpesvirus-induced "immediate early" polypeptides. Virology. 1980 Jul 15;104(1):230–234. doi: 10.1016/0042-6822(80)90381-5. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchington P. R., Reinhold W. C., Casey T. A., Straus S. E., Hay J., Ruyechan W. T. Inversion and circularization of the varicella-zoster virus genome. J Virol. 1985 Oct;56(1):194–200. doi: 10.1128/jvi.56.1.194-200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchington P. R., Remenick J., Ostrove J. M., Straus S. E., Ruyechan W. T., Hay J. Putative glycoprotein gene of varicella-zoster virus with variable copy numbers of a 42-base-pair repeat sequence has homology to herpes simplex virus glycoprotein C. J Virol. 1986 Sep;59(3):660–668. doi: 10.1128/jvi.59.3.660-668.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Quinlan M. P., Spang A. E. Characterization of two conformational forms of the major DNA-binding protein encoded by herpes simplex virus 1. J Virol. 1982 Nov;44(2):736–741. doi: 10.1128/jvi.44.2.736-741.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Knipe D. M. Thermolabile in vivo DNA-binding activity associated with a protein encoded by mutants of herpes simplex virus type 1. J Virol. 1983 Jun;46(3):909–919. doi: 10.1128/jvi.46.3.909-919.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler E., Purifoy D., Minson A., Powell K. L. Herpes simplex virus non-structural proteins. III. Function of the major DNA-binding protein. J Gen Virol. 1983 May;64(Pt 5):983–995. doi: 10.1099/0022-1317-64-5-983. [DOI] [PubMed] [Google Scholar]
- Littler E., Yeo J., Killington R. A., Purifoy D. J., Powell K. L. Antigenic and structural conservation of herpesvirus DNA-binding proteins. J Gen Virol. 1981 Oct;56(Pt 2):409–419. doi: 10.1099/0022-1317-56-2-409. [DOI] [PubMed] [Google Scholar]
- Merrifield R. B. Solid-phase peptide synthesis. Adv Enzymol Relat Areas Mol Biol. 1969;32:221–296. doi: 10.1002/9780470122778.ch6. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Littler E., Purifoy D. J. Nonstructural proteins of herpes simplex virus. II. Major virus-specific DNa-binding protein. J Virol. 1981 Sep;39(3):894–902. doi: 10.1128/jvi.39.3.894-902.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. DNA-binding proteins of cells infected by herpes simplex virus type 1 and type 2. Intervirology. 1976;7(4-5):225–239. doi: 10.1159/000149955. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. DNA-binding proteins induced by herpes simplex virus type 2 in HEp-2 cells. J Virol. 1976 Aug;19(2):717–731. doi: 10.1128/jvi.19.2.717-731.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., McGeoch D. J. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the genes for DNA polymerase and the major DNA binding protein. Nucleic Acids Res. 1985 Nov 25;13(22):8143–8163. doi: 10.1093/nar/13.22.8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon F. J., Atkinson M. A., Hay J. Intranuclear distribution of herpes simplex virus type 2 DNA synthesis: examination by light and electron microscopy. J Gen Virol. 1983 Sep;64(Pt 9):2087–2092. doi: 10.1099/0022-1317-64-9-2087. [DOI] [PubMed] [Google Scholar]
- Roberts C. R., Weir A. C., Hay J., Straus S. E., Ruyechan W. T. DNA-binding proteins present in varicella-zoster virus-infected cells. J Virol. 1985 Jul;55(1):45–53. doi: 10.1128/jvi.55.1.45-53.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T. The major herpes simplex virus DNA-binding protein holds single-stranded DNA in an extended configuration. J Virol. 1983 May;46(2):661–666. doi: 10.1128/jvi.46.2.661-666.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyechan W. T., Weir A. C. Interaction with nucleic acids and stimulation of the viral DNA polymerase by the herpes simplex virus type 1 major DNA-binding protein. J Virol. 1984 Dec;52(3):727–733. doi: 10.1128/jvi.52.3.727-733.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A. E., Godowski P. J., Knipe D. M. Characterization of herpes simplex virus 2 temperature-sensitive mutants whose lesions map in or near the coding sequences for the major DNA-binding protein. J Virol. 1983 Jan;45(1):332–342. doi: 10.1128/jvi.45.1.332-342.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus S. E., Aulakh H. S., Ruyechan W. T., Hay J., Casey T. A., Vande Woude G. F., Owens J., Smith H. A. Structure of varicella-zoster virus DNA. J Virol. 1981 Nov;40(2):516–525. doi: 10.1128/jvi.40.2.516-525.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan P. J., Banks L. M., Purifoy D. J., Powell K. L. Interactions between herpes simplex virus DNA-binding proteins. J Gen Virol. 1984 Nov;65(Pt 11):2033–2041. doi: 10.1099/0022-1317-65-11-2033. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Lee K. J., Sabourin D. J., Schaffer P. A. Genetic analysis of temperature-sensitive mutants which define the gene for the major herpes simplex virus type 1 DNA-binding protein. J Virol. 1983 Jan;45(1):354–366. doi: 10.1128/jvi.45.1.354-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo J., Killington R. A., Watson D. H., Powell K. L. Studies on cross-reactive antigens in the herpesviruses. Virology. 1981 Jan 30;108(2):256–266. doi: 10.1016/0042-6822(81)90434-7. [DOI] [PubMed] [Google Scholar]